Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- ResearchBible
- Cosmos IF
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Elucidation of the biochemical and functional properties of an unknown novel protein from Arabidopsis thaliana
5th International Conference on Agriculture & Horticulture
June 27-29, 2016 Cape Town, South Africa
S K Sehlabane, T B Dikobe, P Chatukuta, D T Kawadza and O Ruzvidzo
North-West University, South Africa
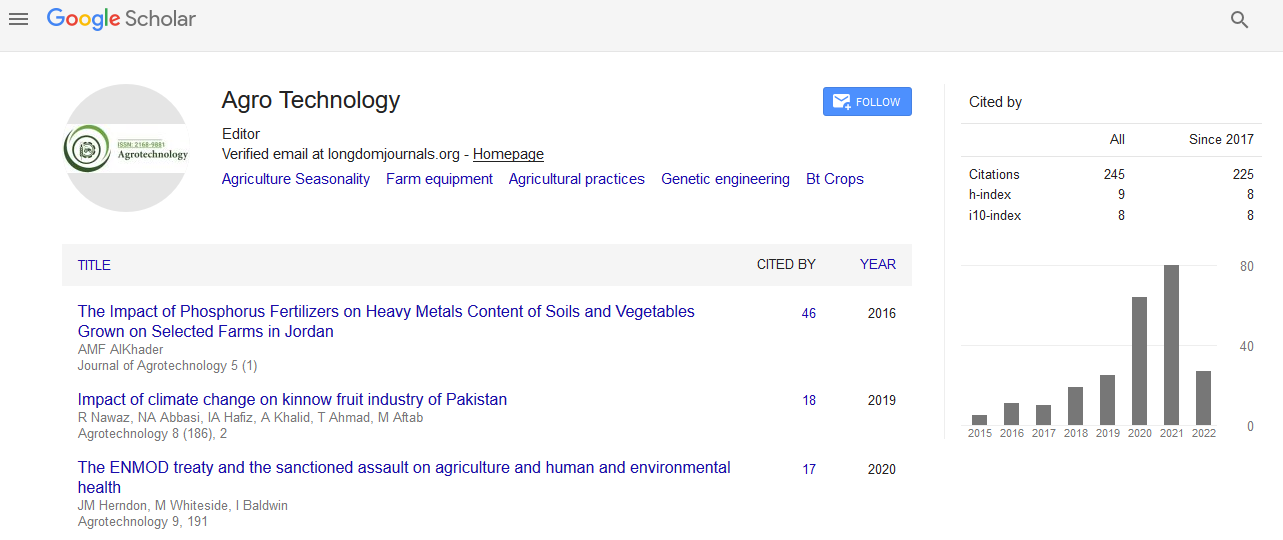
Posters & Accepted Abstracts: Agrotechnol
Abstract:
Adenylate cyclases (ACs) are a special group of enzymes capable of catalyzing the conversion of adenosine 5-triphosphate (ATP) into the signaling molecule cyclic 3,5-adenosine monophosphate (cAMP), which in turn acts as a second messenger in various cellular and metabolic pathways. Apparently, while the presence of ACs and their functional roles in animals and prokaryotes have since been well-documented, their presence and/or functional roles in higher plants has somewhat remained a matter of serious debates and controversy. Notably and in a recent BLAST search of the Arabidopsis genome using a 14-mer motif with specificity for ATP binding and catalysis, an AC-like protein coded for by the At3g21465 gene has been identified. However, even though the AC-like protein does contain the AC catalytic core motif, it notably has not yet been shown to possess any known putative AC catalytic function and/or share any similarities with any annotated and/or experimentally confirmed ACs, but instead, it only appears to be transcriptionally up-regulated in response to biotic stress factors. Therefore in an attempt to test and determine whether this putative protein candidate has any functional AC activity, total mRNA of the 4-6 weeks old Arabidopsis thaliana plants was extracted and used as a template for the complementary synthesis and amplification of a 384 bp AC-like gene fragment via a specialized Reverse Transcriptase - Polymerase Chain Reaction (RT-PCR) system. The amplified fragment was then cloned into a pTrcHis2- TOPO expression vector and the resultant recombinant expression vector eventually transformed into chemically competent E. cloni express BL21 (DE3) pLysS expression host cells. Positive clones were determined by confirmatory PCR and further validated by nucleotide-specific sequencing. The 18.0 kDa C-terminus His-tagged recombinant AC-like protein was then over-expressed following an induction with isopropyl-β-D-1-thiogalactopyranoside (1 mM, IPTG) and purified over a nickel-nitrilotriacetic acid (Ni-NTA) affinity matrix system. The endogenous and in vitro AC activities of the resultant recombinant AC-like protein were then tested via a cAMP-linked enzyme immunoassaying system while its inherent in vivo AC activity was also concurrently tested via a complementation testing system using the cyaA SP850 mutant Escherichia coli cells. Results from these three independent assays collectively indicated that the AC-like protein encoded for by the At3g21465 gene from A. thaliana possesses the endogenous, in vitro and in vivo AC activities, and thus unequivocally confirming it as a bona fide higher plant AC molecule with a possible cAMPmediated signaling system.
Biography :
S K Sehlabane is currently working in North-West University, South Africa Katlego.
Email: sehlabane@gmail.co.za