Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2021) Volume 12, Issue 4
Why Nose and Mouth Coverings are Highly Recommended to Impede SARS-Cov-2 Spread
J.L. Morán-López1* and A. Calles22Department of Physics, Faculty of Science, National Autonomous University of Mexico, Mexico City, Mexico
Received: 14-May-2021 Published: 04-Jun-2021, DOI: 10.35248/2155-9597.21.12.402
Abstract
In fourteen months the number of infected people with SARS-COV-2 has reached more than 159 millions and from those more than 3 million have resulted in death. There is now a consensus that the airborne saliva droplets, that are produced while speaking, coughingor sneezing by infected people is one of the most likely routes of transmission of the corona virus disease (COVID-19). The expelled droplets can measure between 0.4 and 450 μm in diameter. Once the droplets are in the air, they are subject to the gravitational, and air frictional forces that dictate their motion. Through exhaustive aerodynamic studies it has been shown that the aerosol droplets (less than 5 μm) can remain in the environment for very long periods of time and be transported by air currents. Larger droplets take shorter times and land within a circle of 1.5 to 2 m radius. Of key importance is the droplet size distribution and many efforts have been done to characterize this. By modeling the production of the number of saliva droplets with log-log Gaussian distributions, the virial load of the expelled droplets is estimated as a function of droplet size. Assuming a constant virus density, we estimate the amount of virus delivered into the environment. The use of face masks reduce drastically the amount of droplets emitted to the air by an infected person and to be inhaled by a healthy one. We emphasize the great importance of using adequate face protection to minimize COVID-19, transmission and to reduce the death toll due to this disease.
Keywords
COVID-19; SARS-COV-2; Virus transmission; Saliva droplets
Introduction
After fourteen months of the first reported case of the SARS-CoV-2 virus infection, by the World Health Organization (WHO), the virus transmission has not been controlled and continues affecting millions of people, all over the world [1]. The number of infected people has reached a record number of 157 millions, with 3.2 million of them resulting in death. The COVID-19 pandemic has caused enormous economic and social disruption. Although several vaccines have been produced in a record time, the number of fully immunized people is still very small. Therefore it is imperative to continue observing sanitary recommendations to reduce, as much as possible, virus transmission.
Although there are still many unknowns, it is now accepted that the most likely route of infection is through the airborne saliva droplets emitted by infected people while breathing, talking, coughing or sneezing. Many studies have shown that the infection can occur by the inhalation of those small saliva droplets [2,3]. This is supported by recent studies, in which high viral loads of SARS-CoV-2 were found in the oral fluids of patients suffering the coronavirus disease [4,5].
Literature Review
This path of virus propagation was discussed more than ten years ago, during the H1N1 pandemic. However, despite several investigations published over the last decade that showed risk of transmission through airborne droplets, health authorities only recently recognized the importance of using face protection, and now recommends its widespread use [6]. The results presented in this study strongly support that recommendation.
There is experimental evidence that saliva droplets exhaled in respiratory events have a diameter (D) that range from less than a micron to about 500 microns. The number of droplets as a function of its size is a key information to estimate the risk that they represent. The characterization of this function is very complex, since it depends on many environmental parameters and the characteristics of the emitting person. Nevertheless, various experimental techniques have been used in order to determine the droplet size distributions while sneezing, coughing or talking [7-10]. Based on these experimental results, one can fit the data by log-log Gaussian distribution functions; the maximum of the emitted particles, the diameter at which this occurs, and the width of the bell curve function depends on the respiratory action. The experimental maxima occur around 10 μm, and the number of droplets expelled with this diameter are approximately 350,000, 1,600, and 78, while sneezing, coughing and talking, respectively. In Figure 1a, we show the fitting of the number of droplets Nd as a function of the droplets diameter D.
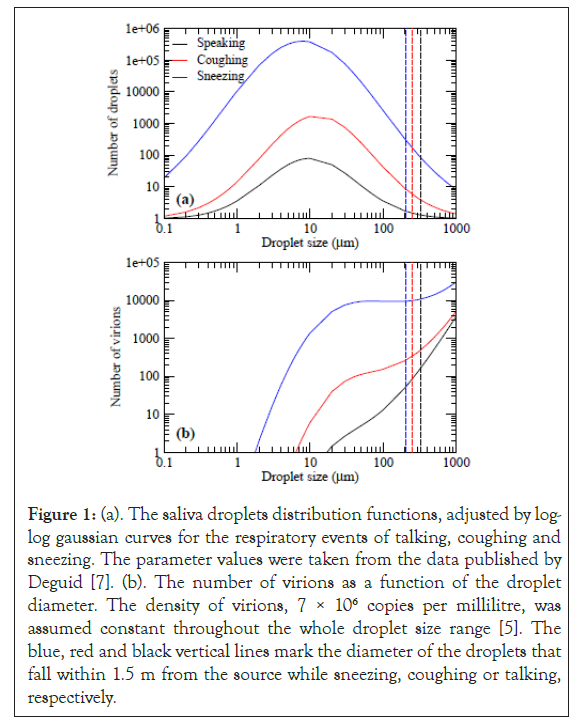
Figure 1: (a). The saliva droplets distribution functions, adjusted by log- log gaussian curves for the respiratory events of talking, coughing and sneezing. The parameter values were taken from the data published by Deguid [7]. (b). The number of virions as a function of the droplet diameter. The density of virions, 7 × 106 copies per millilitre, was assumed constant throughout the whole droplet size range [5]. The blue, red and black vertical lines mark the diameter of the droplets that fall within 1.5 m from the source while sneezing, coughing or talking, respectively.
These fits reproduce the maximum amount of droplets, the D where this occurs, and the number of droplets with D=100 μm.
Discussion
DOne of the key differences in the three respiratory actions is the velocity at which the droplets are ejected. Those velocities are approximately 120, 60, and 30 km/hr, while sneezing, coughing and talking, respectively. With these parameters, through exhaustive aerodynamic calculations that take into account the gravitational and drag forces, it has been shown that, in the absence of air currents, for droplets with D=0.4 μm, the time of flight is 3.83 days for the three starting velocities and the horizontal distances traveled are 3.07 × 10-5; 1.54 × 10-5, and 7.75 × 10-6 m, when, sneezing, coughing and talking, respectively. These droplets would remain suspended in the air for several days in the absence of air currents, and the distance traveled horizontally would be negligible. For larger droplets measuring 1 μm in diameter, still in the aerosol regime, the time needed to fall to the ground decreases to 14.7 hours and the distance traveled increases to 1.74 × 10-4, 8.8 × 10-5, and 4.43 × 10-5 m, for sneezing, coughing, and speaking, respectively. This is still a very long residence time and the distance traveled is now near the millimeter regime. The 10 μm droplets stay in the air 8.82 min and travel 4.17, 7.83 and 14.1 mm. Although the particles remain suspended only minutes, there is enough time for events characteristic of daily life activities to impact their travel distance. These droplets move some millimeters away from the source. Much larger droplets with diameter D=100 μm, would be in the air 3.28 seconds and will land 26.8 cm, away when speaking, 40.6 cm away, when coughing, and 50 cm away, when sneezing from the emitting person. Finally, the 450 μm droplets ejected at the talking, coughing, and sneezing velocities take only 0.66 s, 0.67 s and 0.68 s, and they travel horizontally 1.82, 2.89 and 3.75 m.
Furthermore, it is found that in a calm environment, the saliva droplets that fall within a distance of 1.5 m, are those with diameters less than 327 μm, 250 μm, and 202 μm emitted while speaking, coughing or sneezing, respectively. By inspecting Figure 1a, these results confirm that most of the droplets fall within the safe distance, recommended by the WHO authorities of 1.5 m. However, two points have to be raised: i) the aerosol droplets, with D<5 μm stay stationary around the source with a residence time that can be of hours or even days. These scenario changes in the presence of wind disturbances, which can transport them much farther. Good ventilation can move the droplets far away and disperse them, reducing thereby the risk. However, weak air currents can only extend the action range of the virus loaded droplets; ii) In the size distribution curves, a portion of droplets, with large sizes fall in a distance longer than 1.5 meters, prompting the question of how dangerous these droplets might be. To answer this question, it is necessary to estimate the viral load of the droplets as a function of their size and combine that information with the size distribution. Virological testing using Reverse Transcription Polymerase Chain Reaction (RT-PCR) found, in saliva samples of sick persons, an average viral load of 7 × 106 copies per milliliter [5]. Thus, to estimate the number of virions expelled in the respiratory events, we calculate from Figure 1a, the volume associated to the number of droplets for each size and multiply them by the average viral density.
In Figure 1b, we show the total number of virions contained in each droplet population (number of droplets × volume × viral load) as a function of size. It is important to note that in the absence of information on how the viral density changes as a function of droplet size, we have assumed a constant value, obtained for milliliter samples. Thus, the estimations are more accurate for large volume saliva droplets. The results for the aerosol regime are probably underestimated. Here, the onset of the curves starts when the volume is big enough to contain a virus unit. In the curves related to coughing and sneezing, one observes a rapid increase that slows down when the number of droplets pases the maximum value. The second increase is due to the cubic dependence of the volume that overcomes the decrease in the number of droplets. The curve for droplets generated by speaking is continuously increases as the droplets volume dominates throughout the whole range. Although the number of particles that land at a distance longer than 1.5 m, is small, they carry an important viral load. It is better to keep a distance of 2 meters between individuals. The size of the droplets that fall within that distance is 431 μm, 316 μm, and 254 μm, while speaking, coughing, or sneezing, and the landing time is 0.66, 0.78, and 0.91 seconds.
Droplets with diameters of 10-100 μm convey the largest viral load due to the abundance of droplets of this size (calculated as 17,500 when talking, 340,000 when coughing and 46,100,000 when sneezing). Those drops stay in the air from 529 to 3.28 seconds, a range of time very dangerous for an infection. However, their horizontal range is less than 1 m, i.e. within the safe distance of 1.5 m, recommended by the WHO.
From our results, one can observe that the best way to reduce the virus transmission is to block the dissemination of viral particles by mouth and nose coverings. We stress the importance that a person with symptoms uses, mouth and nose protection, in order to avoid the contamination of its immediate environment. On the other hand, equally important is that a healthy person protects mouth, nose and eyes, to avoid the eventual inhalation of the airborne virus and the contact with eyes. This measure should be observed any time out of home and by all the public.
A more dangerous scenario is that of group gatherings in confined environments like hospitals, schools, airplanes, or public transportation, where air ventilation might not be sufficient and should be maximized. The use of mouth and nose protection must be enforced.
An important observation that supports the recommendation of the implementation of face coverings, is that, common fabric materials, like cotton or silk, can be effectively used in the production of protective face masks [11]. A mask made with a combination of cotton and silk provides high filtration efficiencies of droplets in the range of 10 nm to 6 μm. Thus, there is no reason for shortage of masks since they should be available at very low cost. These low cost measures, can be implemented easily in developing countries to effectively reduce the virus propagation.
In summary, we have modeled the virus load of airborne saliva particles expelled by SARSCOV-2 infected people. We analysed three different respiratory events, sneezing, coughing and speaking. The major risk is represented by the medium size, 10-100 μm emitted while sneezing. A topic under study is the size dependence of the virus concentration as a function of droplet size: in particular in aerosol droplets.
Conclusion
The use of face covering is a low cost measure that can control the virus spread by infected people and protect healthy ones. The enforcement of mask-wearing reduces the virus transmission and thereby could minimize the death toll. We expect that this report motivates further experimental studies to continue the understanding of airborne transmission of respiratory infections.
Acknowledgments
AC acknowledges the financial support of DGTIC-UNAM, under projects LANCADUNAM-DGTIC-055 and 0385, and UNAM- PAPIT IN114318 and IN120120. The calculations were carried out in the Mitzli UNAM supercomputer. Technical assistance by S.López-Moreno and critical discussions with J.M. Morán-Mirabal, are also acknowledged.
REFERENCES
- Rolling updates on coronavirus disease (COVID-19). World Health Organization. 2020.
- Morawska L, Junji C. Airborne transmission of SARS-CoV-2: The world should face the reality. Environmental Int. 2020;139(1):105730.
- Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. PNAS. 2020;117(26):14857-14863.
- Chan JFW, Yip CCY, To KKW, Tang THC, Wong SCY, Leung HY, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310-e00320.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
- Weber TP, Stilianskis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57(5):361-373.
- Duguid JP. The size and the duration of air-carriage of respiratory droplets and dropletnuclei. J Hyg (Lond). 1946;44(6):471-479.
- Morawska L, Johnson G, Ristovski Z, Hargreaves M, Mengersen K, Corbett S, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(3):256-269.
- Johnson G, Morawska L, Ristovski Z, Hargreaves M, Mengersen K, Chao C, et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42(12):839-851.
- Han ZY, Weng WG, Huang QY. Characterizations of particle size distribution of the droplets exhaled by sneezing. J R Soc Interface. 2013;10(1):20130560.
- Konda A, Prakash A, Moss GA, Schmoldt M, Gram G, Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;11(1):0c03252.
Citation: Morán-López JL, Calles A (2021) Why Nose and Mouth Coverings are Highly Recommended to Impede SARS-Cov-2 Spread. J Bacteriolc Parasitol. 12: 402.
Copyright: © 2021 Morán-López JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

