Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 5
Virulence Diversity and Physiological Race Composition of Wheat Stem Rust (Puccinia graminis f.sp. tritici) in Tigray Region, Northern Ethiopia
Gizachew Hirpa Regasa1* and Netsanet Bacha Hei22Ethiopian Institute of Agricultural Research Head Offices, Addis Ababa, Ethiopia
Received: 23-Apr-2021 Published: 30-May-2021, DOI: 10.35248/2157-7471.21.12.555
Abstract
Wheat stem rust (black rust) is one of the most important airborne diseases of wheat (Triticum aestivum) caused by Puccinia graminis f. sp. tritici remains a constraint to the world’s wheat production. Because of the sudden changes in stem rust race patterns, commercial varieties tend to become vulnerable globally at large and particularly in Ethiopia. It was responsible to cause 6.2 million metric tonnes per year or higher losses under severe epidemics at global level. Therefore, this study was initiated to identify the physiological races and virulence diversity of Puccinia graminis f. sp. tritici in Tigray region. Race identification through inoculation of stem rust isolates, multiplication of single-pustule of the pathogen and race designation by inoculating on a set of wheat differential lines were done in the greenhouse. Forty-seven stem rust isolates were analyzed on the twenty stem rust differentials and resulted in the identification of six races namely; TTTTF, TKTTF, TRTTF, TTRTF, RRTTF and TKPTF. In this study, race TTRTF was detected for the first time in Ethiopia during 2017 cropping season. Out of the six races identified, TTTTF was detected from 25 (53.19%) isolates and TKTTF from 15 (31.91%) isolates. The most virulent race that made 18 stem rust resistant genes non-effective was TTTTF, which virulent on 90% of stem rust resistance genes. Differential hosts carrying Sr24 and Sr31 were effective genes which confers resistance to all of the races identified. Hence, the stem rust resistance gene Sr24 and Sr31 can be used as sources of resistance in the wheat breeding program.
Keywords
Gene; Puccinia graminis tritici; Race; Resistant; Stem rust; Virulence
Introduction
Wheat (Triticum spp.) is one of the most important staple cereal crops cultivated in the world particularly, Ethiopia and produced in different agro-ecological zones [1,2]. It is the leading source of cereal proteins in human food and one of the primary staple foods throughout the planet, having higher protein content than maize and rice [3]. Wheat plays an important role in everyday life of the world’s population and provides over 21% of the food calories and 20% of the protein to more than 4.5 billion people, thereby playing a fundamental role in food security [4]. Globally, wheat is cultivated on over 244 million hectares of land with a production of about 881 million metric tonnes and the productivity of wheat worldwide on the average reaches 3.65 t/ha [4].
In Ethiopia, it ranks 4th after teff, maize, and sorghum in area coverage and 3rd in total production after maize and teff. It is cultivated on 1.70 million hectares of land with total production of 4.64 million tonnes which makes the country the largest wheat producer in Sub-Saharan Africa [5]. The total wheat area and production in 2017 cropping season in the Tigray region was 107,929.86 ha and 214,003.14tonnes, respectively [5]. However, the national average yield of wheat in Ethiopia is estimated at 2.74 t/ha while it is only 1.98 t/ha in Tigray region [5], which is lower than the country’s and world's average yield of 3.65 t/ha [3]. The low productivity is attributed to a number of factors caused by biotic, abiotic and socioeconomic constraints [6].
The major biotic factors that constraints wheat production in the country include diseases, insect pests and weeds [2]. Among the fungal diseases, stem rust (Puccinia graminis f. sp. tritici Eriks. & E. Henn), leaf rust (P. triticina Eriks) and stripe rust (P. striiformis Westend. tritici Eriks) are the most important diseases reducing wheat production in Ethiopia. Wheat Stem rust (black rust) caused by Puccinia graminis f. sp. tritici is one of the most devastating air borne diseases of wheat which causes considerable yield losses in the world, particularly in our country Ethiopia. The pathogen is capable to produce new physiological races that attack resistant varieties and develop epidemic under optimal environmental conditions which causing up to 100% yield losses over wide areas during epidemic years [7-9].
The last major stem rust epidemic occurred in Ethiopia during 1993/94 [10] when a popular wheat variety ‘Enkoy’ fell out of production, the rest of the world has practically remained unhurt from stem rust for over three decades [11] until the appearance of a new virulent race named Ug99 that overcomes the previously effective stem rust resistance gene Sr31. Even if, Ug99 in Ethiopia first occurred in 2003 in a few locations, it has now become the dominant race across all regions [12]. Ug99 race has broad virulence, mutates and expanded quickly. Currently, there are 13 variants of Ug99 races have been found that are derivatives from the original race TTKSK and rendering additional resistance genes ineffective having an almost identical DNA pattern, only differing in their avirulence/virulence formula [13]. Three additional variants of Ug99 have been detected in Ethiopia are TTTSK, PTKSK and PTKST races [14]. Again, in 2013/14 and 2014/15 severe localized stem rust epidemics occurred on variety Digalu, caused by race TKTTF. Ug99 (race TTKSK) was first confirmed in 2003 and continued to be the predominant pathotypes until 2014 when TKTTF (Digalu) race became dominant [15,16].
Furthermore, changing temperature and rainfall patterns have encouraged the emergence of new stem rust races that overcome the currently resistant wheat varieties and popularly grown wheat varieties remain at constant stake of losing their resistance to it [17]. Therefore, virulence spectrum and diversity were important for studying the evolution of new races and forecasting the virulence shifts in a population.
Materials and Methods
Collection of rust samples
Stems and/or leaf sheath of wheat plants infected with stem rust were cut into small pieces of 5-10 cm in length using scissors and placed in paper bags after the leaf sheath was separated from the stem in order to keep stem and/or leaf sheath dry. This technique was used in order to prevent spores germination before processing in the greenhouse. The collected samples were labeled with the name of the zone, district, variety and date of collection and kept in a refrigerator at 4oC until the surveys in all districts completed and used for the virulence analysis. Once sample collection has completed the samples were kept in the icebox and transported to Ambo Agricultural Research Center’s (AmARC) laboratory for race analysis and it was done in the greenhouse of Ambo Agricultural Research Center.
Isolation of the pathogen
Seedlings of variety “McNair”, which does not carry known stem rust resistance gene, were raised in 5 cm diameter pots for inoculation in the growth chamber. For raising seedlings, sterilized soil composed of three different materials; soil, sand and farmyard manure mixed at the ratio of 2:1:1 by volume were used. Seedlings were raised by spreading the seeds on filter paper in Petri dishes, moisten with water and close the lid to pre-germinate seeds. On the third day, the seeds germinate and the radicles were seen. Then, these germinated seeds were planted in pots using forceps. Inoculation in the greenhouse was done to revitalize the spores collected from the field, to multiply the isolates and to inoculate the differential lines sets for race identification. Greenhouse inoculations were done using the methods and procedures developed by Stackman et al. [18].
Leaves of seven-day-old McNair seedlings or seedlings with fully expanded primary leaves and second leaves beginning to grow were rubbed gently with clean (disinfected with 70% alcohol) moistened (with distilled water) fingers to remove the waxy layer from the surface of the leaves that hinders the penetration of the spores. Spores from the stem rust infected sample was collected using atomized spore collector/vacuum pump in the cubicle, then suspended in lightweight mineral oil (Soltrol 170) and then sprayed onto seven-day-old seedlings of McNair [19]. Inoculation of the susceptible check McNair was done late in the afternoon when the ambient temperature is low and cool. Cool temperatures help moisture to stay longer on the leaves, thus facilitating the germination of spores resulting in infection [20].
The inoculated seedlings were placed on a table for 30 minutes until the Soltrol evaporates and leaves have dried out. Following this, the seedlings were moistened with fine droplets of distilled water produced with an atomizer and placed in the incubation chamber for 18 hours in a dark at 18-22°C followed by exposure to light for 3-4 hours to provide a condition for infection and seedlings were allowed to dry their dew for about 1-2 hours. The incubation chamber has light, dew chambers and humidifiers. The incubation chamber was wooden boxes covered with the white polyethylene sheet and again the black polyethylene sheet was coved white polyethylene sheet in order to create darkness in the wooden box. The humidifier on for about 1:30 hours, so the seedlings have enough moisture for the whole dark period, this condition facilitates the initial infection process successful.
Then after, the seedlings were transferred from the dew chamber to glass compartments in the greenhouse where conditions are regulated at 12 hours photoperiod, at a temperature of 18-25°C and relative humidity of 60-70%. The remaining rust spore samples were kept in the refrigerator at 4°C, in order to substitute for samples that failed to produce infection on McNair in the greenhouse. After seven to ten days of inoculation (when the flecks/symptoms are clearly visible) leaves containing a single fleck that produces a single pustule were selected from the base of the leaves and the remaining seedlings within the pots were removed using scissors. Leaves with a single pustule were separately covered with cellophane bags (145×235 mm) and tied up at the base with a rubber band to avoid cross contamination [21].
Multiplication of single pustules
After two weeks of inoculation (when a pustule is well developed), spores from each pustule were collected using power-operated vacuum aspirator and stored separately in gelatine capsules. A suspension, prepared by mixing urediospores with lightweight mineral oil (Soltrol), was inoculated on seven-day-old seedlings of the susceptible variety 'McNair' for multiplication purpose for each of the single pustules on separate pots. Immediately after inoculation, the seedlings were placed in an incubation chamber in dark condition at 18-22°C for 18 hours and light for 3-4 hours, after which they were transferred to a greenhouse where the temperature varied between 18-25°C and RH of 60-70% following the procedures mentioned earlier. Then, 14 days after inoculation, the spores of a single pustule were collected in separate test tubes and stored at 4°C until they were inoculated on the standard differential sets. The spore multiplication procedure was repeated again until sufficient spores are produced to inoculate the set of stem rust differential hosts (Figure 1).
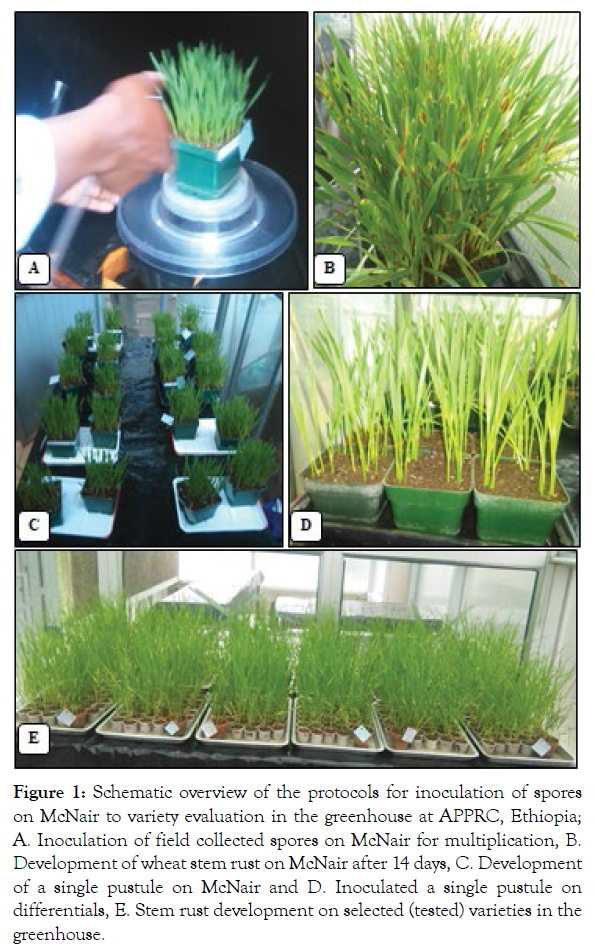
Figure 1: Schematic overview of the protocols for inoculation of spores on McNair to variety evaluation in the greenhouse at APPRC, Ethiopia; A. Inoculation of field collected spores on McNair for multiplication, B. Development of wheat stem rust on McNair after 14 days, C. Development of a single pustule on McNair and D. Inoculated a single pustule on differentials, E. Stem rust development on selected (tested) varieties in the greenhouse.
Inoculation of Wheat Stem Rust Differential Host Lines
Five seeds of the twenty wheat stem rust differentials (Table 1) with known resistance genes and one susceptible variety McNair were grown in 5 cm diameter pots separately in the greenhouse. Each rust isolate derived from a single pustule was suspended in Soltrol 170. The suspension was adjusted to 4-5 mg spores per 1 ml lightweight mineral oil suspension and inoculated onto seedlings of the differentials using spore inoculators. After inoculation, plants were moistened with fine droplets of distilled water produced with an atomizer and placed in an incubation chamber for 18 hrs dark period at 18-22°C and 98-100% relative humidity.
| Differential host lines | Stem rust genes | Pedigree |
|---|---|---|
| LcSr24Ag | 24 | Little Club/Agent (Cl 13523) |
| W2691SrTt-1 | 36 | Cl12632 T. timopheevii |
| ISr7b-Ra | 7b | Hope/Chinese Spring |
| ISr8a-Ra | 8a | Rieti/Wilhelmina//Akagomughi |
| CnSSrTmp | Tmp | Triumph 64(Cl 13679)/ Chinese Spring |
| Sr31(Benno)/6*LMPG | 31 | Kavkaz |
| CnS-T-.mono-deriv | 21 | Einkorn Cl 2433 |
| Trident | 38 | Spear*4/VPM (Pl519303) |
| ISr9a-Ra | 9a | Red Egyptian/Chinese Spring |
| ISr9d-Ra | 9d | Hope/Chinese Spring |
| Combination VII | 17 | Esp 518/9 |
| ISr5-Ra | 5 | Thatcher/Chinese Spring |
| ISr6-Ra | 6 | Red Egyptian/Chinese Spring |
| W2691Sr9b | 9b | Kenya 117A |
| Vernsteine | 9e | Little Club//3*Gabo/2* |
| W2691Sr10 | 10 | Marquis*4/Egypt NA95/2/2*W2691 |
| BtSr30Wst | 30 | Festival/Uruguay C10837 |
| CnsSr9g | 9g | Selection from Kubanka (Cl1516) |
| ISr11-Ra | 11 | Kenya C6402/Pusa4/Dundee |
| McNair 701 | McN | Cl 15288 |
Table 1: Wheat stem rust differential lines with their corresponding stem rust resistant gene used for the present study.
Upon the seedling removed from the incubation chamber, plants were exposed to 3-4 hrs of light to facilitate the infection process and seedlings were allowed to dry/remove their dew for about 1-2 hrs. Inoculated plants were placed in separate glass compartments in the greenhouse to avoid contamination and produce infection. Greenhouse temperature was maintained between 18°C and 25°C. Natural daylight was supplemented with an additional 4 hrs/day that emitted by cool white fluorescent tubes arranged directly above plants [22].
Determination of Stem Rust Races
Seedling infection types (ITs) were scored 14 days after inoculation using 0 to 4 scoring scale described by Stakman et al. [18]. The IT readings of 3 (medium-size uredia with/without chlorosis) and 4 (large uredia without chlorosis or necrosis) were regarded as susceptible. Other readings, i.e., 0 (immune or fleck), 1 (small uredia with necrosis) and 2 (small to medium uredia with chlorosis or necrosis) were resistant (Figure 2). The variations were refined by modifying characters as follows: -, uredinia somewhat smaller than normal for the infection type; +, uredinia somewhat larger than normal for the infection type. Hence, ITs were then grouped into low (“0”, “;”, “;1”, “1”, “1+”, “2-”, “2”, “2+”) and high (“3-”, “3”, “3+”, and “4”) infection types [18].
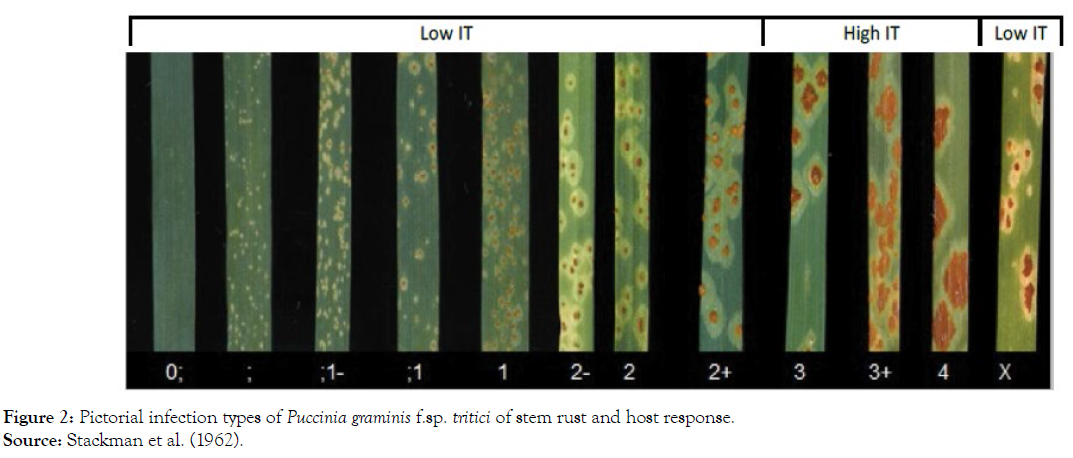
Figure 2: Pictorial infection types of Puccinia graminis f.sp. tritici of stem rust and host response. Source: Stackman et al. (1962).
Race identification was done using the North American nomenclature system for Puccinia graminis f. sp. tritici [23]. Races were identified based on their reaction on differential hosts. Race designation was done by grouping the differential lines into five subsets as indicated in Table 2. Each isolate was assigned using a combination of a three letter code of Roelfs and Martens [23] and an additional two letter race code by Jin et al. [24] which finally give a five letter of designation based on its reaction on the differential lines [21].
| Wheat Pgt gene differential sets and infection phenotype of pathogen coding | ||||
|---|---|---|---|---|
| Sets | Differential lines identified by Pgt resistance gene | |||
| Set 1 | 5 | 21 | 9e | 7b |
| Set 2 | 11 | 6 | 8a | 9g |
| Set 3 | 36 | 9b | 30 | 17 |
| Set 4 | 9a | 9d | 10 | Tmp |
| Set 5 | 24 | 31 | 38 | McN |
| Pgt-code | Infection phenotype; High = virulent reaction (susceptible) and low = avirulent reaction (resistant) | |||
| B | Low | Low | Low | Low |
| C | Low | Low | Low | High |
| D | Low | Low | High | Low |
| F | Low | Low | High | High |
| G | Low | High | Low | Low |
| H | Low | High | Low | High |
| J | Low | High | High | Low |
| K | Low | High | High | High |
| L | High | Low | Low | Low |
| M | High | Low | Low | High |
| N | High | Low | High | Low |
| P | High | Low | High | High |
| Q | High | High | Low | Low |
| R | High | High | Low | High |
| S | High | High | High | Low |
| T | High | High | High | High |
Table 2: North American nomenclature of Puccinia graminis f. sp. tritici based on 20 differential wheat lines.
Depending on the above illustration: race TTTTF where;
Set 1 = T (No genes in set are effective)
Set 2 = T (No genes in set are effective)
Set 3 = T (No genes in set are effective)
Set 4 = T (No genes in set are effective)
Set 5 = F (Only genes Sr24 and Sr31 are effective)
For instance, low infection type (IT) on all four hosts in a set was assigned the letter B, while high IT on the four hosts assigned T. Hence, an isolate produces low IT (resistant reaction) on each of the 20 differential lines; the race was designated with a five letter race code BBBBB. In the same way, an isolate that produces a high IT (susceptible reaction) on the 20 differential lines had a race code TTTTT. If an isolate produces a low IT on Sr31 and Sr24, but a high infection type on the remaining 18 differential lines, the race was designated as TTTTF. The frequency of each race identified was also recorded.
Results and Discussion
Sixty-six infected wheat stem samples were collected from Southern, Eastern and Southeast zones of Tigray region. Of these, 19 did not yield viable isolates at the time of inoculation in the laboratory; hence, 47 isolates were used for the race spectrum analysis. Of these isolates, 6 races namely TTTTF, TKTTF, TRTTF, RRTTF, TTRTF and TKPTF were identified (Figure 3). All the 6 races were found in the Southern zone. Three races (TTTTF, TKTTF, and TKPTF) were identified from Southeast zone while 2 races TTTTF and TKTTF were detected in Eastern zone. Only 1 race TKTTF was detected in Saesia Tsaedaemba district while in the other districts 2 or more races were detected. TKTTF race was a common race detected in all the studied districts of the three zones and also TTTTF was detected from all districts except Saesia Tsaedaemba district.
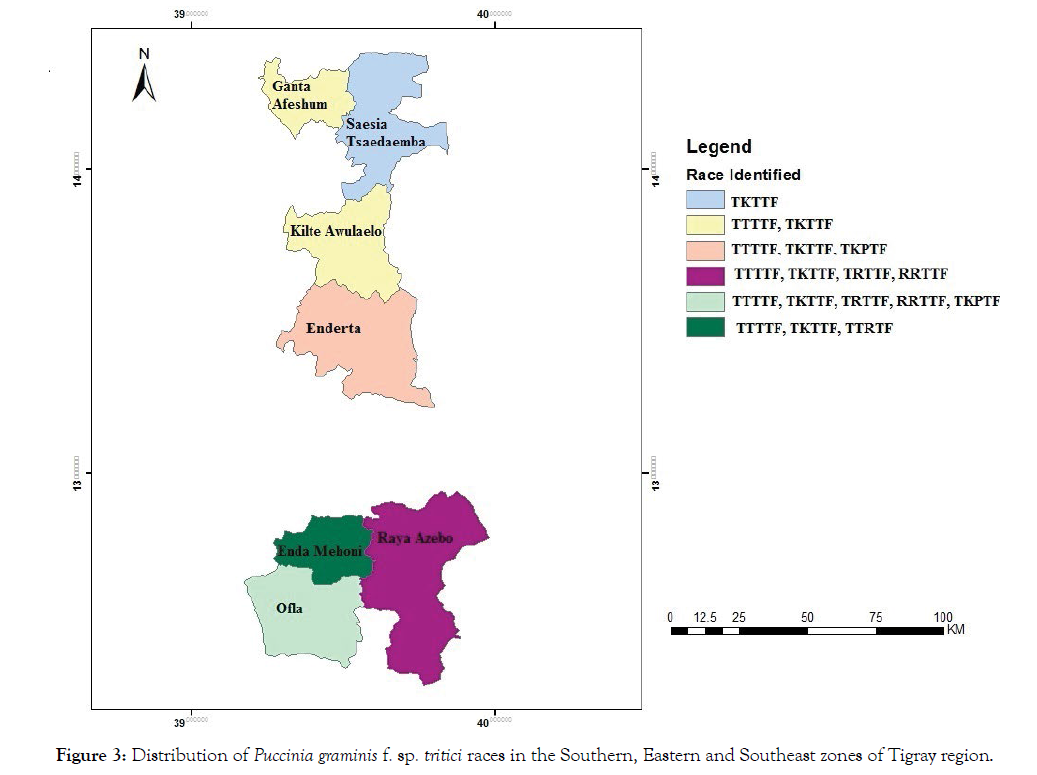
Figure 3: Distribution of Puccinia graminis f. sp. tritici races in the Southern, Eastern and Southeast zones of Tigray region.
Out of the 47 viable stem rust samples collected, TTTTF race was identified from 25 isolates while TKTTF detected from 15 isolates indicating that TTTTF and TKTTF were the most abundant races in the study areas in the season. TTTTF with virulence to Sr9e and Sr13 attacked thousands of hectares of wheat, resulting in the largest burst of wheat stem rust in the world and a large number of spores produced by it may continue the epidemic [25]. This race has a virulence formula which is almost similar to TKTTF but is clearly different from stem rust race TTKSK (Ug99) as it has avirulence to Sr24 and Sr31. TTTTF race was first detected from samples collected in 2009 in Eastern Shoa zone of central Ethiopia at trace level [26]. It was also detected in Iran from samples collected during 2010-2014 [27,28]. TTTTF hit several thousands of hectares of durum wheat on the Italian Islands of Sicily in 2016, causing the largest stem rust outbreak that Europe has seen in decades [13].
This study revealed that TKTTF race was the second most dominate stem rust population in the season. TKTTF was detected for the first time at a trace level in 2012 main cropping season, samples collected from Arsi and Bale zones of Oromia region and was found to be primary cause of the epidemics in the Southeastern parts of the country in 2013 and 2014 cropping seasons [29]. The detection of this race in 2012 was the first report of virulence to SrTmp in the country. This non-effective gene is present in the most popular and widely grown bread wheat variety Digalu [29]. According to Mert et al. [30] as cited by Singh et al. [14] races similar to TKTTF occurred in Turkey in the 1990s and still are predominant races in country. TKTTF has been detected in Iran in 2010, Lebanon in 2012, Egypt in 2013, Azerbajan, Eriteria and Yemen [16]. It is also detected in Kenya from samples collected in 2014 and 2015. The presence of stem rust race with identical virulence profiles throughout this vast region implies that there are inoculum exchanges and the race is a serious threat to the wheat production to wider scale and needs monitoring [31]. Studies indicated that TKTTF does not belong to Ug99 lineage based on avirulence to Sr11 and Sr31 and molecular fingerprints [16].
The frequency of each race was calculated as a percentage of the total number of isolates analyzed. The races identified from major wheat grown areas in the zone of the region had wide virulence spectra (Table 3). Out of the six races identified, the most frequently and predominantly occurred race was TTTTF with a frequency of 53.19%. The second most frequently detected race was TKTTF with a frequency of 31.91%. The remaining 14.91% fields infected by other races such as TRTTF (4.26%), RRTTF (4.26%), TKPTF (4.26%), and the least frequent registered race was TTRTF with 2.13%. This study confirmed the presence of wider virulence diversity within the Puccinia graminis f. sp. tritici population in the study area and in agreement with the previous studies conducted in the country.
| Zone | District | No. of isolates | Identified races | Wheat varieties | Altitude (m) |
|---|---|---|---|---|---|
| Southern | Ofla | 1 | RRTTF | Kakaba | 2465 |
| 1 | TKPTF | Mekelle II | 2471 | ||
| 1 | TRTTF | Hidassie | 2475 | ||
| 3 | TKTTF | Kakaba, Hidassie and Dashen | 2460-2497 | ||
| 4 | TTTTF | Kakaba, Hidassie, Mekelle I and Shehan (local) | 2458-2480 | ||
| Raya Azebo | 6 | TTTTF | Kingbird, Gambo, Fentale and Shehan | 1567-1758 | |
| 1 | TKTTF | Shehan local | 1663 | ||
| 1 | RRTTF | Shehan local | 1762 | ||
| 1 | TRTTF | Dashen | 1650 | ||
| Enda Mehoni | 3 | TKTTF | Hidassie and Shehan | 2400-2480 | |
| 5 | TTTTF | Kakaba, Dashen and Shehan | 2303-2511 | ||
| 1 | TTRTF | Hidassie | 2493 | ||
| Eastern | Kilte Awulaelo | 4 | TTTTF | Kakaba, Pavon-76, Ares local | 1950-2194 |
| 3 | TKTTF | Kakaba, Mekelle I, Ares local | 1950-1955 | ||
| Ganta Afeshum | 3 | TTTTF | Mekelle II Mekelle I, Kakaba | 2451-2567 | |
| 2 | TKTTF | Mekelle III | 2444-2447 | ||
| S/Tsaedaemba | 2 | TKTTF | Kakaba | 2024-2519 | |
| Southeast | Enderta | 3 | TTTTF | Ares local and Shehan local | 1981-2332 |
| 1 | TKPTF | Kakaba | 2124 | ||
| 1 | TKTTF | Mekelle I | 1998 | ||
| Total | 47 | 6 | 1567-2567 |
Table 3: Puccinia graminis f. sp. tritici races identified from samples collected and wheat varieties grown in varying altitude ranges of zones of Tigray region in 2017 main cropping season.
The most important virulent race TTTTF was isolated from 25 wheat fields grown with Kakaba, Shehan, Ares local, kingbird, Gambo, Fentale, Pavon-76, Mekelle II, Mekelle I, Dashen, and Hidassie of which 5 (50%), 6 (66.67%), 3 (75%), 2 (100%), 2 (100%), 1 (100%), 1 (100%), 1 (100%), 2 (50%) 1 (33.33%), and 1 (16.67%) were infected with this race, in the same orders. For instance, nine viable sampled wheat fields with Shehan local bread wheat variety, 6 (66.67%) were infected with TTTTF in all surveyed zones. Out of 10 samples taken from Kakaba variety, 5 (50%) of wheat fields were infected with TTTTF. This race was detected from an altitude range of 1567-2567 m.a.s.l which show that it is adapted to wider wheat growing agro-ecological zones. TKTTF was the second most virulent race on bread wheat varieties and it was isolated from 15 field sampled with Mekelle III, Dashen, Hidassie, Mekelle I, Shehan, Kakaba, and Ares local of which, 2 (100%), 1 (33.33%), 3 (50%), 3 (60%), 2 (22.22%), 3 (30%) and 1 (25%) were infected in the same orders. This race was distributed in the altitude range of 1663-2519 m.a.s.l. It is rapidly spreading to a wide altitude ranges, this might be due to favorable environmental conditions as well as cultivation of susceptible wheat varieties in those districts. On the other hand, TKPTF was detected from Mekelle II and Kakaba varieties. Similarly, new race TTRTF was detected only at a single location from Hidassie variety. RRTTF race was also obtained from Kakaba and Shehan local varieties. Lastly, TRTTF race was detected from Dashen and Hidassie variety from a single wheat field each. TKPTF was detected from elevations of 2124 and 2471 m.a.s.l and TTRTF was from 2493 m.a.s.l.
The virulence spectra of detected stem rust races were varied between 16 and 18 stem rustresistancegenes (Table 4). The broadest virulence spectrum was recorded for the race TTTTF that exhibited virulence on 18 stem rust resistance genes. Based on a set of twenty North American wheat differential lines, this race was virulent to all differential lines except Sr24 and Sr31. The most devastating stem rust race TTTTF was first detected in the U.S. in 2000 [32,33] and had been spread to most of the wheat growing areas of our country now a day. Out of the races detected, it was the most virulent race that producing high infection types (ITs) on the majority of stem rust differential lines in the study. In agreement with this finding, the presence and potential of TTTTF race to wheat production in Ethiopia has been already reported by Lemma et al. [34].
| Race | Virulence spectrum (ineffective Sr gene) | Avirulence spectrum (effective Sr genes) | No of isolates | Races frequency (%) | Virulence of races on Sr gene (%) |
|---|---|---|---|---|---|
| TTTTF | 5, 21, 9e, 7b, 11, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 24, 31 | 25 | 53.19 | 90 |
| TKTTF | 5, 21, 9e, 7b, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 11, 24, 31 | 15 | 31.91 | 85 |
| RRTTF | 5, 21, 7b, 11, 6, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 9e, 8a, 24, 31 | 2 | 4.26 | 80 |
| TKPTF | 5, 21, 9e, 7b, 6, 8a, 9g, 36, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 11, 9b, 24, 31 | 2 | 4.26 | 80 |
| TRTTF | 5, 21, 9e, 7b, 11, 6, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 8a, 24, 31 | 2 | 4.26 | 85 |
| TTRTF | 5, 21, 7b, 11, 6, 8a, 9g, 36, 9b, 30, 17, 9a, 9d, 10, Tmp, 38, McN | 24, 31, 9e | 1 | 2.13 | 85 |
| Total | 47 | 100 | |||
Table 4: Virulence/Avirulence spectrum and frequency of Puccinia graminis f. sp. tritici races collected from Southern, Eastern and Southeast zones of Tigray region.
The second widest virulence spectrum was recorded from TKTTF, TRTTF and TTRTF races that showed virulence on 17 stem rust resistance genes. The new race TTRTF was recorded broadest virulence on 17 Sr resistance gene. Similarly, RRTTF and TKPTF races were virulence on the 16 stem rust resistance gene of differential lines. Abebe et al. [2] also reported RRTTF race was a virulent spectrum on the 16 resistance gene of differential lines in the Southern zone of the Tigray region. The same to this previous report, RRTTF race was detected from Southern Tigray zone; Raya Azebo and Ofla districts.
About 16.67% of the races (TTTTF) identified showed virulence to 90% of the Sr genes and 50% (TRTTF, TKTTF, and TTRTF) of the races showed virulence on 85% Sr genes. The remaining 33.33% (TKPTF and RRTTF) of the races identified were virulent on 80% of the 20 Sr genes. The new race TTRTF defeated 85% of the Sr resistance genes in the wheat differential lines including Sr30, Sr11, Sr36, and Sr38. The virulence pattern observed in this study confirmed the presence of a wider range of virulence in the study area. Moreover, the detected races had a wider range of virulence in the study areas and high virulence diversity of stem rust races were reported by many authors earlier in Ethiopia [12,34]. Co-evolution of Puccinia graminis f. sp. tritici along with wheat being the reason for high virulence diversity in Ethiopian Puccinia graminis f. sp. tritici populations. Virulence diversities within Pgt were also reported from abroad countries such as South Africa, Mexico, USA and Canada [32].
The stem rust virulence spectrum in Ethiopia was definitely different from other parts of the world. For instance, surveys in Canada, USA, Russia and South Africa detected fewer races such as 15, 5, 6 and 7, respectively [35]. However, more races were identified from Ethiopia; for example, 15, 40 and 88 races were reported in Bale, Arsi, Sidamo and Harargie [36] and 17, 22 and 20 races were detected from Arsi, Bale and Southern Tigray [37]. However, the present study was dissimilar to the previous works conducted in Ethiopia as evident from the fact that only 6 races have been identified from three zones of Tigray region.
Virulence frequency of Puccinia graminis f. sp. tritici isolates on stem rust resistant genes
The study revealed that most of the races identified in the present study were virulent to many of the resistance genes. Fourteen differential lines carrying stem rust resistance gene Sr5, Sr21, Sr7b, Sr6, Sr9g, Sr36, Sr30, Sr17, Sr9a, Sr9d, Sr10, SrTmp, Sr38 and SrMcN were found to be 100% ineffective to all races. Similarly, four stem rust differentials carrying resistance genes Sr9b, Sr9e, Sr8a and Sr11 were found to be ineffective against most of the races detected, with virulence frequencies of 95.74, 93.62, 91.49 and 63.83%, respectively (Table 5).
| Stem rust resistance gene (Sr gene) | Virulence frequency (%) | Stem rust resistance gene (Sr gene) | Virulence frequency (%) |
|---|---|---|---|
| 5 | 100 | 30 | 100 |
| 21 | 100 | 17 | 100 |
| 9e | 93.62 | 9a | 100 |
| 7b | 100 | 9d | 100 |
| 11 | 63.83 | 10 | 100 |
| 6 | 100 | Tmp | 100 |
| 8a | 91.49 | 24 | 0 |
| 9g | 100 | 31 | 0 |
| 36 | 100 | 38 | 100 |
| 9b | 95.74 | McN | 100 |
Table 5: Virulence frequency of Puccinia graminis f. sp. tritici isolates on the 20 stem rust resistance genes.
Of the 20 stem rust resistance genes, the differential hosts carrying Sr11, Sr8a, Sr9e and Sr9b were resistant to 36.17%, 8.51%, 6.38% and 4.26% of races identified, respectively. Stem rust resistance gene Sr24 was effective against all of the races. Likewise, Sr31 also effective in this study due to the reason TTKSK (Ug99) was not detected in the present study. Admassu et al. [12] also indicated that no virulent race was detected against Sr24 gene in Ethiopia. This study confirms the report of Abebe et al. [2] which stated, the Sr24stem rust resistance gene is amongst the effective genes to all stem rust collected from the Southern zone of Tigray region, which have an adequate and some immediate values to almost all races in the world. However, virulence to Sr24genewas reported in Kenya in 2006. A variant of Ug99 group that added virulence on stem rust gene Sr24 (Ug99+Sr24virulence, called TTKST) has further increased the vulnerability of wheat to stem rust worldwide [24].
TTTTF race was avirulent to Sr24 and Sr31 while TKTTF (Digalu race) was avirulent to Sr11, Sr31, and Sr24. Thevirulenceof race TKTTF on the resistance gene SrTmp was considered as the main factor behind the complete susceptibility of the variety “Digalu” to this race. TTRTF was avirulent to Sr24, Sr31 and Sr9e while TKPTF was avirulent to Sr24, Sr31, Sr11 and Sr9b. The breakdown of the Sr31resistant gene in Ethiopia is reported by many authors previously which were evidences for the existence of Ug99 (TTKSK) [22]. In contrast, this study revealed that there was no TTKSK race and its variants detected in the study area during the 2017 cropping season. This finding disagreed with the previous report that Sr31were 100% effective for the races detected. Similarly, surveys carried out by other colleagues nationally during 2017 main cropping season also indicated there were no race TTKSK and its variants in the season. This result might be due to unfavorable environmental condition and resistant varieties grown in the surveyed areas. In conclusion, Sr24 and Sr31 resistance gene were 100% effective for all stem rust races detected in the season. Therefore, use of these genes for breeding program is pertinent.
According to Admassu et al. [12] and Abebe et al. [2], most of the races in Ethiopia varied from one another by single-gene changes. Such single-step changes in virulence were reported to be the main process of evolutionary change in Puccinia graminis f. sp. tritici populations. In agreement with this previous finding, two races identified were varied by single gene changes. For instance, TKTTF was similar to TRTTF with virulence to Sr11 and Sr8a, respectively. However, most of the identified races were not varied by single step changes. These might be due to other factors for race variation in the studied area like, parasexualism, migration, and selection pressure and gene combination.
Conclusion
Forty-seven stem rust isolates were analyzed on 20 stem rust differentials and resulted in the identification of six races namely; TTTTF, TKTTF, TRTTF, TTRTF, RRTTF and TKPTF. TKTTF was a common race detected from all districts of the three zones and TTTTF was detected from all districts except Saesia Tsaedaemba district. TTRTF race was first detected in Ethiopia and it has high virulence spectrum that makes 17 (85%) stem rust differential resistance gene non-effective. The study confirmed the presence of high virulence spectrum and high variable populations among the six identified stem rust races.
The highly virulent race TTTTF was detected with a higher frequency of 53.19% of the races identified and virulent to 90% of the stem rust resistance genes. TKTTF was the second most virulent race with 38.30% frequency and showed 85% virulence to stem rust resistance genes. The study showed that the majority of the resistance genes were ineffective against most of the races identified. The stem rust resistance gene Sr24and Sr31were the only effective gene that showed resistance to all identified races. Hence, the stem rust resistance gene Sr24 and Sr31 can be used as sources of resistance in the wheat breeding program. Therefore, it is mandatory to monitor the virulence composition and dynamics in the stem rust population and utilize currently effective stem rust resistance genes in the wheat improvement program.
REFERENCES
- Braun HJ, Atlin G, Payne T. Multi-location testing as a tool to identify plant response to global climate change. Climate change and crop production. CABI, Wallingford, UK. 2010;1:115–138.
- Abebe T, Woldeab G, Dawit W. Analysis of pathogen virulence of wheat stem rust and cultivar reaction to virulent races in Tigray, Ethiopia. Afr J Plant Sci. 2012;6(9):244-250.
- FAO. Food and Agricultural Organization of the United Nations (FAO): FAO Global Statistical Year book. 2017a.
- Kumar P, Yadava RK, Gollen B, Kumar S, Verma RK, Yadav S. Nutritional contents and medicinal properties of wheat: a review. Life Sciences and Medicine Research. 2011;22(1):1-10.
- CSA. Report on Area and production of Major Crops. The Federal Democratic Republic of Ethiopia; Central Statistics Agency Agricultural Sample Survey for 2017/2018. Addis Ababa, Ethiopia. 2018;1:1-125.
- Haile D, Nigussie D, Ayana A. Nitrogen use efficiency of bread wheat: Effects of nitrogen rate and time of application. J Soil Sci Plant Nutr. 2012;12(3):389-410.
- Admassu BE, Friedt W, Ordon F. Stem rust seedling resistance genes in Ethiopian wheat cultivars and breeding lines. Afr Crop Sci J. 2012;20(3):149-162.
- Hodson D. Keeping track of rust. 2014.
- Gizachew HR, Girma AS, Netsanet BH. Evaluation of Ethiopian bread wheat varieties to dominant stem rust races (Puccinia graminis f. sp. tritici) at seedling stage under greenhouse condition. IJAB. 2019;8(4): 210-216.
- Badebo A. Breeding Bread Wheat with Multiple Disease Resistance and High Yielding for the Ethiopian Highlands: Broadening the Genetic Base of Yellow Rust and Tan Spot Resistance. PhD Dissertation, Gottingen University, Germany. 2002:115.
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, et al. Will stem rust destroy the world's wheat crop?. Adv Agron. 2008;98:271-310.
- Admassu B, Lind V, Friedt W, Ordon F. Virulence analysis of Puccinia graminis f. sp. tritici populations in Ethiopia with special consideration of Ug99. Plant Pathol. 2009;58(2):362-369.
- FAO. Food and Agricultural Organization of the United Nations (FAO). News article: Spread of damaging wheat rust continues: new races found in Europe, Africa, and Central Asia. 2017b.
- Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, Bhavani S, et al. Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. J Phytopathol. 2015;105(7):872-884.
- Haile JK. Genetic mapping of resistance to race Ug99 of Puccinia graminis f. sp. tritici, diversity analysis and identification of stem rust resistance genes in Ethiopian tetraploid wheats. Cuvillier Verlag; 2013:1-9.
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, et al. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013–14. J Phytopathol. 2015;105(7): 917–928.
- Ambika R, Meenakshi D. Wheat stem rust race Ug99: A shifting enemy. Int J Curr Microbial App Sci. 2018;7(1):153.
- Stakman EC, Stewart DM, Loegering WQ. Identification of physiologic races of Puccinia graminis var. tritici. Washington: USDA; 1962:5-50.
- Roelfs AP. Rust diseases of wheat: Concepts and methods of disease management. Cimmyt. 1992:81.
- Woldeab G, Hailu E, Bacha N. Protocols for Race Analysis of Wheat Stem Rust (Puccinia graminis f. sp. tritici). 2017:1-26.
- Fetch Jr TG, Dunsmore KM. Physiologic specialization of Puccinia graminis on wheat, barley, and oat in Canada in 2001. Can J Plant Pathol. 2004;26(2):148-155.
- Hailu A, Woldeab G, Dawit W, Hailu E. distribution of wheat stem rust (Puccinia Graminis F. Sp. Tritici) in West and Southwest Shewa Zones and identification of its physiological races. Advances in Crop Science and Technology. 2015;3(4):189.
- Roelfs AP, Martens JW. An International System of Nomenclature for Puccinia graminis f. sp. tritici. Phytopathology. 1988;78(5):526-533.
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch Jr T. Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2008;92(6):923-926.
- Bhattacharya S. Deadly new wheat disease threatens Europe’s crops. Nature News. 2017;542(7640):145.
- Lemma A, Woldeab G, Semahegn Y, Dilnesaw Z. Survey and virulence distribution of wheat stem rust (Puccinia graminis f. sp. tritici) in the major wheat growing areas of central Ethiopia. Sci-Afric Journal of Scientific Issues, Research and Essays. 2014;2:474-78.
- Patpour M, Afshari F, Hasan Bayat Z, Nazari K. Pathotype identification of Puccinia graminis f. sp. tritici, the Causal Agent of Wheat Stem Rust under Greenhouse Condition. 2014.
- Afshari F, Aghaee M, Jalal MR, Roohparva R, Malihipour A, Khodarahmei M, et al. Surveillance and Puccinia graminis f.sp. tritici race analysis in Iran, 2014. Poster presented at Borlaug Global Rust Initiative Technical Workshop. 2015.
- Hodson D. Summary of Ethiopia 2014/15 rust situation. Re-current, localized stem rust epidemics caused by race TKTTF (“Digalu” race) in Ethiopia. Extreme caution & vigilance needed in East Africa. Rust tracker. org, Global wheat rust monitoring system. 2015.
- Mert Z, Karakaya A, Dusunceli F, Akan K, Cetin L. Determination of Puccinia graminis f.sp. tritici races of wheat in Turkey. Turkey Journal of Agriculture and forestry. 2012;36:107-120.
- Hodson D. Molecular diagnostics indicate presence of race TKTTF (“Digalu” race) in Kenya. Rust tracker.org, Global Wheat Rust Monitoring System. 2016.
- Jin Y. Races of Puccinia graminis identified in the United States during 2003. Plant Dis. 2005;89(10): 1125–1127.
- Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, et al. Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2007;91(9):1096-1099.
- Lemma A, Woldeab G, Semahegn Y. Virulence Spectrum of Wheat Stem Rust (Puccinia graminis f. sp. tritici) in the Eastern Showa of Central Ethiopia. Adv Crop Sci Tech. 2015:1-6.
- Pretorius ZA, Bender CM, Visser B, Terefe T. First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant dis. 2010;94(6):784-785.
- SPL. Scientific Psychopathological laboratory. Annual Report of 1985-1988. Ambo, Ethiopia. 1988.
- Serbessa N. Wheat Stem Rust (P. graminis f. sp. tritici) Intensity and Pathogenic Variability in Arsi and Bale zones of Ethiopia. Plant Science MSc. Thesis presented to School of Graduate Studies of Alemaya University, Ethiopia. 2003.
Citation: Gizachew HR, Bacha Hei N (2021) Virulence Diversity and Physiological Race Composition of Wheat Stem Rust (Puccinia graminis F. sp. tritici) In Tigray Region, Northern Ethiopia. J Plant Pathol Microbiol 12:555.
Copyright: © 2021 Gizachew HR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.