Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 13, Issue 5
Utilization and Purification of Tallow, Grease, and Lard Byproducts from Abattoir and Meat Processing Plant for Soap Production: Case Study at Elfora Agro-Processing Industry, Kombolcha, Wollo, Ethiopia
Talbachew Tadesse Nadew1*, Aklilu Assefa1, Shimeles Nigussie2, Ebrahim Kamus3 and Melak Muchie32Department of Food Process Engineering, School of Chemical and Food Engineering, Kombolcha Institute of Technology, Wollo University, Dessie, Ethiopia
3Department of Food Process Engineering, Debre Berhan University, Debre Berhan, Ethiopia
Received: 04-Sep-2023, Manuscript No. ACE-23-22863; Editor assigned: 07-Sep-2023, Pre QC No. ACE-23-22863 (PQ); Reviewed: 21-Sep-2023, QC No. ACE-23-22863; Revised: 28-Sep-2023, Manuscript No. ACE-23-22863 (R); Published: 05-Oct-2023, DOI: 10.35248/2090-4568.23.13.311
Abstract
Converting byproducts into useful goods with value has an important issue for all industries. Abattoir and meat processing plant at Kombolcha, Ethiopia produces a huge amount of tallow fat, grease and lard byproducts. In this work, the byproduct from the factory was utilized for making soap. The study was conducted by taking 600 g fat samples and made refined and used for a bar soap-making experiment. Refining process of tallow fat was conducted by settling, degumming, and deodorization to remove residues, phosphatides, and water. The citrus vinegar was also prepared from lemon fruit to inhibit the growth of bacteria and to make a good odor. The odor taste of the refined fat was reduced as better when vinegar was added to the refined fat. The saponification value, acid value, and pH of purified fat and prepared soap were also determined and found to be 194.32 mg KOH/g fat, 7.5%, and 5-6.5 respectively for tallow fat and AV of 14.9%, Free fatty content of 0.75%, a total fatty matter of 67.5%, pH of 9.67 and moisture content of 10.4 wt% for produced soap were obtained. All others physio-chemical characteristics of the produced soap were studied and showed similar properties with commercial soap.
Keywords
Tallow fat; Saponification value; Fatty acid; Soap
Introduction
ELFORA is a subsidiary of MIDROC Ethiopia Investment Group and a private agro-industrial company established in December 1997 with a total value of US$ 54.7 million [1,2]. ELFORA produces and exports Livestock and Meat products. The company also produces very large byproducts such as tallow, grease, and lard.
By-products such as tallow, lard, and grease utilization are an important aspect for the ELFORA Agro-Industries to enhance the profitability of the plant. Those byproducts provide many of the feed ingredients and raw materials used to make pharmaceutical, cosmetic, household, and industrial products in addition to such products as lubricants, plastics, soaps, glycerin, and gelatins [3].
In every day to day, processes in the red meat industry processing generate large amounts of offal and meat by-products. Traditionally, they are not as highly valued as prime cuts of meat, and they can pose a threat to the environment and human health if not properly disposed of or processed. They can thus represent a cost rather than a potential revenue stream [4,5]. Animal fat is a valuable byproduct of the meatpacking industry. Lard and tallow are the most common edible animal fats. Lard is a fat made from the clean tissues of healthy pigs. Tallow is a type of hard fat made from the fatty tissues of cattle or sheep. Lard and edible tallow are made by either dry or wet rendering. The fatty tissues are heated in the presence of water, usually at a low temperature, during the wet rendering process.
Tallow and lard were traditionally used for deep frying. However, due to consumer health concerns, this use is declining in the fast- food industry. Because less fat is absorbed, an alternative liquid tallow product has been developed for the preparation of French fries and other fast foods. Tallow and lard are also used in the production of margarine and shortening. Some edible lard is used in sausages and emulsions [6].
In the past century, and now a day, tallow is an important raw material for producing biofuels, such as biodiesel, and can be obtained by rendering animal fats, oil, and grease. Meat processing wastewater contains animal fats, oil, and grease as waste products that must be removed to clarify the wastewater [7]. The company owns a considerable size of production facilities and adding some value to agro-industrial effluents is more attractive and more important, satisfying the essential to encourage resources’ use efficiency and a more sustainable, eco-friendly, and globular economy.
Converting byproducts into useful goods with value has an important issue for the industry captures additional revenue that otherwise would have been unrealized and the costs of disposing of these secondary items are avoided. Intrinsically, byproducts such as tallow fat serve as an extra means for packers to earn revenue or as a cushion to cover losses should the cost of purchasing the live animal exceed the selling price of the carcass. In this work, a preliminary study was conducted according to the feedback information obtained from Abattoir and meat processing plant at Kombolcha, Ethiopia. The company produces a huge amount of tallow fat byproducts; however, its characteristic difficulty and the unpleasant odor of those byproducts make struggle to produce value-added products. So, the current study deliberates on improvement in purifying tallow fat byproducts and producing a valuable product.
The odor and color of tallow and other byproducts were a big problem in the company courtyard as the previous study stated the reason for this issue [8]. Soaps are detergents or emulsifiers made by reacting animal or vegetable fats or oils with solutions of potassium or sodium hydroxide, or salts of alkali metals or heavy metals and alkaline earth. When we try to wash it, an insoluble soap is formed. It is well known that soap is made by mixing oils and fats with an aqueous solution of sodium hydroxide (caustic soda) or potassium hydroxide (caustic potash). Sodium soaps are always harder than potash soaps when the same fat or oil is used in both cases. Soaps often contain dyes that emulsify oils and lower the surface tension of water. It is easy to penetrate and remove dirt [9].
Tallow is a fatty substance that is mostly composed of triglycerides. Each triglyceride comes from different sources, one of which is stearic, palmitic, and oleic acids. Because of its high proportion of saturated fatty acids and traces of unsaturated fatty acids, tallow is sometimes used instead of vegetable oils to make soap [10]. These fatty acids provide soap with more lather stability, hardness, and solubility. Excess tallow can be used to make soap cheaper and more lather-stable and make it harder than normal soap [11]. Tallow is also inexpensive, easily accessible, and lowers the cost of making soap. Glycerin is a byproduct of the exothermic process that occurs when tallow and an alkali (either sodium hydroxide or potassium hydroxide) are combined to make tallow soap [12].
Studies made so far have focused on the agricultural sector at the national level. Annual publications of government ministries and agencies provide data and information at the macro level. Very little research has been made on specific company issues relating to operational efficiency, marketing and sales strategies, new product development, and other areas of importance for the survival and growth of the company.
Soap making from tallow fat or by-products of Abattoir and Meat processing factories is not a common trend. However, tallow is mostly used for biodiesel production by many industrial methods which are classified as chemical and biotechnological techniques. Even though, the production costs of fuel are still rather high compared to soap production costs without considering the final function of the products [13].
In a process known as saponification, natural fats or oils (obtained from plants or animals) are hydrolyzed in the presence of a strong alkali reagent, typically sodium hydroxide, NaOH (commonly referred to as caustic soda or lye), or potassium hydroxide, KOH (commonly referred to as caustic potash) [14]. Soap making is based on alkaline hydrolysis reaction and saponification as given by the following stoichiometric equation as fat reacted with three moles of sodium hydroxide which gives us glycerol plus crude soap. That is said to be a saponification reaction or process.
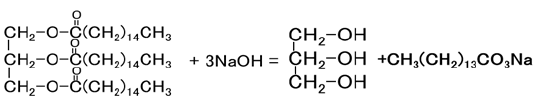
Having said that, the company produces a huge amount of tallow fat byproducts; however, its characteristic difficulty and the unpleasant odor of those byproducts make struggle to produce value-added products. This study was to deliberate on improvement in purifying of Tallow fat byproducts for bar soap production using citrus lemon vinegar.
Materials and Methods
Materials and chemicals
The main raw material for this study was by-products (tallow fat, lard, and grease) collected from ELFORA Agro-Processing Industry (PLC), Kombolcha, Ethiopia. Chemicals and ingredients such as Ethanol (purity-97%), Sodium hydroxide (NaOH) (purity-98%), and Hydrochloric acid (HCl) (purity-98%), all obtained at analytical grade from the chemical supplier from Addis Ababa, Ethiopia. Other citrus juice obtained from citrus fruit (Citrus lemon), Baker yeast (Saccharomyces cerevisiae) and sugar are used for the purification of the raw materials and used as additives for removing the Odor and for the saponification process. Equipment such as a beaker, separation funnel, soap mold, heating and steering mantle, conical bottom flask, stirring spoon, waxed paper, and woven cloth was used.
Preparation and pretreatment process of raw materials (tallow fats)
The tallow, lard and grease, and other bone parts are separated by heating and a high-pressure mechanical pressing machine as shown in Figure 1. The tallow fat was collected using stainless steel pot and taken into Laboratory for analysis and further studies. The tallow fat was filtered with filter cloths (woven cloth) to remove some solid impurities. The process is called rendering.
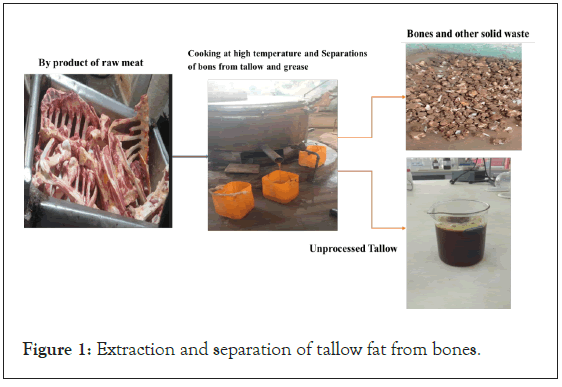
Figure 1: Extraction and separation of tallow fat from bones.
The treatment process involves a series of purifying steps followed by modification into more usable products and finally packaging. The raw tallow obtained from the factory is heated up to a temperature of 65 °C and then allowed for successive washing with water in the magnetic stirrer with a heating plate.
Settling and degumming
Applied to remove residues, phosphatides, and water. Settling involves storing heated fats quiescently in a conical bottom flask. The presence of phospholipids causes the formation of water-in-fat emulsions and these emulsions make the fat cloudy the water can present a hazard when the fat is heated to a temperature above 100 °C. Degumming is a process that removes phospholipids by the addition of distilled water at 1%-3% at 60-80 degrees for 30-60 minutes. The citric acid of 0.1% of fat in weight was added to increase the separation of the phospholipids in it [10,15].
Water and materials associated with water are denser than melted fat and settle into the separation funnel, where they are drawn off, thus, the gum is formed by phospholipid and water is removed. Then heating at 110 °C in the presence of adsorbents and then filtered using woven cloth. The fat can be separated from the separatory funnel by gravity washed with water and separated again by centrifugation.
Deodorization and citrus juice preparation
It is an important oil refining step because of consumer demand for fats and shortenings that has a very bland or practically non-existent flavor [16]. Neutral fats and hydrogenated contain substances contributing to undesirable flavor and odor and these substances must be removed. This is achieved by heating the degummed fat at a high temperature (above 80 °C). This has the effect of removing odoriferous volatile compounds leaving the fat almost tasteless. In this study, the method employed to provide an additional method of lowering even removing the unpleasant taste and/or smell of tallow products is using vinegar produced from Citrus fruit (Citrus lemon).
For citrus juice preparation the lemon fruit was cut into wedges (save some for the garnish). Hold the wedge over the glass and squeeze to release all of the juice from the fruit. The vinegar is prepared according to the method adopted by Adrian L. et al. [17].
Characterization of purified tallow fat
Saponification value determination: It shows how many milligram of sodium hydroxide or potassium hydroxide are needed to saponify 1 gram of fat under the given circumstances. It is a measurement of the overall fatty acids' combined average molecular weight (or chain length). The three fatty acids make up the majority of a fat's or triester's mass, allowing for a comparison of the typical fatty acid chain length [18].
In this study, 10 grams of tallow fat was placed in a 1-liter Erlenmeyer flask. 0.5 M KOH was added to the solution and heated to 55 °C. on a water bath with continuous stirring. After that, the temperature was raised to 100°C and boiled for 1 hour to complete the saponification treatment. Excess KOH was titrated into the mixture using a phenolphthalein indicator until a pink color was observed. The Saponification value of the purified tallow fat was calculated using (Equation 1), as usual in many papers [19,20].
Where, Co and Ce are the initial and equilibrium concentrations (mg/L) of the adsorbate, V (L) the volume of the adsorbate solution agitated and m the mass of the adsorbent used.
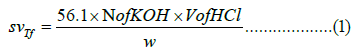
Where svTf is the saponification value of tallow fat, N is the normality of KOH, V is the net volume of HCl used between the real titration and blank (ml) and w is the weight of the tallow fat (g).
Acid value determination
Acid values of the purified tallow fat were determined using the volumetric titration method according to the meth Fod used by Mata T. et al. [21].
To determine the acid value, a 1-liter conical flask with 10 mL of alcohol was prepared, 10 grams of fat were added, and the mixture was then cooked in a water bath. When pink color appeared as the endpoint, two drops of phenolphthalein indicator were added and titrated against 0.1 KOH. The acid value was calculated using (Equation 2).
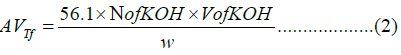
Where AVTf is the acid value of tallow fat, N is the normality of KOH, V is the standard volume of KOH solution (ml) and w is the weight of the tallow fat sample (g).
pH determination
To determine the pH range of the tallow fat sample; an empty beaker was cleaned with distilled water and dried, then the purified tallow fat was poured into the dried beaker, and its basicity or acidity value was determined by using the pH meter [22].
Saponification process (Soap preparation experiment)
When a fat or oil chemically combines with one of the caustic hydrates in the presence of water, the process is called ‘saponification’ and the newly formed compounds are soap and glycerin. The stoichiometric equation for the saponification process is given by the following reaction [23,24].
Characterization of prepared soap
Saponification value: The Saponification Value (SV) of extracted soap was determined in the same method as that for SV of purified tallow fat using equation (1).
Total fatty matter
The Total Fatty Matter (TFM) present in the tallow fat soap was obtained by following the method described by C. Mwanza and Z. Kadango [14]. 10 g of extracted soap from tallow fat was weighed dissolved in 150 mL of water and heated in a water bath for 25 min until a fatty acid layer was formed. 20 mL of H2SO4 (15% concentration) was added with vigorous shaking. The resulting solution was filtered by using filter paper and transferred into a pre-known-weight petri dish. The content was evaporated using an electric oven (700LT-model No. TD-1315, Cooper Technology, UK) and the residue was weighed. The percentage of total fatty matter (%TFM) was calculated using Equation 3.
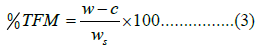
W is the weight of the crucible and sample after oven drying, C is the weight of the empty crucible and ws is the initial weight of the sample. All are in grams.
Free fatty acid content
Free Fatty Acid Content (FFAC) is quantified as a share of free oleic acid in terms of the sample's overall weight. All fats include some free carboxylic acids, but the refining process gets rid of these. To monitor the effectiveness of the refining process, the level of these free fatty acids is regularly evaluated. Alcohol is used to dissolve the carboxylic acids but not the fat in the mixture of fat. The alcohol layer is then scraped off and titrated with sodium hydroxide, a catalyst for this phenolphthalein indicator. The quantity of FFAC in tallow is a sign of how much deterioration has occurred. The FFAC of the sample was conducted according to the modified method followed by Nurulain et al. standards [25,26].
pH value determination: The determination of pH of bar soap produced is small different from that of tallow fat. In this case, the soap should be first dissolved well in distilled water in an empty cleaned beaker. A 10 g soap sample was taken and dissolved with 100 ml of distilled water for optimum soap dissolution, it was left undisturbed for 24 hours. Then pH- meter was inserted and read the pH of the soap extracted. The method is adopted from the previous study by Tarun et al. [27].
Moisture content determination: 20 g of produced soap for the tallow fat was weighted using an electronic analytical balance (AD- 300-3, US) and taken in a dried tarred dish and put in an oven (700LT-model No. TD-1315, Cooper Technology, UK) for 24 hr. at 105°C. The moisture content (Mc) (% wet basis) was calculated using Equation 4 [28]. When the mixture reached a medium to heavy trace, the soap was poured into a mold. The mold was covered with the lid and left for 24 hours before removing the coverings. The soap recipes were checked out after 24 hours. The cold and firm recipes were taken out from the mold letting the soap air. Finally, the Physio-chemical characterization of the bar soap was conducted.
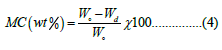
Where, Wo is the initial weight of the soap (g), and Wd is the dry weight of the sample of soap after 24 hours of drying (g).
Foam stability and hardness of soap
The amount of time it took for the foam to collapse was timed using a stopwatch after the generated soap was used to create lather in water. One 500 ml measuring cylinder was filled with 100 ml of distilled water and 2.0 g of the extracted soap. To produce foams, the mixture was shaken ferociously. The cylinder was shaken for about two minutes, and then let to stand for around ten minutes. We measured and noted the height of the foam in the solution [29,30].
Hardness of soap
The hardness of the soap is a determinant factor to sustainably use while washing until to finished uniformly without breaking or leakage. A needle (6.4 cm long, 1 mm in diameter) with a lead fishing weight (130 g) mounted was placed onto the soap to assess its hardness. The depth to which the needle penetrated the soap after 30 seconds was recorded as a measure of the soap's hardness. This was done three times, and the average and standard deviation were then calculated [31].
Test for effectiveness in cleaning
A drop of oil was applied to four separate strips of filter paper to test the produced soaps' cleansing ability. The oil-stained filter papers were placed in separate test tubes containing a soap solution (2 g soap/100 ml distilled water), which was strenuously shaken for 1 minute. The filter papers were then removed, and rinsed with distilled water, and the level of cleanliness in each filter paper was assessed [32].
Sensory evaluation
The color and smell of samples of produced bar soap, refined tallow, and raw tallow were examined. Four members of the chemical engineering department staff from Wollo University's Kombolcha Institute of Technology in Kombolcha, Ethiopia, conducted sensory evaluations.
Washing property of soap
The washing property of tallow soap was studied by washing of our hands using a small bit of soap with deionized water. The lathering qualities and "feel" of the soap were assessed and classified as extremely greasy, normal, or around average [33,34].
Testing solubility of soap
Any soap's solubility can be used to determine how easily it will dissolve in water during washing operations. The amount of foggy film that forms provides a clue as to how many micelles (soap molecules) were created by the soap in the water. Soap can be clean because of the chemical composition of its molecules, which are lipophilic on one end and hydrophilic on the other [23].
2 grams of soap sample were measured and allowed to dissolve in 100 ml of water with vigorous stirring for around 2 minutes to test the extracted soap's solubility. We took note of and looked closely at the extent of uniform soap solution generation [35].
Results and Discussion
Analysis of purified tallow fat
The Saponification Value (SV) and Acid Value (AV) presented in the tallow fat provide information on the glyceride type and quantity or weight of acid that are the major components of soap. The comparison of purified and unpurified tallow fat is shown in Figure 2. The SV and AV of the purified tallow fat are found as 194.32 mg KOH/g fat, and 1.98 mg KOH/g respectively. In the previous study by Chizoo et al, the SV of beef tallow was found 199 mg KOH/g fat [36]. Again, by the researcher Warra et al., the SA value of tallow was reported as 140 mg KOH/g fat [33]. The SA values of fat and oil should be in the range of 180 mg-220 mg KOH/ g fat [37]. The result obtained in this study was found in the range given set by the soap-making manual [24]. The profound thing here is, that the higher the SV will give a better soap or good soap-making process due to more glyceride molecules or long carbon chains in the fatty acid [37].
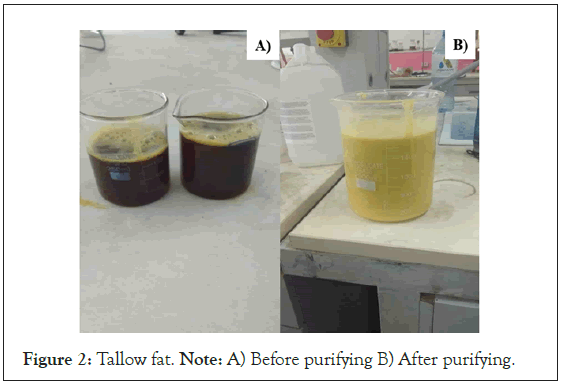
Figure 2: Tallow fat. Note: A) Before purifying B) After purifying.
The AV of the purified tallow fat was also low and below the maximum allowable acid value (2.5 mg KOH/g) set by the FAO/ WHO (2015) report [38]. So, low acid values indicate that the triacylglycerols have not been hydrolyzed, which could indicate good stability and safe for making skin-related products [39,40]. It is also good for shelf-life as its resistance to rancidity, gum formation, and corrosion resistance is good and they can be stored for a longer time [41]. So, the results of AV and SV of purified tallow fat obtained in this study signify its suitability for soap production.
The pH value is also important for determining the quality of soap since it indicates the alkaline or acidic nature of the material. The pH value for the purified tallow fat obtained in this work is 5.9. The range for safe conditions for the human body even for clothes is in the mild alkaline value near neutral (pH 7). A pH below 5 (more acidic) and above 10 (more alkaline) had a negative effect on skin desiccation, touchiness, and alteration [42].
Characterization of produced soap
The physio-chemical characteristics (FFAC, TFM, SV, AV, pH, and Mc) of the extracted soap from tallow fat were determined and shown in Table 1.
| Properties analyzed | Values |
|---|---|
| Free fatty acid content (%) | 0.75 |
| Total Fatty Matter (%TFM) | 67.5 |
| Acid value (mg KOH/g) | 14.9 |
| pH value | 9.67 |
| Moisture content (% wt.) | 10.4 |
Table 1: Physio-chemical properties of extracted soap from tallow fat.
Other properties (Sensory/odor evaluation, washing property, foam stability, and cleaning properties) of tallow soap are shown in Table 2.
| Properties of soap produced | Observed characteristics of soap |
|---|---|
| Solubility property | Completely soluble in water |
| Sensory evaluation and color | Good odor/very good with a yellowish color |
| Foam stability | Foam height over water 4.5 ± 1.2 cm |
| Cleaning property | Very good and effective cleaning |
| Hardness of soap | Depth of needle penetration 2.9 ± 0.5 cm |
| Washing property | Normal with adorable feeling in washing |
Table 2: Physical characteristics of the tallow fat soap.
The FFAC is a prominent parameter used to determine the abrasiveness of soap. The higher value of FFAC indicates a more conversion reaction. The result obtained in this study was in the range of free fatty acids as tabulated in the report by Popescu et al.[30].
The important property of the soap is TFM and AV. The AV of the soap was quite different from that of the purified tallow fat (14.9 mg KOH/g fat in the soap and 1.98 in the purified tallow). This difference would have resulted from the added ingredients such as KOH, Lemon vinegar, and other chemicals. The AV of soap extracted was within the standard range set by FAO/WHO [38]. This indicates that tallow soap has a good efficiency in cleaning [37].
The % TFM of the soap is an important characteristic describing the quality of soap and is the ratio of the mass of fatty matter to the total mass of the soap. According to the study by M. Issa et al. study for various soap type in Nigeria, the values for total fatty matter (TFM) for animal and human soaps ranges between 7.60%-36.10% and 11.72%-49.65% respectively. Also indicated that the values were below that of the Standard Organization of Nigeria (SON) of ≥ 76% set for human soap. Since the higher, the value of TFM the higher the cleaning power of the soap, this suggests that the soap analyzed has less cleaning ability [43]. In this study, the TFM was 67.5% and it was also below the standard value stated. However, it is reasonable to suggest that the cleaning power of soap extracted from tallow fat would have good cleaning ability.
The pH of the extracted soap from tallow fat was in the alkaline range (pH 9.67 greater than pH 7.0). Several studies formulated different standard specifications for the pH value of soap. Owoicho I stated pH standard specification in the range of pH 7–10 [44]. According to M. Issa et al. study, the pH range as a standard set was described as pH of 6.5–8.5 and analyzed the pH of four soap types and obtained a pH value from 9.7 to pH of 10.02 for human soap [43]. Whatever the standards and specifications stated, the pH of extracted soap from tallow fat was good enough for cleaning and washing as this pH value was found in the acceptable range of pH studied by Tarun et al. [27].
Moisture content is a parameter used to estimate the shelf life of a product. High moisture content in soap would cause excess water to react with un-saponified fat to form free fatty acid and glycerol in a process called hydrolysis of soap during storage [45]. The values of moisture content obtained for extracted tallow soap appear 10.4%. This result lay in the acceptable range (10%-15%) for moisture according to the previous study supported by the FAO/WHO report (2009) [46,47].
The other physical properties of tallow soap such as solubility test, foam stability, effectiveness in cleaning, hardness, and washing properties were examined in comparison with the commercial soap and gave the overall acceptability of the soap produced as given in Table 2.
Tallow soap's solubility tells us how easily it will dissolve in water when used for washing. How many micelles (soap molecules) were produced by the soap in the water is shown by the quantity of fog that forms. The tallow soap extracted was completely dissolved and confirmed that the solubility of soap would not be separable from other commercial soap. The foam height and waiting above the water was observed at 4.5+1.2 cm while for the commercial soap (Top soap) it was 5.3+1.6 cm. In the previous study by Warra et al., the foam height of soap from animal fat was found 2.5 cm [33]. The result observed in this study seemed better and showed good features.
The hardness of soap or extent of penetration of the loaded needle on the soap was 2.9+0.5 cm for produced soap and 3.2+0.7 cm for commercial soap (sunlight soap). The difference was not much but this may happen due to the extracted soap was analyzed not more than daily life after extraction. In addition to its good hardness properties, the texture of the soap was soft and good feeling. The other properties (odor, cleaning, and washing properties) of tallow soap showed good overall acceptability in comparison to commercial soap. The produced soap in this work is shown in Figure 3.

Figure 3: Soap produced on molding plate.
Conclusion
Utilization of tallow fat from Abattoir and Meat processing factories for soap making was a vital key to enhancing the plant's profitability. This study was conducted with the suggestion of ELFORA Agro-Processing Industries, Kombolcha Abattoir, and Meat Processing Plant workers. The study is a milestone for those who are new to producing soap from the meat processing sector, which will also find useful for professionals, entrepreneurs, and those studying and researching in this important area. Rural demand for soap is growing, with more and more soap brands being launched in the discount segment targeting the lower socio- economic group of consumers. The first beneficial of the study would be ELFORA Agro-industry PLC company, Kombolcha branch, and other meat processing sectors. Then the study would also solve employee cleaning soap scarcity and reduce the expenditure for soap shopping tasks in the company. Converting byproducts into valuable products should be an important trend for all industries. Therefore, this work was accomplished based on the way forward to be no waste or effluent disposal whereas converting into a monetary value should be done.
Data Availability
The data used to support the findings of this study are included in the article and can be submitted upon request.
Acknowledgements
The authors would like to thank the Department of Chemical Engineering, College of Food and Chemical Engineering, Wollo University, Kombolcha Institute of Technology, Kombolcha, Ethiopia, and ELFORA Agro-Processing Industries, Kombolcha Abattoir, and Meat Processing Plant, Kombolcha, Ethiopia, for their collaboration during the laboratory work.
Author Contribution
Aklilu Assefa-Conceptualization, methodology, formal analysis, investigation, resources, and data curation; Ebrahim Kamus- writing-original draft preparation; Shimeles Nigussie-writing-review and editing, Talbachew Tadesse-visualization, and supervision, and Melak Muchie validation and supervision. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Taye M. The effect of training on employee performance: The case of Elfora agro industries plc (Doctoral dissertation, ST. Mary’s University).
- Bethelehem N. An assessment of labour union leadership practices in Elfora agro industry plc at midroc Ethiopia by Bethlehem negussu (Doctoral dissertation, ST. Mary's University).
- Sutton J, Kellow N. An enterprise map of Ethiopia. International growth centre; 2010.
- Executive Research Associates, Corporate social resonsibility an African case study. 2009:8;36.
- Shirsath AP, Henchion MM. Bovine and ovine meat co-products valorisation opportunities: A systematic literature review. Trends Food Sci Technol. 2021; 118:57-70.
- Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J Food Sci. 2012; 49:278-93.
[Crossref] [Google Scholar] [PubMed]
- Han J, Elgowainy A, Wang M. Development of Tallow-based Biodiesel Pathway in GREET. Systems Assessment Group, Energy Systems Division, Argonne National Laboratory. 2013.
- Igile GO, Ikpeme-Emmanuel CA, Egbung GE, Mgbeje BI. Effect of washing red palm oil and low grade tallow fat with hot water on oil bleachability and bleached oil color and odour stability. Aust j basic appl sci. 2013; 7(11):64-70.
- Okiki PA, Ojo AA, Onyibe HT, Oso O, Ajiboye BO. A comparative study of the physicochemical properties and antimicrobial qualities of Abuad moringa soap with conventional medicated soaps. Potravinarstvo. 2017.
- Sharma H, Giriprasad R, Goswami M. Animal fat-processing and its quality control. J Food Process Technol. 2013; 4(8):1000252.
- Salihu SO, Waziri J, Bello SI, Audu N, Asli UA, Isah AN. Laundry soap production from the respective tallows of goat, sheep and cow: Evaluation of physicochemical properties for the best. Int res j pure appl chem. 2021; 22(3):22-33.
- Tukiran NA, Anuar NA. Halal Cosmetics: A Review on Halalan Toyyiban Concept in Soap Production. Halalpshere. 2022; 2(2):86-93.
- Vafakish B, Barari M. Biodiesel production by transesterification of tallow fat using heterogeneous catalysis. Kem. Ind. 2017; 66(1-2):47-52.
- Mwanza C, Zombe K. Comparative evaluation of some physicochemical properties on selected commercially available soaps on the Zambian market. oalib. 2020; 7(3):1-3.
- Paisan S, Chetpattananondh P, Chongkhong S. Assessment of water degumming and acid degumming of mixed algal oil. J Environ Chem Eng. 2017; 5(5):5115-5123.
- de Araujo-Silva R, Vieira AC, de Campos Giordano R, Fernandez-Lafuente R, Tardioli PW. Enzymatic synthesis of fatty acid isoamyl monoesters from soybean oil deodorizer distillate: A renewable and ecofriendly base stock for lubricant industries. Molecules. 2022; 27(9):2692.
[Crossref] [Google Scholar] [PubMed]
- Leonés A, Durán-Guerrero E, Carbú M, Cantoral JM, Barroso CG, Castro R. Development of vinegar obtained from lemon juice: Optimization and chemical characterization of the process. Lwt. 2019; 100:314-321.
- Patterson HB. Quality and control. In Hydrogenation of fats and oils 2011; 329-350. AOCS Press.
- Jiang FL, Ikeda I, Ogawa Y, Endo Y. Rapid determination of saponification value and polymer content of vegetable and fish oils by terahertz spectroscopy. J Oleo Sci. 2012; 61(10):531-535.
[Crossref] [Google Scholar] [PubMed]
- Dalla Nora FM, Oliveira AS, Lucas BN, de Freitas Ferreira D, Duarte FA, Mello RO, et al. Miniaturized, high-throughput and green determination of the saponification value of edible oils using thermal infrared enthalpimetry. Analytical methods. 2018; 10(30):3770-3776.
- Mata T, Cardoso N, Ornelas M, Neves S, Caetano N. Sustainable production of biodiesel from tallow, lard and poultry fat and its quality evaluation. Chem Eng. 2010; 19(3).
- Kulkarni VS, Shaw C. Miscellaneous Physical, Chemical, and Microbiological Test Methods. Essential chemistry for formulators of semisolid and liquid dosages. 2016:193-221.
- Zauro SA, Abdullahi MT, Aliyu A, Muhammad A, Abubakar I, Sani YM. Production and analysis of soap using locally available raw-materials. Appl Chem. 2016; 96(7):41479-41483.
- Thomssen EG. Soap-making manual: A practical handbook on the raw materials, their manipulation, analysis and control in the modern soap plant. Van Nostrand; 1922.
- Nurulain S, Aziz NA, Najib MS, Salim MR, Manap H. A review of free fatty acid determination methods for palm cooking oil. In Journal of Physics: Conference Series 2021; 1921(1): 012055. IOP Publishing.
- Hishamuddin E, Sulaiman N, Bustamam FK, Beng YC. Recent Updates on the codex standard for named vegetable Oils (CXS 210-1999) in relation to palm oil and palm kernel oil. 2020.
- Tarun J, Susan J, Suria J, Susan VJ, Criton S. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol. 2014; 59(5):442.
[Crossref] [Google Scholar] [PubMed]
- Febriani A, Syafriana V, Afriyando H, Djuhariah YS. The utilization of oil palm leaves (Elaeis guineensis Jacq.) waste as an antibacterial solid bar soap. IOP Conf. Ser. Earth Environ. Sci. 2020;572(1):012038.
- Warra AA, Ibrahim HM, Komo JI, Babatola LJ, Adejuwon OM, Salami TA. Qualitative analysis of soap samples prepared from shea butter. Adv. Res. 2020;21(4):19-24.
- Popescu V, Soceanu A, Dobrinas S, Stanciu G, Epure DT. Quality control and evaluation of certain properties for soaps made in Romania. Sci. Study Res. 2011;12(3):257-261.
- Ameh AO, Muhammad JA, Audu HG. Synthesis and characterization of antiseptic soap from neem oil and shea butter oil. African J. Biotechnol. 2013;12(29):4656-4662.
- Adeyemi AF. Evaluation of sodium and potassium soaps prepared from beeswax: Adding value to material. Int. J. Chem. Mater. Res. 2022;10(1):1-10.
- Warra AA, Hassan LG, Gunu SY, Jega SA. Cold-process synthesis and properties of soaps prepared from different triacylglycerol sources. Niger. J. Basic Appl. Sci. 2010;18(2):315-321.
- Paphane BD, Nkoane B, Oyetunji O, Bwaacha F. Production and characterization of soaps from Croton megalobotrys and Ricinus communis seed oils indigenous to Botswana with high levels of antimicrobial and antioxidant activity. IOSR J Appl Chem. 2022;15(9): 20-30.
- Legesse A, Habtamu A, Tegene T. Use of jatropha seed oil and alkali solution obtained from its ash for soap making. Appl Sci Environ Manag. 2020;24(12):2005:15.
- Esonye C, Ume CS, Esonye MC, Okafor VN, Ofoefule AU. Extraction of Nigerian beef tallow by wet rendering process and its characterization. World News Nat. Sci. 2017;15:129-138.
- Betsy KJ, Jilu M, Fathima R, Varkey JT. Determination of alkali content and total fatty matter in cleansing agents. Asian J. Sci. Appl. Technol. 2013;2(1):8-12.
- A. C. CODEX. Report of the twenty forth session of the codex committee on fats and oils, Melaka, Malaysia. 2017.
- Silva Ferreira B, Pereira Faza L, Le Hyaric M. A comparison of the physicochemical properties and fatty acid composition of indaiá (Attalea dubia) and Babassu (Orbignya phalerata) oils. Sci. World J. 2012;2012.
[Crossref] [Google Scholar] [PubMed]
- Kumar A. Physico-chemical and natural products investigations of essential oil from the rhizomes of Kaempferia galanga L. Der Chem. Sin. 2014;5(2):91-94.
- Belsare GW, Badne SG. Study on physico-chemical characterization of edible oils from agencies of Buldhana district. Int. J. Res. Pharm. Chem. 2017;7(4):525-529.
- Xiao JJ, Jiang W. Study the influence of soap solution’s pH value on the modified asphalt emulsion performance. Appl. Mech. Mater. 2013;253(1):312-316.
- Issa M, Isaac I, Matthew O, Shalangwa B, Sunday M. Physicochemical analysis for quality and safety of some selected animal soaps compared to human soaps in plateau state, Nigeria. IOSR J. Appl. Chem. 2020;13(3):25-28.
- Owoicho I. Quality assessment of soaps produced from bleached palm oil and Moringa oleifera seed oil. Glob. J. Eng. Technol. Adv. 2021;7(1):001-5.
- Vivian OP, Nathan O, Osano A, Mesopirr L, Omwoyo WN. Assessment of the physicochemical properties of selected commercial soaps manufactured and sold in Kenya. Open J. Appl. Sci. 2014;4(8):433-440.
- Adegbe AA, Larayetan RA, Omojuwa TJ. Proximate analysis, physicochemical properties and chemical constituents characterization of Moringa oleifera (Moringaceae) seed oil using GC-MS analysis. Am. J. Chem. 2016;6(2):23-28.
- Glenn E, John A. Process for making high moisture content soap bars. 1997.
Citation: Nadew TT, Assefa A, Nigussie S, Kamus E, Muchie M (2023). Utilization and Purification of Tallow, Grease, and Lard Byproducts from Abattoir and Meat Processing Plant for Soap Production: Case Study at Elfora Agro-Processing Industry, Kombolcha, Wollo, Ethiopia. Adv Chem Eng. 13:311.
Copyright: © 2023 Nadew TT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

