Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Brief Report - (2024) Volume 16, Issue 1
Use of Reverse Blood Grouping to Identify Plasma Samples Switched between Subjects in a Pharmacokinetic Study
Sandeep Shah, Hiren Mehta, Charles E. DiLiberti and Keith D. Gallicano*2Veeda Clinical Research Ltd. Satyamev Corporate, Prahlad Nagar Road, Ahmedabad. Gujarat-380015, India
3Montclair Bioequivalence Services, LLC P.O. Box 1585 Arroyo Grande, CA, 93421, USA
4Scientists Advancing Affordable Medicines, USA
Received: 17-Jan-2024, Manuscript No. JBB-24-24631; Editor assigned: 22-Jan-2024, Pre QC No. JBB-24-24631 (PQ); Reviewed: 05-Feb-2024, QC No. JBB-24-24631; Revised: 12-Feb-2024, Manuscript No. JBB-24-24631 (R); Published: 19-Feb-2024, DOI: 10.35248/0975-0851.24.16.556
Abstract
Background: Inadvertent switching of plasma samples between two subjects is a leading cause of implausible pharmacokinetic profiles in bioequivalence studies, and may cause erroneous bioequivalence conclusions. Such events typically go unnoticed until review of the pharmacokinetic data. A key objective was to find an in vitro test that, in lieu of real-time documentation, and independent of the drug plasma concentrations themselves, could provide definitive evidence of such a putative sample swap.
Methods: We present a case of a post-hoc analysis of two plasma samples from a bioequivalence study using a Reverse Blood Grouping (RBG) procedure to demonstrate unequivocally that a sample switch had occurred between two study subjects with different ABO blood types.
Results: The bioequivalence study failed with the original incorrect assignment of subject identities, but passed following correction of subject assignments based on RBG analysis and the statistical re-analysis of the data.
Conclusion: RBG testing can support correction of aberrant pharmacokinetic data when there is no real-time documentation demonstrating a protocol violation during the clinical and/or analytical phase of the study.
Keywords
Reverse blood grouping; Switched plasma samples; Pharmacokinetic outlier; Bioequivalence
Introduction
Aberrant/implausible pharmacokinetic data can take various forms and arise from various causes. Regulatory agencies generally discourage excluding data from the statistical analysis of a bioavailability or bioequivalence study solely based on a statistical test or pharmacokinetic reasons alone [1]. Guidances published by the United States Food and Drug Administration state that the only instance outlier data should be removed from the statistical analysis of a bioavailability or bioequivalence study is when there is real- time documentation demonstrating a protocol violation during the clinical and/or analytical phase of the study [2,3]. However, such supportive documentation is rarely available in real time, and only after review of the pharmacokinetic data may aberrant results even be suspected. Conventional post-hoc investigations into the root cause of the aberrant drug concentrations often reveal no assignable cause, leaving sponsors and regulators at a loss to determine how to handle the aberrant data.
Inadvertent switching of plasma samples between subjects, particularly during manual sample processing operations in the clinic, is a leading cause of physiologically implausible drug concentrations in pharmacokinetic profiles from bioequivalence studies. Such events, where samples are mistakenly associated with the wrong subject, typically go unnoticed until review of the pharmacokinetic data, and the aberrant drug concentrations may bias the conclusions of the study.
A key objective was to find an in vitro test that, in lieu of real-time documentation, and independent of the drug plasma concentrations themselves, could provide definitive evidence of such a putative sample swap. A commercially available, inexpensive, sensitive, clinical biomarker, with minimal sample volume requirements, and that could reliably distinguish plasma from different subjects would be ideal for this purpose.
Although genomic testing has been used to detect incorrect assignment of samples to the wrong donor and correct mislabeled samples in genomic datasets [4,5], a simpler, more widely available, and less expensive test would be preferred for plasma samples from pharmacokinetic studies. In this communication, we describe a case of a post-hoc analysis of plasma samples from a bioequivalence study using a Reverse Blood Grouping (RBG) procedure to demonstrate unequivocally that a sample interchange had occurred between two subjects with different ABO blood types.
The ABO blood grouping system classifies blood into four main types (A, B, AB and O). Normal healthy adult subjects not expressing a given blood group antigen (A and/or B) on their erythrocytes will have antibodies in their serum/plasma toward the unexpressed blood group antigen(s) (Table 1). When plasma or serum containing these antibodies is treated with erythrocytes bearing the corresponding antigens, antigen-antibody reactions cause hemagglutination that can be observed microscopically. Absence of Rh factor on an individual’s erythrocytes does not induce anti-Rh antibody formation reliably, so testing for anti-Rh antibodies would not be definitive [6].
| ABO blood group | Antigen(s) present on erythrocyte surface | Antibodies present in plasma |
|---|---|---|
| Type O | Neither A nor B antigen | Anti-A and Anti-B |
| Type A | A antigen | Anti-B but not Anti-A |
| Type B | B antigen | Anti-A but not Anti-B |
| Type AB | A and B antigens | Neither Anti-A nor Anti-B |
Table 1: ABO blood group system and reciprocal relationship between antigens and antibodies.
Materials and Methods
During review of the plasma concentration profiles of subjects that participated in a 2-group, 2-period, 2-sequence, 2-formulation, crossover bioequivalence study comparing a test generic formulation to the reference drug product, Subjects 36 and 37 appeared to have their 5-hour samples from period 2 inadvertently interchanged (Figure 1A); in period 2, Subject 36 received the test formulation and Subject 37 received the reference product. The study marginally failed bioequivalence criteria for maximum observed plasma drug concentration (Cmax).
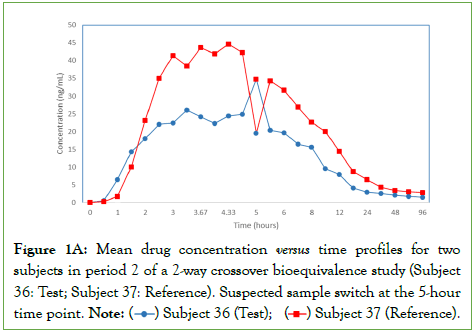
Figure 1A: Mean drug concentration versus time profiles for two subjects in period 2 of a 2-way crossover bioequivalence study (Subject
36: Test; Subject 37: Reference). Suspected sample switch at the 5-hour
time point.
Note: ( ) Subject 36 (Test); (
) Subject 36 (Test); ( ) Subject 37 (Reference).
) Subject 37 (Reference).
To investigate whether the samples were switched, an RBG procedure was applied to seven period-2 samples from each subject: the suspect 5-hour sample and three control samples flanking the 5-hour time point. For each sampling time point, Aliquot 1 was used for RBG analysis to ensure that the analysis was done on the same aliquot as the original bioanalysis for drug concentrations.
These samples were, according to a pre-specified protocol, randomized, blinded, and shipped from the bioanalytical facility at Veeda to Supratech Micropath Laboratory and Research Institute Pvt. Ltd. Ahmedabad, India, which is a certified facility to conduct both automated and manual RBG analysis. All personnel at Supratech were blinded to sample identity at all times.
Initially RBG testing was performed using a fully automated analyzer QWALIS®3 (DIAGAST, Loos, France), but all samples yielded inconclusive results due to inadequate sensitivity.
The more sensitive, clinically-approved manual RBG method, involving treatment of 100-150 μL aliquots of the plasma samples with freshly prepared red cells from Rh-negative donors of known ABO blood types as reagents, followed by microscopic examination for hemagglutination, was then proven to be fit for purpose by analysis, under a pre-specified protocol, of 10 randomized, blinded plasma samples (2 type A, 3 type B, 3 type AB, and 2 type O) from other subjects in the same study, with 100% accuracy.
Following this validation, the original Subject 36 and 37 samples (still blinded) were re-analyzed using the more sensitive manual method, with definitive hemagglutination results from all samples.
Results
The expected ABO blood grouping, antigen and antibody assignments for the two subjects are presented in (Table 2).
| Subject ID and blood group | Antigen(s) present on erythrocyte surface | Antibodies present in plasma |
|---|---|---|
| Subject 36 (AB positive) | A and B antigens | Neither Anti-A nor Anti-B |
| Subject 37 (B positive) | B antigen | Anti-A but not Anti-B |
Table 2: Expected ABO blood group, antigen and antibody assignments for the two subjects.
For the RBG results, all 6 plasma control samples collected from Subject 36 (known type AB positive) had neither anti-A nor anti-B antibodies and all 6 plasma control samples collected from Subject 37 (known type B positive) had anti-A but not anti-B antibodies, confirming the RBG match of the controls to the subjects’ known blood types; however, the 5-hour, period-2 sample from Subject 36 showed anti-A but not anti-B antibodies, and the 5-hour, period-2 sample from Subject 37 showed neither anti-A nor anti-B antibodies, the results of which were not consistent with the subjects' known blood types or their corresponding control samples, but were consistent with the blood type of the other subject.
Correcting the assignment of the subject-mismatched pair of samples produced the expected pharmacokinetic profile (Figure 1B) and the statistical re-analysis of the data resulted in a passing bioequivalence study.
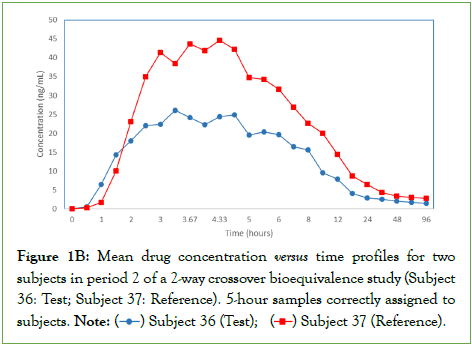
Figure 1B: Mean drug concentration versus time profiles for two subjects in period 2 of a 2-way crossover bioequivalence study (Subject
36: Test; Subject 37: Reference). 5-hour samples correctly assigned to
subjects.
Note: ( ) Subject 36 (Test); (
) Subject 36 (Test); ( ) Subject 37 (Reference).
) Subject 37 (Reference).
Discussion
The period-2, 5-hour sample from subject 36 had been flagged for incurred sample repeat analysis, and yielded a repeat result comparable to the original result. Therefore, the sample switch likely occurred in the clinic during the plasma sample transfer/ segregation following centrifugation of the respective vacutainers and not in the bioanalytical laboratory. This is a logical assumption, considering the plasma tubes for the two samples were adjacent to each other in the sample rack holding the 5-hour samples. However, a specific test, independent of the drug plasma concentrations themselves, was required to prove that the samples were swapped.
The implications for swapped plasma samples in a bioequivalence study are crucial. A bioequivalence study that otherwise would have failed, could, due to a single sample switch, erroneously pass bioequivalence criteria, leading to the regulatory approval of a nonbioequivalent product. Conversely, a study that otherwise would have passed, could, due to a single sample switch, erroneously fail bioequivalence criteria, leading to unnecessary repeat studies, increasing project costs, and delayed timelines.
RBG testing is inexpensive and widely available in clinical laboratories. It is a reliable scientific method to evaluate ABO blood group and has utility in detecting mismatched plasma samples across subjects to help explain physiologically implausible drug concentrations in pharmacokinetic studies. That all 24 samples tested using the manual RBG method yielded accurate and definitive hemagglutination results, despite having undergone multiple freeze-thaw cycles, underscores its robustness with realworld plasma samples. This testing is particularly important when aberrant drug concentrations affect the outcome of a borderline passing or failing bioequivalence study and there is no real-time documentation demonstrating a protocol violation during the clinical and/or analytical phase of the study, though a limitation of the test is that the subjects involved in a putative sample switch must have different ABO blood types.
Conclusion
The totality of evidence from the RBG results and observation of the drug concentration profiles unequivocally shows that the two 5-hour plasma samples were switched between the two subjects. We conclude that manual RBG testing is a reliable and practical method to discern sample switches between subjects of different ABO blood types in a pharmacokinetic study. Moreover, RBG testing can support correction of aberrant pharmacokinetic data when there is no real-time documentation demonstrating a protocol violation during the clinical and/or analytical phase of the study.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
Reverse blood group testing was supported by Veeda Clinical Research Ltd.
Acknowledgments
Keith Gallicano wrote the initial draft of the manuscript. Hiren Mehta reviewed the manuscript and prepared the figures. Charles DiLiberti devised the RBG testing and validation protocols and contributed to the writing of the manuscript. Sandeep Shah supervised the RBG testing and approved the manuscript. Hiren Mehta is an employee of Veeda Clinical Research Ltd. Sandeep Shah is an employee and founder director of Supratech Micropath Laboratory and Research Institute Pvt. Ltd.
References
- Chen ML, Blume H, Beuerle G, Mehta M, Potthast H, Brandt A, et al. Summary report of second EUFEPS/AAPS conference on global harmonization in bioequivalence. Eur J Pharm Sci. 2019;15(127):24-28.
[Crossref] [Google Scholar] [PubMed]
- Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an abbreviated new drug application. 2021.
- Bioavailability Studies Submitted In Ndas Or Inds-General Considerations. 2022.
- Schröder J, Corbin V, Papenfuss AT. HYSYS: Have You Swapped Your Samples?. Bioinformatics. 2017;33(4):596-598.
[Crossref] [Google Scholar] [PubMed]
- Javed N, Farjoun Y, Fennell TJ, Epstein CB, Bernstein BE, Shoresh N. Detecting sample swaps in diverse NGS data types using linkage disequilibrium. Nat Commun. 2020;11(1):3697.
[Crossref] [Google Scholar] [PubMed]
- The Royal Children’s Hospital Melbourne. The Royal Children’s Hospital Melbourne. Blood groups and compatibilities.
Citation: Shah S, Mehta H, DiLiberti C, Gallicano K (2024) Use of Reverse Blood Grouping to Identify Plasma Samples Switched between Subjects in a Pharmacokinetic Study. J Bioequiv Availab.16:556.
Copyright: © 2024 Shah S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

