Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 8, Issue 3
Use of Antiplatelet and Lipid Lowering Agent Therapies as Primary Cardiovascular Disease Prevention Strategy and their Determinant Factors among Type 2 Diabetes Mellitus Patients in University of Gondar Comprehensive Specialized Hospital, Gondar: A Prospective Cross- Sectional Study
Tewodros Solomon1*, Sumeya Tadesse2, Abebech Tewabe2 and Tessema Tsehay22Department of Clinical Pharmacy, University of Gondar, Gondar, Ethiopia
Received: 30-Mar-2023, Manuscript No. DCRS-23-20542; Editor assigned: 03-Apr-2023, Pre QC No. DCRS-23-20542(PQ); Reviewed: 24-Apr-2023, QC No. DCRS-23-20542; Revised: 01-May-2023, Manuscript No. DCRS-23-20542(R); Published: 08-May-2023, DOI: 10.35841/2572-5629.23.8.159
Abstract
Background: Patients with type 2 diabetes mellitus have two to four times increased risk of cardiovascular events compared with those without diabetes. Anti-platelet therapy and lipid lowering therapies have a significant contribution to prevent primary cardiovascular disease to achieve optimal patient outcomes.
Objective: This study aimed to assess the use of antiplatelet and lipid lowering agent therapies as primary cardiovascular disease prevention strategies and their determinant factors among type 2 diabetes mellitus patients in University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia.
Methods: A prospective cross sectional study was conducted among 405 type 2 diabetes mellitus patients selected using a systematic random sampling method. Semi-structured questionnaire and data abstraction format were used for data collection. The data was collected from May 1–July 30, 2022. Multivariable binary logistic regression analysis was done for identifying factors associated with use antiplatelet and lipid lowering agent. Statistical significance was declared at 95% confidence interval.
Result: Out of 405 study participants, the majority of study participants 301 (74.3%) had low (<10%) 10-year cardiovascular disease risk and moderate risk was 75 (18.5%). Statin was used for 180 (44.4%) of the study participants. While 81 mg aspirin was used for 38 participants. About 58.8% using statin appropriately while 89.9% of them using aspirin appropriately. Ages between 65-69 years old (AOR=3.76, 95% CI: 1.33-10.61), taking alcohol (AOR=0.38, 95%: 0.23-0.64), being hypertensive (AOR=2.30, 95%CI: 1.38-3.86) and using two or more glucose lowering agent (AOR=4.60, 95%CI: 2.72-7.78) were significantly associated with use of statin. On the other hand, use of more than one glucose lowering agent (AOR=4.36, 95%CI: 1.64-11.61) and being hypertensive (AOR=3.34, 95%CI: 1.24-8.96) were associated with the use of aspirin.
Conclusion: The appropriate indication of lipid lowering agent was low. So, this population is at high potential risk for the development cardiovascular disease and predisposed to unwanted medication side effects. Also, aspirin was used for 10% of patients who were scored under high risk, which implicates these populations are at high risks for bleeding and Reye syndrome, despite the low benefit. These results emphasize the necessity of considering the cardiovascular disease risk of the patients and apply an appropriate measure for primary prevention.
Keywords
Type 2 diabetes mellitus; Primary cardiovascular diseases prevention; Anti-platelet; Lipid lowering agent
Introduction
Diabetes Mellitus (DM) is a metabolic disorder manifests as high blood glucose as a result of insulin deficiency due to defects in secretion, insulin resistance, or both. Diabetes is associated to longterm harm, dysfunction, and failure of many organs, particularly the heart, blood vessels, kidneys, nerves, eyes, and kidneys [1].
The American Diabetic Association (ADA) has classified diabetes into four categories, which are Type 1 diabetes mellitus, Type 2 Diabetes Mellitus (T2DM), gestational diabetes mellitus and other forms of diabetes mellitus. T2DM is the most common form of diabetes, affecting about 85%–90% of individuals with diabetes. T2DM is a complex disease characterized by hyperglycemia, insulin resistance, and variable degrees of insulin deficiency [2].
Diabetes affected 422 million people in 2014, up from 108 million in 1980. Compared to high-income countries, the prevalence has been increasing more quickly in low- and middle-income nations. With an estimated 1.5 million deaths directly related to diabetes in 2019, it was the ninth most common cause of death. Throughout the past few decades, diabetes has been rapidly increasing in both instances and prevalence [3].
When compared to non-diabetic persons, people with T2DM have a two to four times higher risk of developing Coronary Heart Disease (CHD), and CHD mortality rates are four times higher than those of the general population. Also, diabetic patients without a history of Myocardial Infarction (MI) are at high risk of developing MI as non-diabetic patients with a history of MI [4]. The World Health Organization (WHO) report shows that Cardio-Vascular Disease (CVD) caused around 9% of all deaths in Ethiopia in 2012 [5]. The high incidence of sudden deaths makes primary prevention particularly relevant in this population.
Age, tobacco use, an unhealthy diet, physical inactivity, obesity (which can be caused by a combination of an unhealthy diet, physical inactivity, and other factors), elevated blood pressure (hypertension), abnormal blood lipids (dyslipidaemia), and elevated blood glucose are risk factors that can contribute to the development of CVD [6]. Among this; Age, smoking, raised mean arterial pressure, hypertension, high serum cholesterol, low levels of High-Density Lipoprotein (HDL), and obesity are the major risk factors for CVD in T2DM patients [7].
The importance of lipid lowering agents as PCVDP is advocated by different randomized control trails. The Collaborative Atorvastatin Diabetes Study; the Heart Protection Study; the Anglo-Scandinavian Cardiac Outcomes Trial and the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese study reported that there is a significant reduction in CV events rate in this T2DM population with 37%, 33%, 23% and 36% reduction in CVD events, respectively [8-11]. So, WHO/ISH and ADA 2021 recommend statins as a primary prevention strategy in those who has WHO/ISH risk stratifications of 10-yr risk is greater than 20% and for all 40-75 years T2DM patients [6-12].
According to the Antithrombotic Trialists’ Collaboration metaanalysis which encompasses about 95,000 study participants, aspirin was associated with a 12% reduction in serious vascular events compared with non-aspirin groups [13]. The Study of Cardiovascular Events in Diabetes trial also supports the use of aspirin as PCVDP with a 12% risk reduction of serious vascular events less frequent in the aspirin group than in the placebo group [14]. A current meta-analysis did show a lower risk of non-fatal myocardial infarction and ischaemic stroke with the use of aspirin, while no reduction in all-cause or CV mortality [15]. The ADA, ACC/AHA and ESC guidelines recommend once-daily, low-dose aspirin for PCVDP in patients with diabetes at very high risk (CVD risk score more than 30%) after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding [6-12].
In Ethiopia, the escalating burden of CVD and its risk factors warrants timely action and could be scaled up at a modest budget increase [15,16]. However, little is known about antiplatelet and statin utilization in the case of Ethiopia for primary prevention of CVD. Lack of inadequate/poor knowledge and malpractice of appropriate treatment regimens for prevention of CVD may contribute to inappropriate time or late drug therapy initiation, incorrect drug choices, incorrect dosing, decreased clinical outcome, increase the expenditure for treatment after diseases once occurred and increase the mortality and morbidity of patients.
The potential inappropriate uses of primary prevention for cardiovascular disease in DM patients are becoming a concern worldwide with their increment in quantity and variety. Inappropriate use may be associated with health risks to the patient and financial crisis for the health facilities and patients.
So this study was undertaken to determine the use of Antiplatelet and Lipid Lowering Agent (ALT) as PCVDP strategies also addresses the appropriate initiation of anti-platelet and lipid lowering agent’s patterns, estimates the ten year CV risk and identify the association factors for the use of anti-platelet and lipid lowering agents.
Methodology
Study setting, area and period
The study was conducted at the University of GONDAR COMPREHENSIVE SPECIALIZED HOSPITAL (UoGCSH). The hospital is located in Gondar city which is 738 km away from Addis Ababa, the capital of Ethiopia. It is one of the tertiary care hospitals in the Amhara regional state, and it offers outpatient, inpatient, and emergency services. Among this, the study was conducted among T2DM patients attending UoGCSH outpatient clinic. The hospital provides services for about seven million people in the catchment areas. The hospital currently has about 2000 medical and non-medical staff and has more than 598 beds [17]. There are about 5000 diabetes patient attendants for follow up at UoGCSH and among these 2730 are T2DM patients. A prospective cross-sectional study was conducted to assess use of ALT as PCVDP strategies on T2DM patients attending UoGCSH outpatient diabetes clinic from May 1-July 30, 2022.
Population
All type 2 diabetes mellitus patients attending UoGCSH outpatient diabetes clinic were taken as source of population. All type 2 diabetes mellitus patients who fulfilled the inclusion criteria of the study and were available during the study period were considered as study population.
Criteria
T2DM patients without a previous history of CVD and dyslipidaemia and willing to participate in the study were included in the study. However, Patients having incomplete charts for clinical characteristics, age less than 40 years and greater than 74 years, pregnant women, patients having a serious mental illness, cannot stand for height and weight measurement and lost appointment date were excluded from the study.
Sample size determination and sampling technique
The sample size was determined using a single proportion formula,
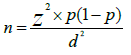
Where, n is the required sample size; d is the marginal error that is 5% (d=0.05); z is the required degree of accuracy at 95% confidence level=1.96 and p is the proportion of ALT use among type 2 DM patients in UoGCSH (0.5 (50%)). The final sample sizes calculated and adjusted were found to be 422. The study population is 1840. So, K=1840/422=4.36 approximately 4. The study subjects were selected by using simple random sampling method with k=4 for each using their sequence of daily follow-up and 2 were selected from 1-4 using the lottery method. Then, it was proceeding with an interval of two mean that 2nd, 6th, 10th etc… participants.
Study variables
Use of anti-platelet therapy and lipid lowering therapy were the dependent variables while socio-demographic characteristics, CVD risk score, type of medications used for DM, number of medications used for DM, compliance with T2DM medications, life style modifications, comorbid conditions, family history of CVD (Genetic factor), contraceptive use and hormonal therapy, glycemic control were the independent variables.
Operational definitions
Cardiovascular disease: Confirmed physician diagnosis of Congenital Heart Defects (CHD) in the form of: stable angina, unstable angina, or acute myocardial infarction. Confirmed physician diagnosis of ischemic or haemorrhagic stroke. Confirmed physician diagnosis of peripheral artery disease (ischemic limb, gangrene, or amputation) [12].
Primary prevention: The actions taken to identify a patient at risk and protect them from emerging of cardiovascular disease through interventions which are ethically acceptable.
Risk stratification: is partitioning of patients by factors other than the treatment given and stratified as low risk patients those have 10 year risk of cardiovascular event<10%; moderate risk patients those have 10% to <20%; high risk patients who have 20% to <30% and very high risk patients who have a 10 year risk of cardiovascular event ≥ 30% [6].
Inappropriate indication: Is a valid reason for using ALT in which the patient risk score status does not feet with the indicated agent or patient’s risk score status needs to use this agent but not taking it currently.
Appropriate indication: Is a valid reason to use ALT in which when patients risk score status feet with the indicated therapy or not using ALT while patients risk score status doesn’t need to use this agent currently.
Use of antiplatelet for PCVDP: Use of 75 mg/day-162 mg/day Aspirin without patient diagnosis of Acute Coronary Syndrome; Ischemic Stroke; Coronary Artery Disease; Pain and fever; and Colorectal Cancer or an indication of aspirin was set for the PCVDP on their medical chart.
Use of lipid lowering agent for PCVDP: Use of simvastatin 10 mg-40 mg, atorvastatin 10 mg-40 mg, rosuvastatin 5 mg-20 mg or lovastatin 20 mg-80 mg PO daily without patient diagnosis of Hyperlipidemia or an indication of statin was set for the PCVDP on their medical chart.
Data collection instrument and procedures
Measurement tool
The criteria for appropriate indication (initiation) of antiplatelet are adopted from WHO/ISH CVD risk prediction tool interventions guide and ADA 2021, in which Aspirin therapy (75 mg/day-162 mg/day) is considered as a primary prevention strategy in those with T2DM at increased cardiovascular risk, whose 10 yr WHO/ ISH risk is greater than 30% after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding [6,12].
The criteria for appropriate indication of lipid lowering agent are adopted from WHO/ISH CVD risk prediction tool interventions guide and ADA 2021 in which moderate intensity statins, which are simvastatin 10 mg-40 mg, atorvastatin 10 mg-40 mg, rosuvastatin 5 mg-20 mg or lovastatin 20 mg-80 mg PO daily is used as a primary prevention strategy in those who has WHO/ISH risk stratifications of 10 yr risk is greater than 20% and for all 40-75 years T2DM patients [6,12].
In the case of determining the risk of CVD for initiation of ALTs, the WHO/ISH risk prediction was used. The WHO/ISH risk chart is a widely validated tool for assessing cardiovascular risk which is categorized in 21 Global Burden of Disease regions with more homogenous grouping of countries, and it had two sets of charts; laboratory-based charts and non-laboratory based charts. Under the laboratory based charts, six variables are included; these are age, sex, smoking, systolic blood pressure, presence or absence of diabetes and total cholesterol. While under non-laboratory-based charts variables like age, sex, smoking, systolic blood pressure and Body Mass Index (BMI) are included.
Smoker: All current smokers and those who quit smoking less than 1 year before the assessment were considered smokers for assessing cardiovascular risk [6,18].
Systolic blood pressure: Taken as the mean of the two most recent readings on each of two occasions [18].
Lipid profile: The mean of two non-fasting measurements of serum cholesterol by dry chemistry, or one non-fasting laboratory measurement, is sufficient for assessing risk [18].
Age: Age was calibrated starting from the age of 40 years with a basis of five years interval until the age of 74 years [6].
Height: Was recorded in a standing position. The subject was asked to stand straight on the plane floor and his head was positioned on the Frankfurt plane. The height was measured in centimetres with the help of a measuring tape.
Weight: The subjects were weighed in digital beam balance and a weight was recorded in kilograms [19].
Body Mass Index (BMI): Was calculated by obtaining the ratio of weight (kg)/height2 (m2).
In addition to the above adopted tool, different literatures are reviewed to assess the factors that are associated with the use of ALTs [6,19-23]. Then data was collected by using a semi-structured interviewer administered questionnaire and reviewing of patient medical records.
The questionnaire was prepared in English language including variables like socio-demographic, risk factor, laboratory monitoring and current medication experience and was translated to Amharic language. Data collection questionnaire papers, pen and pencil were used during data collection.
After all preliminary preparations were completed; the data was collected based on the data collection format by trained data collectors.
Data quality assurance
The data was collected by trained data collectors using semistructured interviewer administrative questionnaire and data abstraction format. Data collectors were trained intensively by the principal investigator on the contents of the questionnaire, data collection methods, and ethical concerns before the actual data collection. The filled questionnaire was checked daily for completeness by the principal investigator. Before starting the data collection, pre-test were done on 10% of the sample size at Felege Himont comprehensive hospital. In addition, we checked the suitability of WHO/ISH risk stratification scale in the pretest of this study with cronbach-alpha value of 0.825. According to the result of the pretest, the data collection tool was modified to the final version. The principal investigator was supervise the data collection process and given a feedback and correction on daily basis. Then the collected data was reviewed and checked for completeness and consistency of the data was made on daily basis.
Data processing and analysis
The data was checked, coded and entered into Epi-Data version 3 and exported to SPSS version 25.0 statistical packages. Descriptive statistics (percentage, mean standard deviations) were used to analyse the respondents' Sociodemographic characteristics, clinical characteristics and other information. To evaluate the relationship between independent variables and anti-platelet and lipid lowering agent use, logistic regression was utilized. Data was treated and expressed with their respective measurements using descriptive (frequencies) and inferential (bivariate and multivariate logistic regression) analysis methods. Bi-variable logistic regression analysis was performed to identify the association of each independent variable with the outcome variables. All variables with a p-value of<0.2 in bi-variable binary logistic regression analysis were entered into the multi-variable logistic regression model. A p-value of<0.05 was considered statistically significant and the adjusted odds ratio (AOR) with a 95% Confidence Interval (CI) was calculated. Goodness of model fitness was checked using the Hosmer-lemshow test and multi-collinearlity was checked using the variance inflation factor. Then the results were presented in the form of table, figures and chart using frequency and summary statistics such as mean, and percentage to describe the study population in relation to relative variables.
Results
Socio demographic characteristics of participants
Out of 422 sample populations, a total of 405 participants responded to the questionnaire with a response rate of 95.97%. The mean age of study participants was 55.86 with an SD of 8.854. More than half of the study participants were female (52.6) with a male to female ratio of 0.906:1. The majority of the study participants were married (76.8%). With regard to their education, the majority of the participants were illiterate and only attended primary, with frequencies of 115 (28.4%) and 112 (27.7%), respectively. Nearly half of study participants were taking governmental health insurance. In addition, the majority of participants were placed in the urban area (88.1) (Table 1).
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Sex | Male | 192 | 47.4 |
| Female | 213 | 52.6 | |
| Age | 40-44 | 50 | 12.3 |
| 45-49 | 47 | 11.6 | |
| 50-54 | 82 | 20.2 | |
| 55-59 | 72 | 17.8 | |
| 60-64 | 78 | 19.3 | |
| 65-69 | 42 | 10.4 | |
| 70-74 | 34 | 8.4 | |
| Marital Status | Single | 6 | 1.5 |
| Married | 311 | 76.8 | |
| Divorced | 19 | 4.7 | |
| Widowed | 69 | 17 | |
| Monthly Income | <1500 ETB | 3 | 1.8 |
| 1500–2999 ETB | 12 | 7.4 | |
| 3000–4999 ETB | 27 | 16.6 | |
| ≥ 5000 ETB | 121 | 74.2 | |
| Educational Level | Illiterate | 115 | 28.4 |
| Primary School | 112 | 27.7 | |
| Secondary School | 82 | 20.2 | |
| College or University Student | 12 | 3 | |
| Diploma or Degree | 84 | 20.7 | |
| Payment Type | Direct payment | 202 | 49.9 |
| Insurance | 196 | 48.4 | |
| Governmental support | 7 | 1.7 | |
| Place of Residence | Urban | 357 | 88.1 |
| Rural | 48 | 11.9 |
Table 1: Socio demographic characteristics of T2DM patients attending UoGCSH Diabetic care clinic from May 1-July 30, 2022.
Table clinical characteristics of participants
Among the 405 Type 2 DM patients who participated in the study, about 62% were diagnosed as T2DM and started treatment before 5 years. About 323 (79.8%) of study participants had uncontrolled debates (FBS>130 mg/dl). The lipid profile of the participants dictates that 60% of participants have low triglycerides and most of participants (67%) have low HDL levels among those who done their lipid profile. The most common comorbid disease and complication was hypertension (49.4%) which is followed by peripheral neuropathy (20%) and RVI (5.2%). Regarding non antidiabetic medications, 41% of the study participants took enalapril, which is followed by amlodipine, amitriptyline and HAART, which accounts for 23.7%, 19.5% and 4.9 respectively. With regard to antidiabetic therapy, 384 (94.8%) patients were taking metformin.
Among the 405 Type 2 DM patients who participated in the study, 55.8% of them took a salt free diet while 32.6% were adding small table salt to their meal (1/2 table spoon full daily) and of- this participants, 45.7% of them were taking alcohol. Among 405 participants, 59.5% of them were doing moderate physical exercises, 50.6% of them had cruddy oil consumption (Table 2).
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Duration of Diabetes | <5 years | 154 | 38 |
| ≥5 years | 251 | 62 | |
| Duration of Diabetes Treatment | < 5 years | 156 | 38.5 |
| ≥5 years | 249 | 61.5 | |
| Alcohol Use | Yes | 185 | 45.7 |
| No | 220 | 54.3 | |
| Physical Activity | Sedentary | 140 | 34.6 |
| Moderate | 241 | 59.5 | |
| Vigorous | 24 | 5.9 | |
| Cigarette | Yes | 14 | 3.5 |
| No | 391 | 96.5 | |
| Salt Intake | Salt free diet | 226 | 55.8 |
| Small table salt | 132 | 32.6 | |
| Normal table salt | 47 | 11.6 | |
| Oil Consumption | Cruddy oil consumption | 205 | 50.6 |
| Liquid oil consumption | 200 | 49.4 | |
| Family history of chronic disease | Yes | 126 | 31.1 |
| No | 279 | 68.9 | |
| Contraceptive use | Yes | 61 | 28.6 |
| No | 152 | 71.4 | |
| BMI | Under weight (<18.5) | 11 | 2.7 |
| Normal (18.5-24.9) | 68 | 16.8 | |
| Over weight (25-29.9) | 99 | 24.4 | |
| Obese (30-34.9) | 114 | 28.1 | |
| Severe obese (35-39.9) | 64 | 15.8 | |
| Morbid obese (40) | 49 | 12.1 | |
| Fasting Blood Sugar | Controlled Diabetes | 82 | 20.2 |
| Uncontrolled Diabetes | 323 | 79.8 | |
| Triglyceride | Normal (<150 mg/dl) | 68 | 59.6 |
| Border High (150 mg/dl-199 mg/dl) | 37 | 32.5 | |
| High (300 mg/dl-499 mg/dl) | 9 | 7.9 | |
| LDL | Optimal (<100 mg/dl) | 21 | 25.6 |
| Near or above Optimal (100 mg/dl-129 mg/dl) | 33 | 40.2 | |
| Boarder High (130 mg/dl-159 mg/dl) | 11 | 13.4 | |
| High (160 mg/dl-189 mg/dl) | 15 | 18.3 | |
| Very High (>=190 mg/dl) | 2 | 2.5 | |
| HDL | Low (< 40 mg/dl) | 27 | 67.5 |
| Normal (40 mg/dl-60 mg/dl) | 13 | 32.5 | |
| High (>60 mg/dl) | 0 | 0 | |
| Hypoglycemic agent | Metformin | 384 | 94.8 |
| Glybenclamide | 148 | 36.5 | |
| Insulin | 74 | 18.3 | |
| Comorbid Diseases | Hypertension | 200 | 49.4 |
| and Complications | Peripheral Neuropathy | 81 | 20 |
| Retroviral infection | 21 | 5.2 | |
| Dyspepsia | 11 | 2.7 | |
| Asthma | 9 | 2.2 | |
| Urinary tract infection | 4 | 1 | |
| Tuberculosis | 3 | 0.7 | |
| Epilepsy | 3 | 0.7 | |
| Toxic multinodular goitre | 3 | 0.7 | |
| Others* | 5 | 1.23 | |
| Hypertensive | Enalapril | 168 | 41.5 |
| Amlodipine | 96 | 23.7 | |
| Hydrochlorothiazide | 10 | 2.5 | |
| Furosemide | 10 | 2.5 | |
| Othersφ | 2 | 0.5 | |
| Other Drug | Amitriptyline | 79 | 19.5 |
| GI Drug | 17 | 4.2 | |
| HAART | 20 | 4.9 | |
| Antiasthmatic drug | 9 | 2.2 | |
| Ciprofloxacin | 4 | 1 | |
| Anti-tuberculosis | 3 | 0.7 | |
| Antiepileptics | 3 | 0.7 | |
| Propylthiouracil | 3 | 0.7 | |
| Othersτ | 4 | 1 | |
| HAART=Highly Active Antiretroviral Therapy; GI=Gastrointestinal; HD=High Density Lipoprotein; LD=Low Density Lipoprotein; GI Drug (Omeprazole, Antacid, Cimetidine); Antiepileptics (Phenytoin, Lamotrigine) *Epilepsy, Erectile Dysfunction, Gout, Joint pain φLosartan, Sacubatril/Valsartan τAntiepileptics, Allopurinol, Meloxicam |
|||
Prediction and evaluation of ten-year total CVD risk
A 10-year CVD risk was calculated using the 2019 WHO/ISH risk prediction chart of a fatal or nonfatal cardiovascular event using gender, age, and systolic blood pressure, and smoking status, presence of diabetes mellitus and either total cholesterol or BMI. The majority of study participants 301 (74.3%) had low (<10%) 10-year CVD risk followed by moderate risk which accounts 75 (18.5%) (Figure 1).
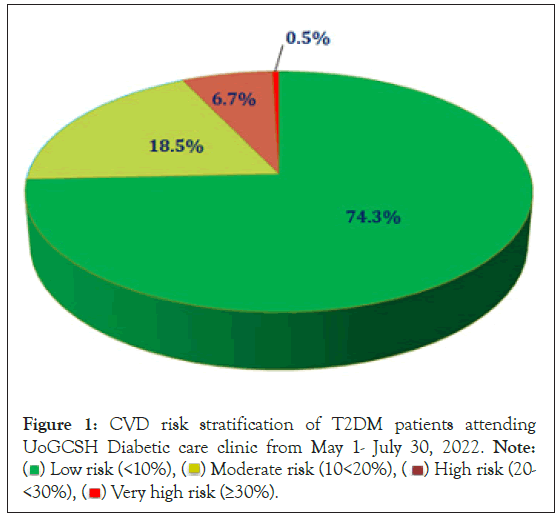
Figure 1: CVD risk stratification of T2DM patients attending
UoGCSH Diabetic care clinic from May 1- July 30, 2022. Note: ( ) Low risk (<10%), (
) Low risk (<10%), ( ) Moderate risk (10<20%), (
) Moderate risk (10<20%), ( ) High risk (20-<30%), (
) High risk (20-<30%), ( ) Very high risk (≥30%).
) Very high risk (≥30%).
Use of antiplatelet and lipid lowering agent
Statin was used by 180 (44.4%) of the study participants. Of which, 116 of them using atorvastatin with a dose of 20 mg which is followed by atorvastatin 40 mg (39) and simvastatin 20 mg [19]. While 81 mg of aspirin was used for all 38 participants as PCVDP (Figure 2 and Table 3).
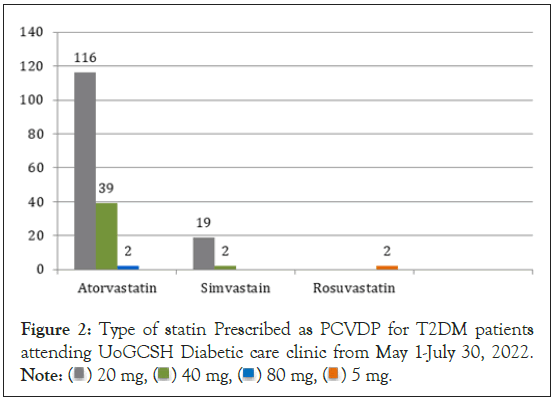
Figure 2: Type of statin Prescribed as PCVDP for T2DM patients
attending UoGCSH Diabetic care clinic from May 1-July 30, 2022. Note: ( ) 20 mg, (
) 20 mg, ( ) 40 mg, (
) 40 mg, ( ) 80 mg, (
) 80 mg, ( ) 5 mg.
) 5 mg.
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Use of Aspirin | Yes | 38 | 9.4 |
| No | 367 | 90.6 | |
| Use of Statin | Yes | 180 | 44.4 |
| No | 225 | 55.6 |
Table 3: Use of ALT as PCVDP for T2DM patients attending UoGCSH Diabetic care clinic from May 1-July 30, 2022.
Appropriate indication of ALT
The appropriate indication for statin and aspirin was measured using the WHO/ISH risk chart stratification and drug recommendation. Among 405 participants, 58.8% of the statins were appropriately indicated. Among the inappropriate indications, 159 used statin even if there was no need to administer it. While only 89.9% of aspirin was appropriately indicated (Table 4).
| Variable | Category | Statin | Total | |
|---|---|---|---|---|
| Indication of antiplatelet | Yes | No | ||
| Appropriate | 21 | 217 | 238 (58.8%) | |
| Inappropriate | 159 | 8 | 167 (41.2%) | |
| Variable | Category | Aspirin | Total | |
| Yes | No | |||
| Indication of antiplatelet | Appropriate | 0 | 364 | 364 (89.9%) |
| Inappropriate | 38 | 3 | 41 (10.1%) | |
Table 4: Appropriate indication of ALTs as PCVDP for T2DM patients attending UoGCSH Diabetic care clinic from May 1-July 30, 2022.
Factors associated with use of statin
Factors associated with the use of statins were assessed using bivariate and multivariate binary logistic regression analysis. In the bivariate analysis, age, residence, duration of diabetes, alcohol use, cigarette smoking, BMI, HTN and number of hypoglycemic agents were associated with statin use on P-value less than 0.2. These variables fulfil the minimum requirements for further multivariate binary logistic analysis.
Among these variables, multivariate logistic regression analysis showed that age between 65-69 years old, being hypertensive and using two or more hypoglycemic agents were positively associated with the use of statins as PCVDP while taking alcohol was negatively associated with the use of statins with p-value less than 0.05. To identify the effect of each variable, a multi-collinearity statistics test was checked and for all variables the VIF was approximated to 1, which is less than 10. The model fitness was tested by the Hosmer- lemshow test and its significance test was 0.96.
In this study, the odds of use of statins among that aged between 65- 69 years old were four times (AOR=3.76, 95% CI: 1.33-10.61). This study also shows that age between 45-49 years old was associated significantly in the p-value but the CI was not likely to have an association, hence, it includes 1. Use of more than one glucose lowering agent was about five times more likely to use statins as compared with taking a single glucose lowering agent (AOR=4.60, 95%CI: 2.72-7.78). In this study, the odds of use of statins among those who took alcohol were 38% less likely to use statins as compared with who didn’t take alcohol (AOR=0.38, 95%: 0.23- 0.64). While being hypertensive was two times more likely to use statins appropriately (AOR=2.30, 95%CI: 1.38-3.86) (Table 5).
| Variable | Category | Use of Statin | COR (95%CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Age | 40-44 | 12 | 38 | 1 | 1 |
| 45 – 49 | 16 | 31 | 0.56 (0.24, 1.34) | 4.39 (0.90, 14.83)* | |
| 50 – 54 | 35 | 47 | 0.42 (0.19, 0.93) | 2.64 (0.80, 8.68) | |
| 55 – 59 | 35 | 37 | 0.33 (0.15, 0.74) | 1.82 (0.62, 5.33) | |
| 60 – 64 | 33 | 45 | 0.43 (0.20, 0.95) | 2.01 (0.69, 5.80) | |
| 65 – 69 | 25 | 17 | 0.22 (0.09, 0.55) | 3.76 (1.33, 10.61)* | |
| 70 – 74 | 24 | 10 | 0.13 (0.05, 0.35) | 1.41 (0.44, 4.51) | |
| Residence | Urban | 166 | 191 | 1 | 1 |
| Rural | 14 | 34 | 0.47 (0.25, 0.91) | 1.97(0.90, 4.34) | |
| Duration of diabetes | <5 years | 45 | 109 | 1 | 1 |
| ≥ 5years | 135 | 116 | 2.82 (1.84, 4.32) | 1.09 (0.61, 1.93) | |
| Alcohol | Yes | 100 | 85 | 2.06 (1.38, 3.07) | 0.38 (0.23, 0.64)* |
| No | 80 | 140 | 1 | 1 | |
| Cigarette | Yes | 8 | 6 | 1.70 (0.58, 4.99) | 0.42 (0.10, .83) |
| No | 172 | 219 | 1 | 1 | |
| Under weight (<18.5) | 5 | 6 | 1 | 1 | |
| Normal (18.5-24.9) | 17 | 51 | 2.50 (0.68, 9.24) | 2.80 (0.61, 12.91) | |
| BMI | Over weight (25-29.9) | 34 | 65 | 1.59 (0.45, 5.60) | 2.06 (0.47, 9.01) |
| Obese (30-34.9) | 49 | 65 | 1.11 (0.32, 3.83) | 1.46 (0.34, 6.28) | |
| Severe obese (35-39.9) | 39 | 25 | 0.53 (0.15, 1.94) | 0.66 (0.14, 2.99) | |
| Morbid obese (≥40) | 36 | 13 | 0.18 (0.04, 0.83) | 0.60 (0.12, 2.94) | |
| HTN | Yes | 124 | 76 | 4.34 (2.85, 6.60) | 2.30 (1.38, 3.86)** |
| No | 56 | 149 | 1 | 1 | |
| Glucose Lowering Agent | Single Agent | 54 | 165 | 1 | 1 |
| Double and Above | 126 | 60 | 6.42 (4.16, 9.91) | 4.60 (2.72, 7.78)* | |
*Statistically Significant: P<0.05 |
|||||
Table 5: Factors associated with the use of lipid lowering agent as PCVDP for T2DM patients attending UoGCSH Diabetic care clinic from May 1-July 30, 2022.
Factors associated with the use of aspirin
The same analysis was conducted to assess factors associated with the use of aspirin. In the bivariate analysis, use of GI drug, HTN, number of hypoglycemic agents, target goal of FBS, physical activity, alcohol use, duration of diabetes and sex were associated with the use of aspirin on P-value less than 0.2. These variables fulfil the minimum requirements for further multivariate binary logistic analysis.
Among these variables, multivariable logistic regression analysis showed that being hypertensive was positively associated with the use of aspirin and using two or more hypoglycemic agents were negatively associated with the use of aspirin as PCVDP with a p-value less than 0.05. To identify the effect of each variable, a multi-collinearity statistics test was checked and for all variables the VIF was approximated to 1, which is less than 10. The model fitness was tested by the Hosmer-lemshow test and its significance test was 0.67.
In this study, use of more than one hypoglycemic agent was four times more likely to use statins as compared with taking a single hypoglycemic agent (AOR=4.36, 95%CI: 1.64-11.61). On the other hand, the odds of use of statins among hypertensive patients was three times higher as compared with non-hypertensive people (AOR=3.34, 95%CI: 1.24-8.96). This study also shows that those who took alcohol were associated significantly in the p-value but the CI was not likely to have association with, hence it includes 1 (Table 6).
| Variable | Category | Use of Aspirin | COR (95%CI) | AOR (95%CI) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Sex | Male | 22 | 170 | 1 | 1 |
| Female | 16 | 197 | 0.63 (0.32, 1.23) | 1.19 (0.552, 2.57) | |
| Duration of diabetes | <5 years | 9 | 145 | 1 | 1 |
| ≥ 5years | 29 | 222 | 2.11 (0.97, 4.58) | 1.41 (0.531, 3.75) | |
| Alcohol | Yes | 26 | 159 | 2.83 (1.39, 5.79) | 0.37 (0.17, 1.12)* |
| No | 12 | 208 | 1 | 1 | |
| Physical Activity | Sedentary | 23 | 117 | 1.34 (0.45, 3.95) | 2.20 (0.58, 8.33) |
| Moderate | 10 | 231 | 6.08 (1.89, 19.61) | 5.93 (1.54, 22.84) | |
| Vigorous | 5 | 19 | 1 | 1 | |
| FBS | Controlled | 5 | 77 | 1 | 1 |
| Uncontrolled | 33 | 290 | 1.75 (0.66, 4.64) | 0.90 (0.29, 2.72) | |
| HTN | Yes | 31 | 169 | 5.19 (2.23, 12.08) | 3.34 (1.24, 8.96)* |
| No | 7 | 198 | 1 | 1 | |
| GI Drug | Yes | 5 | 12 | 0.22 (0.07, 0.67) | 0.30 (0.08, 1.05) |
| No | 33 | 355 | 1 | 1 | |
| Glucose lowering Agent | Single Agent | 7 | 212 | 1 | 1 |
| Double Agent | 31 | 155 | 6.06 (2.60, 14.11) | 4.36 (1.64, 11.61)* | |
*Statistically Significant: P<0.05 |
|||||
Table 6: Factors associated with the use of antiplatelet agent as PCVDP for T2DM patients attending UoGCSH Diabetic care clinic from May 1-July 30, 2022.
Discussion
This is a prospective observational study to assess the use of antiplatelet and lipid lowering agents as primary cardiovascular disease prevention strategies which addresses the appropriate indication and use of anti-platelet and lipid lowering agent’s patterns and identify the association factors for the use of anti- platelet and lipid lowering agents [20].
This study reveals that 44.4% of study participants were used statins as PCVDP. Among for this, most of them (77.33%) use a low dose statin, while only about 21.67% and 1.1% of them use moderate and higher dose statin therapy, respectively. The majority of participants (88.27%) were use atorvastatin [21]. There are many reports on the use of statins for PCVDP among T2DM patients in which their prevalence varies from 32% to 65% [22-29]. This study finding is in accordance with the study reported by the United States of America (40%) (24) and Denmark (47%) [23-25]. While this study has a low rate of statin use compared with the finding of an insight study conducted in Ethiopia (55.7%) [26], India (55.2%) [27] and Malaysia (65%) [28]. This discrepancy could be explained by the difference in number of risk factors in subjects involved in the study in which higher participants were with total cholesterol ≥ 200 mg/dL (34.9% vs. 5.9%) and cigarette smoking (21.2% vs. 3%) compared to the current study; the difference in study design and patient selection of this study and the Malaysian study in which it uses a cohort study design and only uses participants which took statins [26-28].
The appropriate indication of statin for PCVDP was measured using the criteria for initiation of statin were adopted from WHO/ ISH CVD risk prediction tool interventions guide and ADA 2021 in which moderate intensity statin is used as a primary prevention strategy in those who has WHO/ISH risk stratifications of 10-yr risk is greater than 20%. Based on this, the result of this study reveals that the appropriate indication of statin for PCVDP was 238 (58.8%). Among 167 inappropriately indicated patients, 159 (95.2%) were taking statin while they were not eligible to take statin for PCVDP. In this study, the appropriate indication of statin was very low when compared with a cross-sectional study on appropriate use of statins for the prevention of cardiovascular disease in 41 low- income and middle-income countries using a WHO/ISH risk chart stratification in which the 82.5% of eligible population take statin appropriately [29]. This discrepancy may be due to the variation in sample participants used in which they took a higher sample study (116,449 individuals) and differences in study settings. On the other hand, when we see the result of African countries in that study, the appropriate indication of statin was 56.3% which was equivalent with this study result [29]. Even though the appropriate indication of statin was achieved for the WHO goal that at least 50% of eligible individuals receive statin therapy to prevent cardiovascular disease, there is still a high prevalence of inappropriate use was their which needs to be corrected [3]. Hence, the inappropriate indication of statins in T2DM populations drops a significant burden by adding unnecessary medication taken by the patients who tend to manifest unwanted side effects and have an impact on their financial condition. This was supported in a study conducted in Bonga [19].
According to this study, about 9.4% of study participants were using antiplatelet as Program of Cardiovascular Disease Prevention (PCVDP). This study result is comparable with the study conducted in Spain (6.0%) while it is low as compared with use of antiplatelet in the United States of America (20%), China (38.6%) and Colombia (44.2%), respectively [30-33]. Some reasons might account for the low prevalence of prescribing aspirin or other antiplatelet medications. First, the less communication between physicians and patients which lacks time to estimate the benefit provided by aspirin and the potential harm attributable to aspirin, including shared decision making between patient and health-care provider [12]. Second, physicians might be less likely to prescribe aspirin or other antiplatelet medications when they expect their patients not to adhere to their advice.
Most of the study participants (90.6%) didn’t use aspirin for PCVDP. Even though initiation of antiplatelet was low (9.4%), all the antiplatelet started for PCVDP were inappropriately initiated. This might be due to inappropriate risk score prediction for the prevention of CVD, which was a common reason for misuse of aspirin in a study conducted in China. Hence, administration of aspirin for low, moderate and high risk patients has low benefit and outweighs the risks of significant bleeding and Reye syndrome [12]. Also, there was a higher use of GI drugs reported in populations who took aspirin for PCVDP, so attention should be considered for those populations to prevent the occurrence of adverse drug reactions associated with aspirin use [31].
In this study, age between 65-69 years was four times more likely to use statins. This result was in line with the study conducted in Jimma Ethiopia [34], Colombia [32], India [27], Spain [33], and Canada [35]. A possible explanation for this might be, as age advances the risk of ASCVD increases, which tends to use statins more frequently than younger ages [32].
The study of this result shows that people being hypertensive were two times more likely to use statins than those non-hypertensive patients. This result was in line with the study conducted in Colombia [32], and Tanzania [36], which is two times and four times more likely to use statin respectively [33]. This could be because the physicians tend to give statins to those diabetic patients with hypertensive comorbid condition. Hence, diabetes patients having a comorbid condition of hypertension should be considered as a high risk of developing ASCVD [34].
Also, this study shows that use of two or more glucose lowering agents were around 5 times more likely to use statin than those who a took single glucose lowering agent were significantly associated with use of statin. This might be because the physician may think that, the more the patients’ blood sugar level is uncontrolled and are taking multiple blood glucose lowering agents, the more they tend to be at high risk for CVD, so they can prone to use this statin. This was supported in the study conducted in Jimma that uncontrolled blood sugar was two times more likely to use statin than those controlled blood sugar level [35].
According to this study, taking alcohol was negatively associated with the use of statins and those who took alcohol were 38% less likely to use statins than those who didn’t take alcohol. This might be because the physicians will counsel focus on the nonpharmacological preventive therapies more focusable on those who have high risk of ASCVD. Tanzanians study were fails to show the association between alcohol and use of statin [36].
Even though cigarette smoking, being overweight and living with diabetes mellitus for longer periods of time were associated positively with the use of statins, they failed to associate in this study. They were only associated with the use of statins in bivariate logistic regression.
In the multivariate analysis of this study, being hypertensive and using two or more hypoglycemic agents were significantly associated with use of aspirin.
Being hypertensive was three times more likely to use aspirin than those non-hypertensive patients. This result was in line with the study conducted in Spain [33], and China [31], in which hypertensive patients were two times and three times more likely to use aspirin respectively. The reason behind this could be because the presence of hypertension was a major risk factor for ASCVD [7], which prone the clinicians to use aspirin for PCVDP.
In this study, use of more than one glucose lowering agent was four times more likely to use aspirin than those who took single glucose lowering agent. The reason behind this might be the physician may think that, the more the patients’ blood sugar level is uncontrolled and are taking multiple blood glucose lowering agents, the more they tend to be at high risk for CVD, so they can prone to use aspirin.
This study have many strengths among this it uses the most validated WHO/ISH risk stratification Eastern Sub-Saharan Africa tool for stratification of CVD risk which has been adopted by doing a survey of countries of different continents, including Ethiopia and also considers different potential independent variables that have a correlation with the use of ALTs and eliminate confounding factors. While, the cross-sectional nature of study design precludes drawing clear conclusions about the relationship between use of ALT and related risk factors and there were limited studies conducted on the appropriateness of ALT as PCVDP which restricts the researcher to discuss by comparing with them were the potential limitation of this study.
Conclusion
The appropriate indication of lipid lowering agent was low. So, this population is at high potential risk for the development of stroke and other CVDs and predisposed to unwanted medication side effects of using irrelevant medication therapy. Also, aspirin was used for patients who were scored at under high risk using a WHO/ISH risk prediction chart which, implicates that this population is high risks for bleeding and Reye syndrome, despite the low benefit. Age between 65-69 years old, taking alcohol, being hypertensive and using more than one glucose lowering agents was significantly associated with use of statins. On the other hand, use of more than one hypoglycemic agent and being hypertensive were associated with the use of aspirin. These results emphasize the necessity of considering the CVD risk of the patients and apply an appropriate measure for the prevention of CVD in each follow-up of the patients.
Ethical Approval and Consent to Participate
An ethical clearance was obtained from the institutional ethical review board of the College of Medicine and Health Sciences, University of Gondar and was given to the Clinical Directorate of UoGCSH in order to get permission to conduct the study. Since the data was obtained from a semi-structured interviewer’s administrative questionnaire and patient's card, verbal informed consent obtained from participants were approved by the institutional ethical review board of the College of Medicine and Health Sciences, University of Gondar and verbal informed consent were also obtained from each participant including the illiterate ones and confidentiality of the information was assured from the participant (patient) after the objective of the study is made clear and permission for cooperation was asked politely by only giving a serial number for a questionnaire. All methods were carried out in accordance with relevant guidelines and regulations of declaration of Helsinki to fulfill the ethical principles for medical research involving human subjects.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
We declare that there is no competing interest.
Funding
The study was conducted for partial fulfillment of degree of masters in clinical pharmacy and study was fully funded by university of Gondar.
Author Contributions
TS conceived the original idea, drafted the proposal and involved in the data acquisition, analysis, interpretation and write up of the paper. Also, drafted the manuscript and prepared the final draft for publication. ST, AT and TT gave an intellectual advice, guidance, constructive comments, and contribution towards preparation, execution, and realization of this study.
Acknowledgments
We would like to thank the study participants and thank UoGCSH follow up clinical nurse staffs for their cooperativeness during the data collection.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62-S69.
[Cross Ref] [Google Scholar] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15-S33.
[Cross Ref] [Google Scholar] [PubMed]
- Global diffusion of eHealth: making universal health coverage achievable: report of the third global survey on eHealth. World Health Organization. 2017.
- Haffner SJ, Cassells H. Hyperglycemia as a cardiovascular risk factor. Am J Med. 2003;115(8):6-11.
[Cross Ref] [Google Scholar] [PubMed]
- Region WW. Ethiopia, Noncommunicable disease. 2014.
- HEARTS: Technical package for cardiovascular disease management in primary health care: Risk-based CVD management. World Health Organization. 2020.
- Culleton BF, Wilson PW. Cardiovascular disease: risk factors, secular trends, and therapeutic guidelines. J Am Soc Nephrol. 1998;9:S5-S15.
[Google Scholar] [PubMed]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696.
[Cross Ref] [Google Scholar] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. Lancet. 2002;360(9326):7-22.
[Cross Ref] [Google Scholar] [PubMed]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158.
[Cross Ref] [Google Scholar] [PubMed]
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163.
[Cross Ref] [Google Scholar] [PubMed]
- American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S125-S150.
[Cross Ref] [Google Scholar] [PubMed]
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-60.
[Cross Ref] [Google Scholar] [PubMed]
- Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J. Effects of aspirin for primary prevention in persons with diabetes mellitus: the ASCEND Study Collaborative Group. J Vasc Sur. 2019;69(1):305.
[Cross Ref] [Google Scholar] [PubMed]
- Abdelaziz HK, Saad M, Pothineni NV, Megaly M, Potluri R, Saleh M, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol. 2019;73(23):2915-2929.
[Cross Ref] [Google Scholar] [PubMed]
- Tolla MT, Norheim OF, Memirie ST, Abdisa SG, Ababulgu A, Jerene D, et al. Prevention and treatment of cardiovascular disease in Ethiopia: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2016;14:1-4.
[Cross Ref] [Google Scholar] [PubMed]
- Masresha N, Muche EA, Atnafu A, Abdela O. Evaluation of warfarin anticoagulation at university of Gondar comprehensive specialized hospital, North-West Ethiopia. J Blood Med. 2021:189-195.
[Cross Ref] [Google Scholar] [PubMed]
- Prevention of cardiovascular disease. Pocket guidelines for assessment and management of cardiovascular risk. Africa: WHO/ISH cardiovascular risk prediction charts for the African region. World Health Organization. 2007.
- Kebede zelalem B, Feyisa D. Determinants of statin initiation among adult diabetic patients in Bonga, Ethiopia. Diabetes Metab Syndr Obes. 2020:4839-4847.
[Cross Ref] [Google Scholar] [PubMed]
- Tamiru S, Alemseged F. Risk factors for cardiovascular diseases among diabetic patients in southwest Ethiopia. Ethiop J Health Sci. 2010;20(2).
[Cross Ref] [Google Scholar] [PubMed]
- Giday A, Wolde M, Yihdego D. Hypertension, obesity and central obesity in diabetics and non-diabetics in Southern Ethiopia. Ethiop J Health Dev. 2010;24(2).
- Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: a cross sectional study. Int J Endocrinol. 2018;2018.
[Cross Ref] [Google Scholar] [PubMed]
- Al-Shamsi S, Regmi D, Govender RD. Incidence of cardiovascular disease and its associated risk factors in at-risk men and women in the United Arab Emirates: a 9-year retrospective cohort study. BMC Cardiovasc Disord. 2019;19:1-9.
[Cross Ref] [Google Scholar] [PubMed]
- Steen DL, Khan I, Becker L, Foody JM, Gorcyca K, Sanchez RJ, et al. Patterns and predictors of lipid-lowering therapy in patients with atherosclerotic cardiovascular disease and/or diabetes mellitus in 2014: insights from a large US managed-care population. Clin Cardiol. 2017;40(3):155-162.
[Cross Ref] [Google Scholar] [PubMed]
- Berthold HK, Gouni-Berthold I, Bohm M, Krone W, Bestehorn KP. Patterns and predictors of statin prescription in patients with type 2 diabetes. Cardiovasc Diabetol. 2009;8:1-2.
[Cross Ref] [Google Scholar] [PubMed]
- Demoz GT, Wahdey S, Kasahun GG, Hagazy K, Kinfe DG, Tasew H, et al. Prescribing pattern of statins for primary prevention of cardiovascular diseases in patients with type 2 diabetes: insights from Ethiopia. BMC Research Notes. 2019;12(1):1-7.
[Cross Ref] [Google Scholar] [PubMed]
- Gupta R, Lodha S, Sharma KK, Sharma SK, Gupta S, Asirvatham AJ, et al. Evaluation of statin prescriptions in type 2 diabetes: India Heart Watch-2. BMJ Open Diabetes Res Care. 2016;4(1):e000275.
[Cross Ref] [Google Scholar] [PubMed]
- Elnaem MH, Nik Mohamed MH, Huri HZ, Azarisman SM. Patterns of statin therapy prescribing among hospitalized patients with Type 2 diabetes mellitus in two Malaysian tertiary hospitals. Trop J Pharm Res. 2017;16(12).
- Marcus ME, Manne-Goehler J, Theilmann M, Farzadfar F, Moghaddam SS, Keykhaei M, et al. Use of statins for the prevention of cardiovascular disease in 41 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data. Lancet Glob Health. 2022;10(3):e369-e379.
[Cross Ref] [Google Scholar] [PubMed]
- Frieden TR. Use of Selected Clinical Preventive Services Among Adults-United States, 2007–2010. MMWR Suppl. 2012;61(2):1-2.
[Cross Ref] [Google Scholar] [PubMed]
- Chen Y, Yin C, Li Q, Yu L, Zhu L, Hu D, et al. Misuse of aspirin and associated factors for the primary prevention of cardiovascular disease. Front Cardiovasc Med. 2021;8:720113.
[Cross Ref] [Google Scholar] [PubMed]
- Machado-Duque ME, Garcia DA, Emura-Velez MH, Gaviria-Mendoza A, Machado-Alba JE. Prevalence of the use of aspirin and statins for preventing cardiovascular events in the colombian population with type 2 diabetes mellitus: comparison of 2008 and 2018. J Prim Care Community Health. 2021;12:21501327211007015.
[Cross Ref] [Google Scholar] [PubMed]
- Rodriguez-Martin S, Garcia-Lledo A, Gil M, Barreira-Hernandez D, Rodriguez-Miguel A, de Abajo FJ. Prescription prevalence of low dose aspirin in primary prevention in the Spanish population, trends over time and associated factors. Med Clin. 2020;155(3):104-111.
[Cross Ref] [Google Scholar] [PubMed]
- Melaku T, Solomon Y, Chelkeba L. Statin utilization patterns among Type 2 diabetes mellitus patients with high cardiovascular disease risks in Ethiopia. J Pharm Care. 2018:44-51.
- Neutel CI, Morrison H, Campbell NR, de Groh M. Statin use in Canadians: trends, determinants and persistence. Can J Public Health. 2007;98:412-416.
[Cross Ref] [Google Scholar] [PubMed]
- Bideberi AT, Mutagaywa R. Statin prescription patterns and associated factors among patients with Type 2 diabetes mellitus attending diabetic clinic at Muhimbili National Hospital, Dar Es Salaam, Tanzania. Diabetes Metab Syndr Obes. 2022:633-646.
[Cross Ref] [Google Scholar] [PubMed]
Citation: Solomon T, Tadesse S, Tewabe A, Tsehay T (2023) Use of Antiplatelet and Lipid Lowering Agent Therapies as Primary Cardiovascular Disease Prevention Strategy and their Determinant Factors among Type 2 Diabetes Mellitus Patients in University of Gondar Comprehensive Specialized Hospital, Gondar: A Prospective Cross-Sectional Study. Diabetes Case Rep. 8:159.
Copyright: © 2023 Solomon T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
