Indexed In
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2021) Volume 18, Issue 2
Unraveling the Sperm Dark Matter: Investing in Female Health
Tomer Avidor-Reiss1,2* and Samantha B Schon32Department of Urology, College of Medicine and Life Sciences, University of Toledo, Toledo, USA
3Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, USA
Received: 18-Jan-2021 Published: 10-Feb-2021, DOI: 10.35248/2090-7214.21.18.375
Abstract
Male-factor infertility affects millions of couples worldwide. Due to a lack of alternatives, many couples ultimately resort to In Vitro fertilization (IVF) with Intracytoplasmic Sperm Injection (ICSI). This treatment puts most of the infertility burden on the female partner and requires an invasive surgical procedure. The availability of IVF/ICSI has inhibited progress into the understanding and treatment of male infertility and put the female at risk. To overcome this negative impact on the female, there is a need to reinvest in the study of sperm biology. As recent examples demonstrate, sperm have many unknown factors – the sperm dark matter-that can help resolve undetermined male infertility. Directing research to uncover the sperm dark matter will ultimately lead to novel treatments of male infertility focused on treating the underlying etiology and not merely focusing on the female partner.
Keywords
Sperm; Infertility;In vitro fertilization; Intra cytoplasmic; Sperm injection
Introduction
Paradoxically, when a male is diagnosed with infertility, it is his female partner that undergoes extensive medical treatments to conceive a child. For example, if a male is found to have immotile sperm, there is generally no treatment or cure for him to overcome his inability to reproduce. Without intervention, this male would be unable to father children [1]. Dr. Gianpiero Palermo developed a revolutionary treatment that allows many such infertile (naturally sterile) men to have children-Intra Cytoplasmic Sperm Injection (ICSI) [2]. Remarkably, rather than treating the sterile men, the ICSI procedure requires the female partner to undergo ovarian hyper stimulation, surgical oocyte retrieval, and ultimately embryo transfer into the uterus. As a result of ICSI’s successes in helping ~50% of the treated couples, research on male infertility has often been overlooked in the last 25 years, halting clinical progress [3-5]. Consequentially, we know very little about male infertility's underlying causes and how to treat them effectively [6]. This lack of progress resulted in minimal advances in treating male infertility, and thus the majority of the treatment burden remains on the female partner.
One of the main reasons women are treated for male infertility is that we do not know the underlying etiology of most sperm defects. While the semen analysis can identify visible sperm defects, it does not provide information on this abnormality's underlying cause. While there are genetic, hormonal, and structural causes of male infertility, in a significant portion of men, no cause can be identified – this is referred to as unexplained male infertility. In other cases, testing fails to find any abnormalities in either the male or female partner that can explain their infertility. This is referred to as unexplained couple infertility.
Approximately 1/3 of couples are diagnosed with unexplained infertility (Pandruvada 2021). Since “known” infertility is contributed evenly by males and females (1/3 of cases for each), a significant portion of unexplained couple infertility is likely due to undetermined male infertility. Indeed, the evidence mounts that unexplained couple infertility analyzed by advanced semen analysis can identify those with male infertility [7,8].
Developing more advanced semen analyses and, in particular, intracellular sperm analyses will help identify additional male factor infertility that is now categorized as unexplained. Therefore, there is a need for vigorous reinvestment in basic research of male reproduction. A critical step forward would be to identify the yet unknown essential components of sperm, a problem we refer to as the sperm dark matter-the sperm matter that accounts for undetermined male infertility. Revealing the nature of sperm dark matter will fuel a wave of translational research into diagnostic and therapeutics for male infertility that spare the female unjust treatment burden.
The Female Burden
In approximately 20% of infertility cases (1.2 million American couples), the male partner is infertile for an undetermined cause [6]. In these cases, the medical treatment burden for the male’s infertility falls on the female partner [5] (Figure 1).
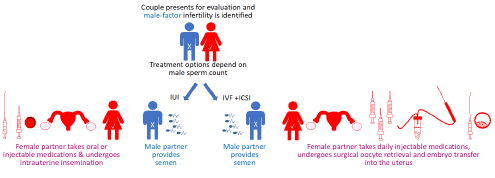
Figure 1: Clinical treatments for male infertility.
Depending on the male-infertility severity, treatments may include either Intra Uterine Insemination (IUI) or Assisted Reproductive Technology (ART) such as IVF/ICSI. Both these treatments involve invasive and time-consuming treatments to the female partner.
In IVF/ICSI, the female undergoes a multistep treatment known as controlled ovarian hyper stimulation followed by transvaginal oocyte retrieval. This process involves the use of daily injectable medications to induce ovarian hyper stimulation. The response of the ovaries is measured by frequent vaginal ultrasound and blood draws. After approximately two weeks of injectable medications and monitoring, the female patient must undergo a transvaginal oocyte retrieval procedure (egg collection). This is an invasive procedure that involves inserting a needle through the vaginal wall and into the ovarian follicle under ultrasound guidance.
Finally, following embryo development in vitro, she undergoes embryo transfer, which involves inserting a catheter loaded with embryo(s) into her uterine cavity through the cervix. National success rates for live birth per retrieval range from 3.2%-42.6%, with a much poorer prognosis in women above 37; thus, many couples may need to undergo multiple IVF/ICSI cycles to obtain a healthy baby [9]. IVF/ICSI generally has a negligible risk to the male (only an ejaculated sample is provided) unless a testicular sample is required. However, IVF/ICSI risks to the female include infection, bleeding, and Ovarian Hyperstimulation Syndrome (OHSS) [10]. Moderate to severe OHSS is estimated to occur in ~1%-5% of IVF/ICSI cycles and can include a spectrum of clinical findings such as ovarian enlargement, abdominal pain, ascites, pleural effusions, electrolyte imbalances, hemoconcentration, and formation of blood clots [11].
The Sperm Dark Matter
A significant reason for the lack of knowledge about the sperm and its content is that the sperm is a highly specialized cell of all the cell types in animals, the sperm is the most morphologically diverse [12]. The sperm has transformed Golgi, highly reduced cytoplasm, only residual ER, and no or inactive cytoplasmic ribosomes [13-16]. Even the chromosomes are unique. For example, in all our cells, DNA is packaged with the help of proteins called histones. In contrast, the sperm DNA is packaged to chromosomes with a sperm-specific protein, protamine’s [17]. These proteins condense the DNA at least six times more than in other cells during mitosis [18]. Because the sperm structure and function are so unique, the study of different cell types in our body does not sufficiently inform us about it. Therefore, there is a need for dedicated research of sperm basic biology, without which we would not know of the opportunities to treat male infertility.
An extreme example of the lack of research is the recent discovery of the sperm atypical centriole-a large structure attached to the sperm tail [19]. Centrioles are structurally and compositionally conserved microtubule-based organelle. They form the centrosome, the cell's major microtubule organization center, and cilium/flagellum, a subcellular compartment involved in signaling and motility. The precise way that centrioles are inherited during fertilization remained an unresolved question for humans and other mammals until 2018. A key reason for this lack of understanding is that many proteins known in the 20 century, like gamma-tubulin, are only transiently present in the sperm centriole due to a process known as Centrosome Reduction [20].
As a result, until recently, the dogma held that in human sperm structural degeneration and functional inactivation occur to one of the centrioles (the distal centriole), resulting in a vestigial remnant [21]. This dogma led to the belief that human spermatozoa only have one functional centriole (the proximal centriole). However, recent studies suggest that two centrioles are present, although the distal centriole has an extremely atypical structure and composition [22-24]. These findings led to an alternative model: Centriole Remodeling, which states that preexisting spermatid centrioles go through a structural and compositional transformation during differentiation resulting in an atypical centriole with novel functions [25]. In bovine zygotes, this atypical distal centriole participates in orchestrating the microtubule's cytoskeleton and is likely essential for embryonic development [26-28].
Conclusion
Considering the deficiency in investment in male reproductive research, it is very likely that many more significant missed essential cellular components are waiting in the sperm. See, for example, the new findings described in a recent paper applying cryo-electron microscopy to study the sperm and recent papers on sperm motility. Therefore, it is likely that an investment in sperm research will correspond with exciting new biology that most likely opens unexpected male infertility diagnostic and treatment opportunities.
REFERENCES
- Royfman R, Shah TA, Sindhwani P, Nadiminty N, Reiss TA. Sterility: An overlooked health condition. Women. 2020;1(1):29-45
- Palermo G, Joris H, Devroey P. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17-18.
- Group CCW. The current status and future of andrology: A consensus report from the cairo workshop group. Andrology. 2020;8(1):27-52.
- De Jonge C, Barratt CLR. The present crisis in male reproductive health: An urgent need for a political, social, and research roadmap. Andrology. 2019;7(6):762-768.
- Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, et al. Male infertility is a women's health issue-research and clinical evaluation of male infertility is needed. Cells. 2020;9(4):990.
- Pandruvada S, Royfman R, Shah TA, Sindhwani P, Dupree JM, Schon S, et al. Lack of trusted diagnostic tools for undetermined male infertility. J Assist Reprod Genet. 2021.
- Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology. 2013;1(3):357-360.
- Garanina AS, Alieva IB, Bragina EE, Blanchard E, Arbeille B, Guerif F, et al. The centriolar adjunct(-)appearance and disassembly in spermiogenesis and the potential impact on fertility. Cells. 2019;8(2):180.
- Prevention CDC. 2017 Assisted reproductive technology fertility clinic success rates report. Atlanta (GA). 2019.
- Klemetti R, Sevón T, Gissler M, Hemminki E. Complications of IVF and ovulation induction. Hum Reprod. 2005;20(12):3293-3300.
- Medicine PCAR. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil Steril. 2016;106(7):1634-1647.
- Pitnick S, Hosken DJ, Birkhead TR. Sperm morphological diversity. Sperm biology. Amsterdam. Netherlands. 2009; 69-149.
- Miller D, Ostermeier GC. Towards a better understanding of RNA carriage by ejaculate spermatozoa. Hum Reprod Update. 2006;12(6):757-767.
- Khawar MB, Gao H, Li W. Mechanism of acrosome biogenesis in mammals. Front Cell Dev Biol. 2019;7:195.
- Avidor-Reiss T, Zhang Z, Li XZ. Editorial: Sperm differentiation and spermatozoa function: Mechanisms, diagnostics, and treatment. Front Cell Dev Biol. 2020;8:219.
- Le Blévec E, Muronová J, Ray PF, Arnoult C. Paternal epigenetics: Mammalian sperm provide much more than DNA at fertilization. Mol Cell Endocrinol. 2020;518:110964.
- Balhorn R. The protamine family of sperm nuclear proteins. Genome Biol. 2007;8(9):1-8.
- Mascorro GF, Serrano H, Rosado A. Sperm chromatin. Arch Androl. 2000;45(3):215-225.
- Reiss TA, Carr A, Fishman EL. The sperm centrioles. Mol Cell Endocrinol. 2020; 518:110987.
- Schatten G. The centrosome and its mode of inheritance: The reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165(2):299-335.
- Manandhar G, Simerly C, Schatten G. Centrosome reduction during mammalian spermiogenesis. Curr Top Dev Biol. 2000;49:343-363.
- Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, et al. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018;9(1):2210.
- Khanal S, Leung MR, Gadêlha BH, Fishman EL, Saltzman B, Gadêlha HB, et al. A dynamic basal complex modulates mammalian sperm movement. 2021.
- Leung MR, Roelofs MC, Ravi RT, Maitan P, Zhang M, Henning H, et al. The multi-scale architecture of mammalian sperm flagella and implications for ciliary motility. bioRxiv. 2020
- Khanal S, Leung MR, Royfman A, Fishman EL, Saltzman B, Gadêlha HB, et al. A dynamic basal complex modulates mammalian sperm movement. Research Square. 2021.
- Schneider I, de Ruijter-Villani M, Hossain MJ, Stout TA, Ellenberg J. Non-rodent mammalian zygotes assemble dual spindles despite the presence of paternal centrosomes. bioRxiv. 2020.
- Cavazza T, Politi AZ, Aldag P, Baker C, Elder K, Blayney M, et al. Parental genome unification is highly erroneous in mammalian embryos. bioRxiv. 2020.
- Gadadhar S, Alvarez Viar G,Hansen JN, Gong A, Kostarev A, Radio CI, et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science. 2021;371(6525):eabd4914.
Citation: Reiss Avidor T , Schon SB (2021) Unraveling the Sperm Dark Matter: Investing in Female Health. Clinics Mother Child Health. 18:375
Copyright: ©(2021) Reiss Avidor T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

