Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2025) Volume 13, Issue 1
Two Natural Product Compositions Improve Lifespan and Healthspan in Caenorhabditis Elegans by Targeting the Hallmarks of Aging
Lawry Han and David Vollmer*Received: 03-Jan-2025, Manuscript No. JASC-25-28270; Editor assigned: 06-Jan-2025, Pre QC No. JASC-25-28270 (PQ); Reviewed: 13-Jan-2025, QC No. JASC-25-28270; Revised: 27-Jan-2025, Manuscript No. JASC-25-28270 (R); Published: 03-Feb-2025, DOI: 10.35248/2329-8847.25.13.395
Abstract
Aging has been increasingly recognized as a global health burden. Current aging research suggests that preventative strategies may increase both lifespan and healthspan. Many natural compounds or natural products have demonstrated promising antiaging benefits and gained popularity in aging research. The present study evaluated two compositions comprised of nicotinamide mononucleotide, quercetin, alpha ketoglutarate, white button mushroom extract (standardized to spermidine), Transfer Factor (TF) (extracts from cow colostrum and chicken egg yolk) with or without milk thistle fruit extract (standardized to silybin) and apigenin for their antiaging effect in Caenorhabditis elegans (i.e., C. elegans). The active ingredients included in the two compositions were screened for their senolytic effects on cell viability of irradiated human umbilical vein endothelial cells. To further explore their potential molecular mechanisms of action, the two compositions were also tested for their binding activity to human sirtuin enzymes and TF on natural killer cell activities in human peripheral blood mononuclear cells from both young and old donors. Both compositions significantly increased the lifespan and healthspan of C. elegans. Both compositions demonstrated significant dose-dependent inhibitory effect on several sirtuins. When treated with TF, both younger and older human peripheral blood mononuclear cells bolstered natural killer cell activity. The present study suggested that these two compositions significantly improved both lifespan and healthspan of C. elegans likely by modulating sirtuin activity and rejuvenating senescent immune cells.
Keywords
Lifespan; Healthspan; Transfer factor; Nicotinamide mononucleotide; Spermidine
Introduction
Aging is a major risk factor for nearly all major chronic diseases, including cardiovascular diseases, cancers, Alzheimer’s, Parkinson’s and other neurodegenerative diseases [1-4]. Age-related decline of immune function, also known as immunosenescence, has been increasingly recognized as an important cause of age- related morbidity and mortality, which may extend beyond its role in infectious diseases. Current aging research suggests that preventative strategies may increase both lifespan and healthspan. Potential restorative interventions reversing the many biological clocks back to the young productive healthy state can help prevent loss of function and possibly result in future performance gains. Many agents including natural compounds or natural products have demonstrated potential antiaging benefits and gained popularity in aging research [5,6].
Nicotinamide Mononucleotide (NMN) is a precursor to Nicotinamide Adenine Dinucleotide (NAD+), a coenzyme that plays an important role in various cellular processes, including energy metabolism and DNA repair. As individuals age, there is a decline in NAD+ levels, which is associated with decreased mitochondrial function, impaired energy production and compromised cellular repair mechanisms. NAD+ is important for mitochondrial function and maintaining optimal levels of this coenzyme may support the efficiency of cellular energy production. NAD+ is also a cofactor for sirtuins, a class of enzymes that play a role in regulating various cellular processes, including DNA repair, apoptosis and energy metabolism. Sirtuins play roles in the extension of lifespan and healthspan. The antiaging effects of NMN are primarily attributed to its ability to boost NAD+ levels within cells. Preclinical and clinical studies have demonstrated that administration of NMN can improve blood NAD+ levels and improve biomarkers of aging. NMN administration was also shown to increase Natural Killer (NK) cell cytotoxic activity [7-9].
Quercetin (from Styphnolobium japonicum) is a flavonoid found in various fruits, vegetables and plants. Recently it has been studied and considered a senolytic, that selectively clear senescent cells. Quercetin has also been reported to modulate the activity of immune cells, including T cells, B cells and NK cells. It may enhance the production of certain cytokines and support a stronger immune response, particularly in the context of immunosenescence [10,11]. Preclinical study showed that quercetin improved both healthspan and lifespan in mice [12]. Apigenin (from Apium gravolens) is another natural flavonoid that has been studied for its antiaging effect and is considered a senolytic [13]. Apigenin induces apoptosis and regulates Mitogen Activated Protein Kinases (MAPK) signaling pathways in mouse macrophage Antinuclear Antibody-1 (ANA-1) cells [14]. Apigenin has also been shown to protect against stress factors and promote lifespan of C. elegans [15].
Alpha Ketoglutarate (AKG) is a small molecule that is naturally present in the human body. It is used as a fuel by mitochondria, thereby improving cellular mitochondrial health. AKG levels decline about 10 fold between 40 and 80 years old [16]. It is not readily available in the diet, thus requiring supplementation and has been shown to extend lifespan and improve healthspan in various organisms, including humans [17].
White button mushroom (from Agaricus bisporus) has been investigated for its potential immunomodulatory, anti- inflammatory and antiaging effects. Spermidine, a naturally occurring polyamine in white button mushroom, has been shown to induce autophagy, a cellular process responsible for the degradation and recycling of damaged cellular components. Autophagy is important for maintaining cellular homeostasis and thus longevity and the mitigation of age-related diseases. The induction of autophagy by spermidine may also contribute to the removal of dysfunctional components within immune cells, promoting their overall functionality [18,19]. Spermidine was shown to improve lifespan in mice, via autophagy induction [20]. Another study further suggested that spermidine might also improve lifespan via protecting against telomere attrition [21]. Milk thistle (from Silybum marianum) fruit extract has been investigated for its potential anti-aging effects, primarily due to its antioxidant, anti-inflammatory, immunomodulatory and senolytic activities [22]. Further, one study performed in transgenic mice and human breast SKBR3 tumor cells suggest that silybin may act as a senolytic based on a senescent like growth arrest on the tumor cells [23].
TF, a combination of cow colostrum filtrates and chicken egg yolk extract, has been shown to modulate immune responses in a variety of human ex vivo and murine immune cells [24-27]. Most recently, it has been used for improving immune functions in humans [28].
Whilst there are many studies of individual natural product ingredients targeting the attributes of aging, there are few that explore this area with combinations of natural product ingredients. In this effort, there lies the potential to realize additive or synergistic benefits in different combinations of ingredients, particularly those that target different attributes of aging. In the present study, two compositions including NMN, quercetin, AKG, white button mushroom extract (standardized to spermidine) and TF with or without milk thistle fruit extract (standardized to silybin) or apigenin were evaluated for their antiaging effect in C. elegans, which is a standard model to assess improvements in lifespan and healthspan. The ingredient composition in the two formulas were designed to towards increasing lifespan and healthspan in C. elegans, taking into account the constraints of providing a convenient and cost- effective capsule delivery format designed for human consumption.
The active ingredients included in the two compositions were screened for their senolytic effects on cell viability irradiated Human Umbilical Vein Endothelial Cells (HUVEC), which represents one of the key attributes of aging. To further explore their potential molecular mechanisms of action, the two compositions were also tested for their binding activity to human sirtuin enzymes, which may play a role in several attributes of aging, including genomic stability, mitochondrial function and loss of proteostasis. TF’s effect on NK cells activities in human Peripheral Blood Mononuclear Cells (PBMCs) from both young and old donors was also evaluated as a surrogate for immune senescence.
Materials and Methods
Test compounds and formulations used in studies
All ingredients and formulas were supplied by 4Life Research. Colostrum filtrates were prepared by defatting whole bovine colostrum and ultra or nano-filtering to concentrate the peptides and proteins with molecular sizes smaller than 10,000 or 5000 Daltons, respectively. The colostrum filtrates were spray dried to a fine powder. Chicken egg yolk extract was prepared by separation of the yolk from the albumen and eggshell, followed by spray drying to a fine powder. TF is a proprietary combination of these colostrum filtrates with the egg yolk extract. NMN is a food grade bulk powder, greater than 98% pure.
Quercetin is extracted from Styphnolobium japonicum buds, standardized to greater than 95% purity. Silybin is extracted from Silybum marianum fruiting body, standardized to greater than 30% purity. Apigenin is extracted from Apium gravolens seeds, standardized to greater than 98% purity. Spermidine is extracted from Agaricus bisporusfruiting body, standardized to greater than 1% purity. Alpha ketoglutarate is a food-grade crystalline powder, greater than 99% pure. Other individual ingredients (e.g., green tea extract, fisetin extract) were also examined in the senescence assay but are not described in detail due to their lack of activity.
The formulas were comprised of various combinations of the above ingredients. Formula 1 is a combination of AKG (>45%), NMN (>25%), colostrum filtrates (>5%), quercetin extract (>2%), spermidine extract (>2%), egg yolk extract (>2%) and apigenin extract (>2%). Formula 2 is a combination of AKG (>75%), colostrum filtrates (>5%), quercetin extract (>2%), spermidine extract (>2%), egg yolk extract (>2%) and silybin extract (>2%).
Screening of natural products for selective cellular senescence activity
HUVECs (Lonza) were thawed from cryopreservation and seeded to T-182 cm2 flasks in growth medium: Vascular Cell Basal Medium (PCS-100-030) supplemented with an Endothelial Cell Growth Kit- VEGF (PCS-100-041). Cells were passaged when confluence reached 80% and were detached using accutase following manufactures guidelines. Incubation conditions were 37.0°C with 50% CO2.
Upon initiation of study, HUVEC were seeded to white, clear bottom 96 well plates at a seeding density of 12,600 cells/well in 100 μL of growth medium for downstream work in dosing with 4Life compounds. Cells were also seeded to black, clear bottom 96 well plates at a seeding density of 12,600 cells/well in 100 μL of growth medium for downstream quality control of senescent phenotype expression. All plates were then subsequently irradiated with a 10 Gy insult to induce cellular senescence. Plates were incubated for 72 h post-irradiation with a complete media change to 100 μL fresh growth medium 24 h post irradiation.
At 48 h post-irradiation of group 1, HUVEC maintained in culture were seeded to white, clear bottom 96 well plates at a seeding density of 6,300 cells/well to match the remaining cells that experienced irradiation from group 1 for downstream use in dosing. Cells were also seeded to black, clear bottom 96 well plates at a seeding density of 6,300 cells/well in 100 μL of growth medium for downstream quality control of senescent phenotype expression.
Study samples were reconstituted in DMSO to 10 mg/mL. White, clear bottom 96 well plates from Group 1 and Group 2 were removed from incubation and dosed in 11 point serial dilution with study samples in triplicate. Plates were imaged directly post- dose and returned to incubation for 72 h.
Upon completion of the 72 h incubation, plates were removed from incubation and imaged for representation of dosing effects. Plates were then subsequently analyzed for viability using CellTiterGlo Luminescent Cell Viability Assay (Promega G7570) following vendor protocols. Raw luminescent values were plotted against study samples concentrations to look for dose-response and Effective Concentration 50 (EC50) values plotted as compared to Navitoclax.
Cellular senescence was verified using HUVEC seeded to black, clear bottom 96 well plates from Group 1 and Group 2 were analyzed the same day as initial dosing for senescent phenotype expression using the following methods: Click-iT EdU Staining (Thermo C10338), SA-β-Gal (Cell Signaling 9860) following vendor recommended procedures [29].
Lifespan and healthspan effects of two formulas in C. elegans
The methods employed are necessarily the same as originally published. Wild type (N2) C. elegans were cultured on 60 mm petri dishes (Fisher Scientific; Austin, TX, USA) on a standard food source of E. coli OP50 and incubated for 48 h at 20°C. For age synchronization, a suspension of gravid adults in 20 mg/mL E. coli OP50 were loaded into microfluidic chips and allowed to lay eggs for 2 h [30]. These progenies were grown for 3 days and then loaded into microfluidic chips along with 20 mg/mL of E. coli OP50 in liquid Nematode Growth Medium (NGM). Two concentrations of Formula 1 (2.088 mg/mL and 4.176 mg/mL) and Formula 2 (2.492 mg/mL and 4.984 mg/mL) were formulated in liquid NGM and mixed with DMSO (Fisher Scientific). The lower concentrations represent the Human Equivalent Dose (1x HED) in C. elegans based on human consumption dosage of these formulas and the higher concentration represents 2x HED [31]. A positive control group with 100 μM Resveratrol and a vehicle control were also included. In all tested solutions, the final concentration of DMSO was maintained at 0.2% v/v and the food concentration was maintained at 20 mg/mL of E. coli OP50.
Each microfluidics assay was conducted in triplicate (three biological replicates) and each biological replicate consisted of 2 technical replicates. One technical replicate is a population of ~60 animals in a microfluidic growth chamber. For each lifespan assay, videos were acquired each day to determine live counts, prior to feeding fresh doxazosin solutions. L4 stage was counted as day 0 of adulthood. Videos were analyzed using the Infinity Code software (NemaLife Inc., TX) for animal survival and motility. The number of living animals in the population was determined based on detectable movement.
Mobility was determined based on the displacement of individual animals from the rectangular area (bounding box) that encloses their whole body. The inverse of the pixel correlation was used to indicate how far the worm has moved outside of the box within 30s. If the animal has entirely left the box, there is no pixel correlation between the two frames, an activity score of 1 was given and were labelled “highly active.” If the worm has not moved at all between the two frames, then the pixel correlation is identical, which gives an activity score of 0.
The percentage of highly active animals in the population was then calculated. Statistical comparisons were performed in GraphPad Prism using two-way Analysis of Variance (ANOVA). Kaplan Meier curves from the lifespan assays were generated using GraphPad Prism.
Sirtuin binding effect of two formulas
Experiments followed standard procedures of Eurofins Cerep (Celle l'Evescault, France). Briefly, the test compound, reference compound or water (control) are preincubated for 5 min at 22°C with enzyme in a buffer containing 45 mM Tris-HCl (pH 8.0), 123.3 mM NaCl, 2.43 mM KCl, 0.9 mM MgCl2 and 0.18% BSA. For stimulated control measurements, the mixture also contains 1 μM of activator resveratrol.
Thereafter, the reaction is initiated by adding 200 μM of the fluorogenic HDAC substrate and 500 μM of β-NAD co-substrate (for Sirtuin-2: 150 μM of the fluorolysine sirtuin 2 deacetylase substrate and 400 μM of β-NAD co-substrate; for Sirtuin-3: 20 μM of the fluorolysine sirtuin 2 deacetylase substrate and 500 μM of β-NAD co-substrate; for Sirtuin-6: 50 μM of the fluorogenic HDAC substrate and 150 μM of β-NAD co-substrate) and the fluorescence intensity is measured at λex=355 nm and λem=460 nm using a microplate reader (Envision, Perkin Elmer). This measurement at t=0 allows the detection of any compound interference with the fluorimetric detection method at these wavelengths. The mixture is then incubated for 20 min at 22°C.
After incubation, the reaction is stopped by adding one assay volume of buffer containing 1 cc HDAC developer (peptidase activity, BPS Bioscience). After 15 min (for Sirtuin 6: 60 min), the fluorescence intensity emitted by the reaction product fluorolysine is then measured at the same wavelengths (for Sirtuin-1, t=35; for Sirtuin-2, t=75; for Sirtuin-3, t=60; for Sirtuin-6: 60 min). The enzyme activity is determined by subtracting the signal measured at t=0 from that measured at the end. The results are expressed as a percent inhibition of the control activity. The standard inhibitory reference compound is tested in each experiment at several concentrations to obtain an inhibition curve from which its Inhibitory Concentration 50 (IC50) value (for activator, EC50) is calculated.
Three compounds (TF, Formula 1 and 2) were tested for IC50 or EC50 determination. Compound enzyme inhibition effect was calculated as a % inhibition of control enzyme activity. Results showing an inhibition or stimulation higher than 50% are considered to represent significant effects of the test compounds.
Activation of NK cell activity by TF in human PBMCs
The methods used are necessarily the same as described previously [32]. Briefly, immune activity of tested samples was evaluated based on ability of treated PBMC (Cellular Technology Limited) samples to kill K562 cells (ATCC), which are identified as health threats. Il-2 serves as the positive control in this assay. PBMC samples were taken from six younger subjects (3 females, 3 males) with an average age of 27 and five older subjects (2 females, 3 males) with an average age of 62.
The samples were treated for 48 h prior to analysis. Killing events are quantified by flow cytometry using DAPI (4′,6-diamidino-2- phenylindole) staining. Results were normalized using PBMC+K562 treatment alone. The treatment group was PBMC+K562+TF. Differences between groups were explored using ANOVA. Statistical significance was determined with the t-test method. Results were considered statistically different if p<0.05.
Results
Screening of natural products for selective cellular senescence activity
Cellular senescence was demonstrated in irradiated HUVEC as compared to non-irradiated controls by EdU staining and β-Galactosidase activity (Figure 1). Irradiated HUVEC showed lower viability compared to non-irradiated HUVEC as measured by luminescence signals via CellTiterGlo®. Navitoclax, as positive control, showed a half maximal EC50 that is 0.0865 μg/mL in non-irradiated HUVEC and an EC50 0.169 μg/mL in irradiated HUVEC (Table 1).
| Sample Name | EC50, non-irradiated HUVEC | EC50, irradiated HUVEC | Difference in EC50 |
Response Difference |
|---|---|---|---|---|
| Spermidine extract | 21.5 | 10.1 | 11.5 | High |
| Silybin extract | 39.7 | 18.4 | 21.3 | High |
| NMN | 4.6 | 3.1 | 1.6 | Medium |
| Quercetin extract | 3.6 | 3.1 | 0.5 | Medium |
| Apigenin extract | 5.0 | 5.0 | 0.0 | Low |
| Transfer Factor | ND | ND | ND | Null |
| AKG | ND | ND | ND | Null |
| Navitoclax (control) | 0.1 | 0.2 | - 0.1 | Low |
Table 1: EC50 (µg/mL) of tested compounds in non-irradiated and irradiated HUVEC cells. Differences between non-irradiated and irradiated cells for each ingredient were not significant.
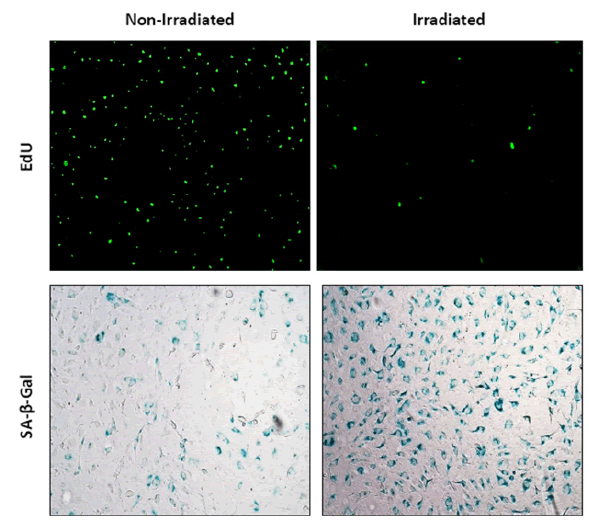
Figure 1: EdU staining and β-Galactosidase activity in non-irradiated and irradiated HUVEC cells. Greater cellular senescence is denoted by a decrease in green pixels in EdU and increased blue staining in β-Galactosidase.
The spermidine and silybin extracts are the only two compounds among all tested compounds that trended, though not statistically significant, towards differential effects in non-irradiated and irradiated HUVEC. NMN, quercetin and apigenin all showed a dose response, but little difference in EC50 values between non- irradiated and irradiated HUVEC. Transfer Factor and alpha ketoglutarate did not show an impact on cell viability, thus no dose-response or EC50 value in either non-irradiated or irradiated HUVEC.
Lifespan and healthspan effects of two formulas in C. elegans
The resveratrol group showed statistically higher survival than the control group on days 12, 18, 21, 23, 25 and 28. Like the resveratrol group, both doses of Formula 1 showed statistically higher survival than the control group. Worthy of note, the low dose of Formula 1 (1x HED) showed the highest survival, higher than the resveratrol group and high dose of Formula 1 group (2x HED). Specifically, the low dose of Formula 1 group showed statistically higher survival than resveratrol group and the high dose of Formula 1 group on days 25 and 28 (Figure 2).
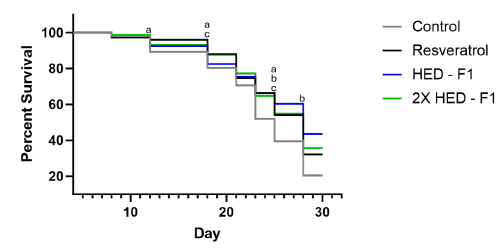
Figure 2: C. elegans survival probability from Day 4 to Day 28, Formula 1. Note: (a): Indicates significant statistical difference between resveratrol and control group (p<0.05); (b): Indicates significant statistical difference between 2.088 mg/mL Formula 1 and control group (p<0.05); (c): Indicates significant statistical difference between 4.176 mg/mL Formula 1 and control group (p<0.05).
Like the resveratrol group, both doses of Formula 2 showed statistically higher survival than the control group on days 12, 18, 21, 23, 25 and 28. Both doses of Formula 2 further showed statistically higher survival than the resveratrol group on days 21, 23 and 25. No statistical difference was found between the two doses of Formula 2. However, the high dose of Formula 2 showed higher survival than both the low dose of Formula 2 group and the resveratrol group on day 28 (Figure 3).
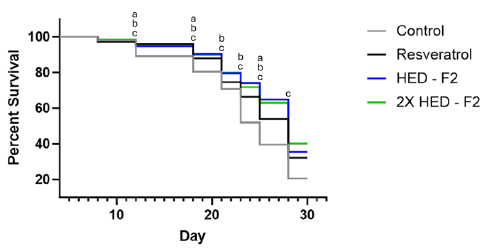
Figure 3: C. elegans survival probability from Day 4 to Day 28, Formula 2. Note: (a): Indicates significant statistical difference between resveratrol and control group (p<0.05); (b): Indicates significant statistical difference between 2.492 mg/mL Formula 1 and control group (p<0.05); (c): Indicates significant statistical difference between 4.984 mg/mL Formula 1 and control group (p<0.05).
The resveratrol group did not show significant improvement in healthspan (defined hereafter as activity) except on day 25, relative to the control group (Figure 4). The low dose of Formula 1 showed statistically higher activity than both the control group and the resveratrol group on days 18, 21, 23, 25 and 28. The high dose of Formula 1 showed statistically higher activity than the control group on days 18, 23, 25 and 28. The low dose of Formula 2 showed statistically higher activity than both the control group and the resveratrol group on days 18, 21, 23 and 25 and statistically higher activity than the control group on day 28. The high dose of Formula 2 showed statistically higher activity than the control group on days 21 and 25.
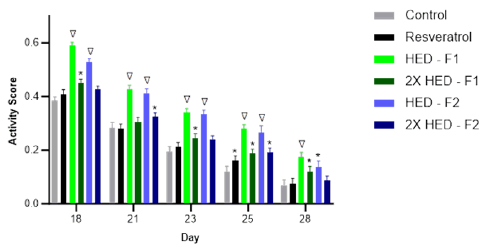
Figure 4: Healthspan (Activity Score) of C. elegans from Day 18 to Day 28. Note: * indicates significant statistical difference compared to control group (p<0.05); ∇ indicates significant statistical difference compared to both control group and resveratrol group (p<0.01).
Sirtuin binding effect of two formulas
Formula 1 showed significant dose-dependent inhibitory effect on both enzymes Sirtuin 3 (IC50=0.7 mg/mL) and Sirtuin 6 (IC50=0.7 mg/mL), but not much effect on enzymes Sirtuin 1 and Sirtuin 2. Formula 2 showed significant dose-dependent inhibitory effect on all four tested enzymes: Sirtuin 1 (IC50=0.7 mg/mL), Sirtuin 2 (IC50=0.6 mg/mL), Sirtuin 3 (IC50=0.8 mg/mL) and Sirtuin 6 (IC50=0.3 mg/mL) (Table 2).
| Assay | IC50 | |
|---|---|---|
| Formula 1 | Sirtuin 3 (h) (inhibitor effect) | 0.7 |
| Sirtuin 6 (h) (inhibitor effect) | 0.7 | |
| Formula 2 | Sirtuin 1 (h) (inhibitor effect) | 0.7 |
| Sirtuin 2 (h) (inhibitor effect) | 0.6 | |
| Sirtuin 3 (h) (inhibitor effect) | 0.8 | |
| Sirtuin 6 (h) (inhibitor effect) | 0.3 |
Table 2: Binding effect (mg/mL) of two formulas to sirtuin enzymes.
IC50 values of Formula 1 and Formula 2 (range from 0.3 mg/ mL to 0.8 mg/mL) are significantly higher than that of Reference Compounds (ADP Ribose for Sirtuin 6, suramin for Sirtuin 1 and 2, niacinamide for Sirtuin 3) for the respective enzymes (range from 0.005 mg/ml to 0.04 mg/mL).
Neither Formula 1 nor Formula 2 showed any activating effect on Sirtuin 1 enzyme. Another tested compound, TF, did not show any inhibitory or activating effect on any of the tested enzymes (data not shown).
Activation of NK cell activity by TF in human PBMCs
When treated with TF, both younger and older PBMC samples bolstered NK cell activity to a statistically similar level, as demonstrated by percent killing of K562 cancer cells (Figure 5). IL- 2, the control for this assay, also showed positive activity.
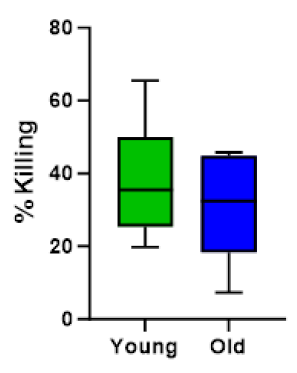
Figure 5: Percentages of K562 killing in PBMC from both young donors and old donors, treated with test compound TF (a combination of colostrum filtrate and egg yolk extract). Young donors, N=6, age<35 with an average age of 27. Old donors, N=5, age>55 with an average age of 62.
Discussion
Screening of natural products for selective cellular senescence activity
Among all tested active ingredients, silybin and spermidine extracts are two compounds that showed similar effect in non- irradiated and irradiated HUVEC to navitoclax, the positive control, suggesting they possess potential to selectively target senescent cells. Comparable results were observed in a senescent human dermal fibroblast model utilizing a Silybum marianumflower extract, including selective targeting of senescent cells [33]. Several studies have also shown spermidine to have senolytic activity [34,35], though most studies utilize wheat-germ extracts [36]. To the best of our knowledge, this is the first standardized extract of spermidine derived from mushroom, which are rich in polyamines to demonstrate selectivity and activity towards cellular senescence [37].
NMN and quercetin and apigenin extracts all showed certain dose- response effect in non-irradiated and irradiated HUVEC, but no difference in EC50 values between the two conditions, suggesting a lack of selectivity in targeting senescent cells. These compound results are partly consistent with other studies demonstrating the senolytic activity in this same cell line or combinations with other senolytic compounds though these studies did not necessarily evaluate selectivity towards non-irradiated and irradiated cell lines and they utilized different means of inducing senescence (e.g., peroxide, doxorubicin) [38-40]. Another quercetin study showed similar lack of selectivity in HUVEC cells and suggested that human coronary artery endothelial cells may be more relevant cell line for this type of work [41].
Colostrum filtrates, egg yolk extract and alpha ketoglutarate did not show any impact on cell viability in non-irradiated HUVEC or irradiated HUVEC. Interestingly, this lack of response could suggest a potential use for protecting HUVEC cells against DNA damage caused by irradiation and has been observed with other natural products [42].
Lifespan and healthspan effects of two formulas in C. elegans
Consistent with previous studies the resveratrol control group showed statistically improved lifespan over the course of the study when compared to the vehicle control group [43]. Both Formulas 1 and 2 showed a significantly greater lifespan when compared to either control groups, demonstrating the strong potential for lifespan improvements. Not surprisingly, comparable results have been observed with some of the individual ingredients that make up these formulas or compounds that utilize the same pathway and was, in part, the basis for selection of these formula ingredients [44,45].
This C. elegans model also uses a novel AI-driven technology to monitor in real-time the activity (e.g., locomotion, behavior, etc.) of the nematodes over the course of the study [46]. While activity can be measured across multiple days that represent early, mid and late- life phases of the worm’s lifespan, in this study, only late-life activity measurements (i.e., days 18, 21, 23, 25 and 28) were recorded and evaluated. The activity results demonstrate an improved healthspan in the late-life phase for both formulas compared to either control group. These overall improvements in healthspan with both formulas are noteworthy considering that other studies have not necessarily seen a correlation between improved lifespan and improved healthspan, though it should be noted these models compared wild-type and long-lived mutants rather than different treatment strategies of wild type worms [47,48].
Sirtuin binding effect of two formulas
To explore the mechanisms involved in the improved lifespan and healthspan of the C. elegans model, the activity of the two formulas was tested in several sirtuin binding as says. C. elegans have four sirtuins one of which has shared homology with humans and are thought to be intimately involved in lifespan improvements [49-51]. Formula 2 shows moderate activity for all sirtuins tested, whereas Formula 1 only shows moderate activity for SIRT3 and SIRT6. TF was also tested and showed no sirtuin activity, likely because it has been observed previously to have little direct activity in different binding assays [52].
The lesser activity of Formula 1 is interesting since it contains an NAD precursor (i.e., NMN) that is important in sirtuin activity and resulting lifespan improvements [53]. The omission of NMN in Formula 2 was compensated for by additional AKG, which has reportedly been shown to coordinate NAD-SIRT1 signaling and results in improved lifespan and healthspan on C. elegans [54]. Further, Formula 2 includes more potent senolytic ingredients, according to the senescence screening assay and can play a role in increased sirtuin activity [55]. Together, these differences in sirtuin activity between the two formulas might partially explain their subtle variances in lifespan and healthspan improvements observed in C. elegans model.
Activation of NK cell activity by TF in human PBMCs
Though TF did not show any sirtuin activity, it has been repeatedly demonstrated for its immunomodulatory properties, including boosting natural killer cell activity [25,56]. The impact of other immunostimulants on natural killer cells in the C. elegans model demonstrates its therapeutic potential and may be particularly relevant given the impact of NK cells on the aging human immune system.
One of the more striking human studies evaluated NK cytotoxicity of centenarians and showed their immune system was more closely related to younger subjects compared to middle-aged subjects. The fact that TF treatment significantly increased NK cell activity in PBMC samples from both younger and older donors suggests that it has the potential to rejuvenate immunosenescent cells into a more youthful and active state. Further investigations are warranted to determine if TF directly translates into improved lifespan and healthspan of C. elegans.
Conclusion
This study highlights the potential of natural compounds and formulations in targeting cellular senescence, improving lifespan and enhancing healthspan. Specifically, spermidine and silybin extracts demonstrated selective senolytic activity in irradiated HUVEC cells, suggesting their potential as therapeutic agents for senescence related conditions. Both Formula 1 and Formula 2 significantly improved lifespan and healthspan in the C. elegans model with Formula 1 showing strong effects at lower doses and Formula 2 demonstrating strong sirtuin activity. Moreover, the immunomodulatory effects of TF on NK cell activity in human PBMCs, especially in rejuvenating immune function in older individuals, further support its therapeutic potential. These findings provide the possibility for further studies targeting the hallmarks of aging and their relevance in aging biology.
There are several limitations to these studies that are worth noting. Foremost is the lack of lifespan and healthspan as well as sirtuin activity data on the individual ingredients contained in the two formulas, which could then lend better insights into additive or synergistic benefits when combining the ingredients. The selection of the formula composition was primarily driven by a combination of existing literature for C. elegans and sirtuin activity, as well as the senolytic activity of the screened ingredients. Further, some of the ingredients chosen in the formulas were trending toward selective senolytic activity but did not reach statistically significant differences in the non-irradiated and irradiated HUVEC cells, which may suggest uncertainty in their selectivity as senolytic agents. Finally, while it is understood there are homologous genes between C. elegans and humans, there is little evidence that improvements in lifespan or healthspan are analogous across those species.
Authors Contributions
Conceptualization, David Vollmer; methodology, David Vollmer; writing original draft preparation, Lawry Han; writing review and editing, David Vollmer and Lawry Han; supervision, David Vollmer; funding acquisition, David Vollmer.
Funding
This research was funded by 4Life Research, LLC.
Institutional Review Board Statement
The Caenorhabditis Elegans study protocol did not require Institutional Animal Care and Use Committee approval as they are considered lower-level in-vertebrates.
Data Availability Statement
The data presented in this study are available on request from the corresponding author upon reasonable request.
Acknowledgement
The authors would like to acknowledge Ichor Life Sciences for the cellular senescence assay, Eurofins Cereps for the sirtuin binding assay and Nemalife Inc. for the C. elegans study.
Conflicts of Interest
The authors are employees of 4Life Research.
References
- Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391.
[Crossref] [Google Scholar] [PubMed]
- Junaid M, Lee A, Kim J, Park TJ, Lim SB. Transcriptional heterogeneity of cellular senescence in cancer. Mol Cells. 2022;45(9):610-619.
[Crossref] [Google Scholar] [PubMed]
- Hwang ES, Song SB. Impaired autophagic flux in glucose-deprived cells: An outcome of lysosomal acidification failure exacerbated by mitophagy dysfunction. Mol Cells. 2023;46(11):655-663.
[Crossref] [Google Scholar] [PubMed]
- Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634-650.
[Crossref] [Google Scholar] [PubMed]
- Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, et al. Aging of the immune system: Focus on natural killer cells phenotype and functions. Cells. 2022;11(16):1017.
[Crossref] [Google Scholar] [PubMed]
- Bakula D, Ablasser A, Aguzzi A, Antebi A, Barzilai N, Bittner MI, et al. Latest advances in aging research and drug discovery. Aging (Albany NY). 2019;11(22):9971-9981.
[Crossref] [Google Scholar] [PubMed]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795-806.
[Crossref] [Google Scholar] [PubMed]
- Yi L, Maier AB, Tao R, Lin Z, Vaidya A, Pendse S, et al. The efficacy and safety of β-Nicotinamide Mononucleotide (NMN) supplementation in healthy middle-aged adults: A randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. GeroScience. 2023;45(1):29-43.
[Crossref] [Google Scholar] [PubMed]
- Fukamizu Y, Uchida Y, Shigekawa A, Sato T, Kosaka H, Sakurai T. Safety evaluation of β-nicotinamide mononucleotide oral administration in healthy adult men and women. Sci Rep. 2022;12(1):14442.
[Crossref] [Google Scholar] [PubMed]
- Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45(10):763-71.
[Crossref] [Google Scholar] [PubMed]
- Kirkland JL, Tchkonia T. Senolytic drugs: From discovery to translation. J Intern Med. 2020;288(5):518-536.
[Crossref] [Google Scholar] [PubMed]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246-1256.
[Crossref] [Google Scholar] [PubMed]
- Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. 2019;20(6):1305.
[Crossref] [Google Scholar] [PubMed]
- Liao Y, Shen W, Kong G, Lv H, Tao W, Bo P. Apigenin induces the apoptosis and regulates MAPK signaling pathways in mouse macrophage ANA-1 cells. PLoS One. 2014;9(3):e92007.
[Crossref] [Google Scholar] [PubMed]
- Elkhedir AE, Iqbal A, Zogona D, Mohammed HH, Murtaza A, Xu X. Apigenin glycosides from green pepper enhance longevity and stress resistance in Caenorhabditis elegans. Nutr Res. 2022;102:23-34.
[Crossref] [Google Scholar] [PubMed]
- Harrison AP, Pierzynowski SG. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art–review article. J Physiol Pharmacol. 2008;59(S1):91–106.
[Google Scholar] [PubMed]
- Demidenko O, Barardo D, Budovskii V, Finnemore R, Palmer FR, Kennedy BK, et al. Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 year reduction in biological aging, after an average of 7 months of use, in the TruAge DNA methylation test. Aging (Albany NY). 2021; 13(22):24485-24499.
[Crossref] [Google Scholar] [PubMed]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305-14.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Alsaleh G, Feltham J, Sun Y, Napolitano G, Riffelmacher T, et al. Polyamines control eIF5A Hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol Cell. 2019;76(1):110-125.e9.
[Crossref] [Google Scholar] [PubMed]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428-1438.
[Crossref] [Google Scholar] [PubMed]
- Wirth A, Wolf B, Huang CK, Glage S, Hofer SJ, Bankstahl M, et al. Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. Geroscience. 2021;43(2):673-690.
[Crossref] [Google Scholar] [PubMed]
- Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules. 2017;22(2):191.
[Crossref] [Google Scholar] [PubMed]
- Provinciali M, Papalini F, Orlando F, Pierpaoli S, Donnini A, Morazzoni P, et al. Effect of the silybin-phosphatidylcholine complex (IdB 1016) on the development of mammary tumors in HER-2/neu transgenic mice. Cancer Res. 2007;67(5):2022-2029.
[Crossref] [Google Scholar] [PubMed]
- Han X, Vollmer D, Enioutina EY. Immunomodulatory effects of modified bovine colostrum, whey, and their combination with other natural products: Effects on human peripheral blood mononuclear cells. Curr Ther Res Clin Exp. 2023;99:100720.
[Crossref] [Google Scholar] [PubMed]
- Vetvicka V, Vetvickova J. Effects of transfer factor supplementation on immune reactions in mice. J Nutr Health Sci. 2019;6(3):301.
- Vetvicka V, Fernandez-Botran R. Non-specific immunostimulatory effects of transfer factor. Int Clin Pathol J. 2020; 8(1):1-6.
- Vetvicka V, Vetvickova J. Antigen-specific immunomodulatory effects of transfer factor. Austin J Clin Pathol. 2020;7(1):1062.
- Yu L, Iloba I, Cruickshank D, Jensen GS. Feasibility trial exploring immune-related biomarkers pertaining to rapid immune surveillance and cytokine changes after consuming a nutraceutical supplement containing colostrum- and egg-based low-molecular-weight peptides. Curr Issues Mol Biol. 2024;46(7):6710-6724.
[Crossref] [Google Scholar] [PubMed]
- Aging and aging-related diseases: From molecular mechanisms to interventions and treatments
- Rahman M, Edwards H, Birze N, Gabrilska R, Rumbaugh KP, Blawzdziewicz J, et al. NemaLife chip: A micropillar-based microfluidic culture device optimized for aging studies in crawling C. elegans. Sci Rep. 2020;10(1):16190.
[Crossref] [Google Scholar] [PubMed]
- Andersen A, Vieira-Brock PL, Vaughan B, Vollmer D. Method development for the analysis of PBMC-mediated killing of K562 cells by bovine colostrum. J Immunol Methods. 2021;499:113175.
[Crossref] [Google Scholar] [PubMed]
- Woo J, Shin S, Cho E, Ryu D, Garandeau D, Chajra H, et al. Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PLoS One. 2021;16(12):e0260545.
[Crossref] [Google Scholar] [PubMed]
- Ni YQ, Liu YS. New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging Dis. 2021;12(8):1948-1963.
[Crossref] [Google Scholar] [PubMed]
- Ueno D, Ikeda K, Yamazaki E, Katayama A, Urata R, Matoba S. Spermidine improves angiogenic capacity of senescent endothelial cells and enhances ischemia-induced neovascularization in aged mice. 2023;13(1):8338.
[Crossref] [Google Scholar] [PubMed]
- Mohajeri M, Ayatollahi SA, Kobarfard F, Goli M, Khandan M, Mokhtari S, et al. Wheat germ, a byproduct of the wheat milling industry, as a beneficial source of anti-aging polyamines: A quantitative comparison of various forms. Food Sci Nutr. 2023;11(11):7242-7254.
[Crossref] [Google Scholar] [PubMed]
- Muñoz-Esparza NC, Costa-Catala J, Comas-Basté O, Toro-Funes N, Latorre-Moratalla ML, Veciana-Nogués MT, et al. Occurrence of polyamines in foods and the influence of cooking processes. Foods. 2021;10(8):1752.
[Crossref] [Google Scholar] [PubMed]
- Zhu N, Liu X, Xu M, Li Y. Dietary nucleotides retard oxidative stress-induced senescence of human umbilical vein endothelial cells. Nutrients. 2021;13:3279.
[Crossref] [Google Scholar] [PubMed]
- Hwang HV, Tran DT, Rebuffatti MN, Li CS, Knowlton AA. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS One. 2018;13(1):e0190374.
[Crossref] [Google Scholar] [PubMed]
- Fan T, Du Y, Zhang M, Zhu AR, Zhang J. Senolytics cocktail Dasatinib and quercetin alleviate human umbilical vein endothelial cell senescence via the TRAF6-MAPK-NF-κB Axis in a YTHDF2-dependent manner. Gerontology. 2022;68(8):920-934.
[Crossref] [Google Scholar] [PubMed]
- Hwang HV, Tran DT, Rebuffatti MN, Li CS, Knowlton AA. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS One. 2018;13(1):e0190374.
[Crossref] [Google Scholar] [PubMed]
- Banse SA, Lucanic M, Sedore CA, Coleman-Hulbert AL, Plummer WT, Chen E, Kish JL, et al. Automated lifespan determination across Caenorhabditis strains and species reveals assay-specific effects of chemical interventions. Geroscience. 2019;41(6):945-960.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Bahramimehr F, Shahhamzehei N, Fu H, Lin S, Wang H, et al. Anti-aging effects of medicinal plants and their rapid screening using the nematode Caenorhabditis elegans. Phytomedicine. 2024;129:155665.
[Crossref] [Google Scholar] [PubMed]
- Peters JD, Peters MP, Bradshaw PC. Nicotinamide riboside functions during development while beta-hydroxybutyrate functions during adulthood to extend C. elegans lifespan. MicroPubl Biol. 2023.
[Crossref] [Google Scholar] [PubMed]
- McIntyre RL, Rahman M, Vanapalli SA, Houtkooper RH, Janssens GE. Biological age prediction from wearable device movement data identifies nutritional and pharmacological interventions for healthy aging. Front Aging. 2021;2:708680.
[Crossref] [Google Scholar] [PubMed]
- Statzer C, Reichert P, Dual J, Ewald CY. Longevity interventions temporally scale healthspan in Caenorhabditis elegans. iScience. 2022;25(3):103983.
[Crossref] [Google Scholar] [PubMed]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112(3):E277-86.
[Crossref] [Google Scholar] [PubMed]
- Viswanathan M, Tissenbaum HA. C. elegans sirtuins. Methods Mol Biol. 2013;1077:39-56.
[Crossref] [Google Scholar] [PubMed]
- Bitto A, Wang AM, Bennett CF, Kaeberlein M. Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb Perspect Med. 2015;5(11):a025114.
[Crossref] [Google Scholar] [PubMed]
- Imai S, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017.
[Crossref] [Google Scholar] [PubMed]
- Wu N, Ma YC, Gong XQ, Zhao PJ, Jia YJ, Zhao Q, et al. The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans. Nat Commun. 2023;14(1):240.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Lee JH, Lee HY, Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52(1):24-34.
[Crossref] [Google Scholar] [PubMed]
- Han X, Vollmer D, Yan X, Zhang Y, Zang M, Zhang C, et al. Immunomodulatory effects of modified colostrum, whey, and their combination with other natural products: Effects on natural killer cells. Curr Ther Res Clin Exp. 2024;101:100750.
[Crossref] [Google Scholar] [PubMed]
- Narayanan S, Baburajan AP, Muhammad M, Joseph A, Vemula PK, Bhat SG. Demonstrating the immunostimulatory and cytokine-augmentation effects of bacterial ghosts on natural killer cells and Caenorhabditis Elegans. Biotechnol Bioeng. 2024;121(3):959-970.
[Crossref] [Google Scholar] [PubMed]
- Chelyapov N, Nguyen TT, Gonzalez R. Autologous NK cells propagated and activated ex vivo decrease senescence markers in human PBMCs. Biochem Biophys Rep. 2022;32:101380.
[Crossref] [Google Scholar] [PubMed]
- Gounder SS, Abdullah BJJ, Radzuanb NEIBM, Zain FDBM, Sait NBM, Chua C, et al. Effect of aging on NK cell population and their proliferation at ex vivo culture condition. Anal Cell Pathol (Amst). 2018;2018:7871814.
[Crossref] [Google Scholar] [PubMed]
- Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82(9):2767-2773.
[Google Scholar] [PubMed]
Citation: Han L, Vollmer D (2025). Two Natural Product Compositions Improve Lifespan and Healthspan in Caenorhabditis elegans by Targeting the Hallmarks of Aging. J Aging Sci. 13:395.
Copyright: © 2025 Han L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

