Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 4
Trehalose Liposomes Inhibit the Growth of Glioblastoma Cell In vitro and In vivo
Keiji Kuwabara, Hideaki Ichihara and Yoko Matsumoto*Received: 24-May-2021 Published: 14-Jun-2021, DOI: 10.35248/2157-2518.21.12.364
Abstract
The inhibitory effects of trehalose liposomes (TL) composed of α-D-glycopyranosyl-α-D-glucopyranoside monomyristate and L-α-dimyristoylphosphatidylcholine on the growth of human glioblastoma (U-87MG) cells were evaluated. Induction of apoptosis was observed after fusion and accumulation of TL in U-87MG cell membranes. Increased membrane fluidity of U-87MG cells treated with TL was observed. TL caused apoptosis in U-87MG cells through the activation of mitochondria and apoptosis-inducing factor via a caspase-independent pathway. Tumor weights markedly decreased in orthotopic graft mouse models of glioblastoma (U-87MG) after intravenous administration of TL compared with those in the control group.
Keywords
Trehalose liposome; Nanomedicine; Therapeutic effects; Glioblastoma
Abbrevations
DMPC: L-α-dimyristoylphosphatidylcholine; TL: Trehalose Liposomes composed of DMPC and TreC14; TreC14: α-D- glycopyranosyl-α-D-glucopyranoside monomyristate
Introduction
Glioblastoma is the most malignant brain tumor. The standard of care is a combination of radiotherapy and chemotherapy with temozolomide after surgical resection [1]. Treatment with bevacizumab during radiotherapy does not improve survival in patients with glioblastoma [2,3].
Trehalose Liposomes (TL) composed of L-α-Dimyristoylphosphatidylcholine (DMPC) and trehalose micelles have been produced using a sonication method [4,5]. TL are effective in inhibiting the growth of tumor cells without including antitumor agents in vitro and in vivo [6-8]. Induction of apoptosis has been observed in colon and gastric cancer, hepatocarcinoma, leukemia, lung carcinoma, and breast cancer after treatment with TL [4-8]. The anti-invasive effects of TL on the proliferation of lung carcinomas have been reported [9]. However, the inhibitory effects of TL on the growth of glioblastoma cells have not yet been examined. In this study, we investigated the inhibitory effects of TL on the growth of glioblastoma (U-87MG) cells in vitro and in vivo.
Materials and Methods
Preparation of TL
TL were prepared using a VS-N300 sonicator (VELVO, Tokyo, Japan) after 30 mol% DMPC (purity>99%; NOF Co. Ltd., Tokyo, Japan) and 70 mol % trehalose C14 (Dojindo Ltd., Japan) were mixed in 5% glucose solution at 45°C and 300 W, and filtered through a 0.20 μm filter.
Dynamic light scattering measurements
The diameter (dhy) of the TL was measured using an ELS-8000 light scattering spectrometer (Otsuka Electronics, Osaka, Japan) equipped with an He-Ne laser (633 nm) at a scattering angle of 90°. The dhy was calculated using the Stokes-Einstein formula (Equation 1), where κ is the Boltzmann constant, T is the absolute temperature, η is the viscosity, and D is the diffusion coefficient:
dhy=κT/3πηD (Equation 1)
Cell culture
The U-87MG human glioblastoma cell line purchased from ATCC (Manassas, VA, USA) were cultured in MEM (Gibco, Thermo Fisher Scientific, Inc. Waltham, MA, USA) containing 10% fetal bovine serum (HyClone Laboratories Inc., Logan, UT, USA), penicillin (100 unit/ml), and streptomycin (50 μg/ml). The cells were kept in a humidified incubator at 37°C in an atmosphere of 5% CO2.
Assessment of half-maximal inhibitory concentration (IC50) of TL in vitro
The IC50 of TL for U-87MG cells was determined using a WST-8[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4 disulfophenyl)- 2H tetrazolium, monosodium salt] assay (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan) [10]. U-87MG cells (0~5.0 × 103 cells/ml) were seeded in 96-well plates and cultured in a 5% CO2 humidified incubator at 37°C for 24 h. Cells were cultured for a further 48 h after the addition of DMPC (0-2.0 × 10-3 M) and TL (0-6.0 × 10-4 M).
The WST-8 solution was then added and incubated for 3 h. The absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific). The inhibitory activity of TL on the growth of U-87MG cells was evaluated by calculating Amean/ Acontrol, where Amean and Acontrol denote the absorbance of water-soluble formazan in the presence and absence of TL, respectively.
Analysis of induction of apoptosis by TL
Cells were seeded at a density of 5.0 × 103 cells/100 ml in a culture dish and incubated in a humidified atmosphere of 5% CO2 at 37°C for 24 h. TL (0.1 5.0 × 10-4 M) was added to each dish and the dishes were incubated. Cells were centrifuged and suspended in PBS (-) containing 1 mg/ml RNase, 0.1% Triton X-100, and 40 μg/ml propidium iodide (Molecular Probes, Eugene, OR, USA) in a dark room. The percentage of apoptotic cells was analyzed using an Epics XL system II flow cytometer (Beckman Coulter, Brea, CA, USA).
Measurement of caspase activity
U-87MG cells (5.0 × 104 cells/ml) were treated with TL. After 24 h of incubation, each substrate solution (PhiPhiLux-GID2, CaspaLux 8-LID2, CaspaLux 9-MID2 for caspase-3, caspase-8, and caspase-9, respectively; OncoImmunin Inc., Gaithersburg, MD, USA) was added to the U-87MG cell suspension [11]. The cells were incubated for 45 min at 37°C, washed twice with 1 ml of ice- cold PBS (-), and resuspended in 1 ml fresh PBS (-). Caspase activity was analyzed by flow cytometry.
Mitochondrial membrane potential
3, 3-Dihexyloxacarbocyanine iodide (DiOC6 (3); Molecular Probes, Eugene, OR, USA) was added to U-87MG cells (5.0 × 104 cells/ml), previously treated with TL (2.5 × 10-4 M), to evaluate mitochondrial transmembrane potential (Δ ψ m). Stained U-87MG cells were examined by flow cytometry using a 15 mW 488 nm air-cooled Ar laser and an FL1 sensor (505-545nm).
Assessment of Apoptosis Inducing Factor (AIF by flow cytometry
U-87MG cells (5.0 × 104 cells/ml) were seeded in dishes and incubated for 24 h. The cells were then treated with TL (2.5 × 10-4 M) during another 24 h incubation. The cells were then fixed with 10% formaldehyde for 30 min in wells, followed by incubation with anti-AIF Alexa Fluor® 488-conjugated antibody (5 μg/ml; Abcam plc, Cambridge, UK) in a shaded box at 4°C for 1 h. The stained U-87MG cells were analyzed by flow cytometry.
Fusion and accumulation of TL in cell membrane
TL fusion and accumulation in the cell membrane was assessed using confocal laser microscopy (TCS-SP; Leica Microsystems, Berlin, Germany) and a fluorescent probe ((1-palmitoyl-2-[12-[(7- nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-glycero-3- phosphocholine (NBDPC; Avanti Polar Lipids, Birmingham, ALUSA)]. U-87MG cells (5.0 × 104 cells/ml) were incubated in a humidified incubator at 37°C and 5% CO2 for 24 h. The cells were then treated with TL/NBDPC ([DMTre]=9.6 × 10-5 M, [NBDPC]= 3.9 × 10-6 M~4.0 × 10-6 M) for 10-60 min and examined by flow cytometry using a 15 mW 488 nm air-cooled Ar laser and FL1 sensor (505-545 nm).
Measurement of cellular membrane fluidity
The membrane fluidity of U-87MG cells was measured by spectrophotometry (F-7100; HITACHI, Tokyo, Japan) based on a previously described fluorescence depolarization method [12]. The cells were labeled with 1 6-diphenyl-1 3 5-hexatriene (DPH; Nacalai Tesque, Tokyo, Japan). The fluorescence depolarization (P) value of DPH in cells treated with TL (5.0 × 10-5 M) was measured.
Assessment of therapeutic effects of TL in vivo
All mice were handled in accordance with the guidelines for animal experimentation mandated by Japanese law. This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals issued by Sojo University. The protocol was approved by the Committee on Ethics of Animal Experiments at Sojo University. BALB/c-R/J mice were kindly provided by Prof. Seiji Okada (Kumamoto University, Japan) [12].
The mice were bred using 100% fresh ventilatory changes (14 times per hour) at a room temperature of 25 ± 1°C, humidity of 50 ± 10%, and a light/dark cycle of 12 h. The mice were randomly grouped based on their body weights using the stratified randomization method. Five mice were included in each group. U-87MG cells (2.5 × 106 cells in 10 μL Matrigel) were orthotopically implanted into the right brains of anesthetized mice.
TL (136 mg/kg for DMPC) was intravenously administered once daily for 29 days starting one day after inoculation with U- 87MG cells. Brain samples were harvested from anesthetized mice and weighed after completion of the TL administration regime.
Statistical analysis
Data are presented as mean ± S.D. For statistical comparison, Student’s t-test was performed. Differences were considered statistically significant at P<0.05.
Results
Physical properties of TL
The diameter of the TL was measured using dynamic light scattering measurements. The hydrodynamic diameter of TL was <100 nm, indicating that TL can avoid recognition by the reticular endothelial system in vivo [13]. The DMPC liposomes were unstable and precipitated.
TL inhibition of U-87MG cell growth leading to apoptosis
The 50% Inhibitory Concentration (IC50) of TL is shown in Figure 1A. The IC50 value was 92 μM, whereas that of the DMPC liposomes was 240 μM. The difference was significant, indicating a greater inhibitory effect of TL compared with that of DMPC liposomes. Induction of apoptosis was investigated using flow cytometry. The amount of apoptotic DNA increased in U-87MG cells treated with TL and reached 100% (Figure 1B).
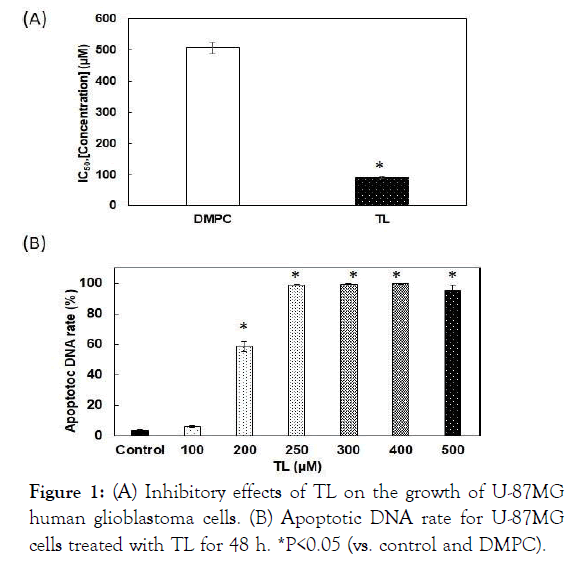
Figure 1: (A) Inhibitory effects of TL on the growth of U-87MG human glioblastoma cells. (B) Apoptotic DNA rate for U-87MG cells treated with TL for 48 h. *P<0.05 (vs. control and DMPC).
Apoptotic signal transduction by TL
The apoptotic pathway in U-87MG cells treated with TL was examined. Caspase-3, -8, and -9 were not activated in U-87MG cells treated with TL, indicating that TL induced apoptosis through a caspase-independent pathway (Figure 2A). The mitochondrial pathway in U-87MG cells treated with TL was also examined. The mitochondrial transmembrane potential of U-87MG cells treated with HL decreased, indicating that TL induced apoptosis in U-87MG cells through the mitochondria (Figure 2B). AIF is involved in initiating the caspase- independent pathway of apoptosis. Thus, the expression of AIF was assessed. The fluorescence intensity of AIF increased in U- 87MG cells treated with TL, indicating that AIF is involved in apoptosis induced by TL (Figure 2C). These results suggest that TL induces apoptosis through mitochondria and AIF in a caspase-independent pathway manner.
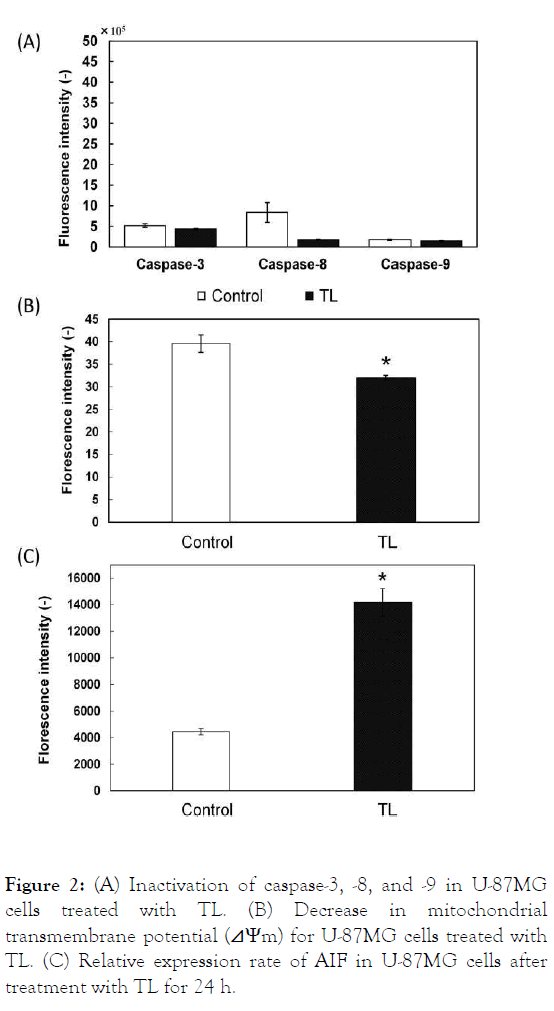
Figure 2: (A) Inactivation of caspase-3, -8, and -9 in U-87MG cells treated with TL. (B) Decrease in mitochondrial transmembrane potential (⊿Ψm) for U-87MG cells treated with TL. (C) Relative expression rate of AIF in U-87MG cells after treatment with TL for 24 h.
Accumulation of TL in cell membrane
The fusion and accumulation of TL in the membrane of U-87MG cells was examined using fluorescence probe-labeled TL. The fluorescence intensity of labeled TL increased (Figure 3A), indicating that TL fused and accumulated in the membrane of U-87MG cells.
The membrane fluidity of the U-87MG cells treated with TL was examined. The P-value decreased for U-87MG cells treated with TL, indicating that the membrane fluidity of U-87MG cells increased after TL treatment (Figure 3B).
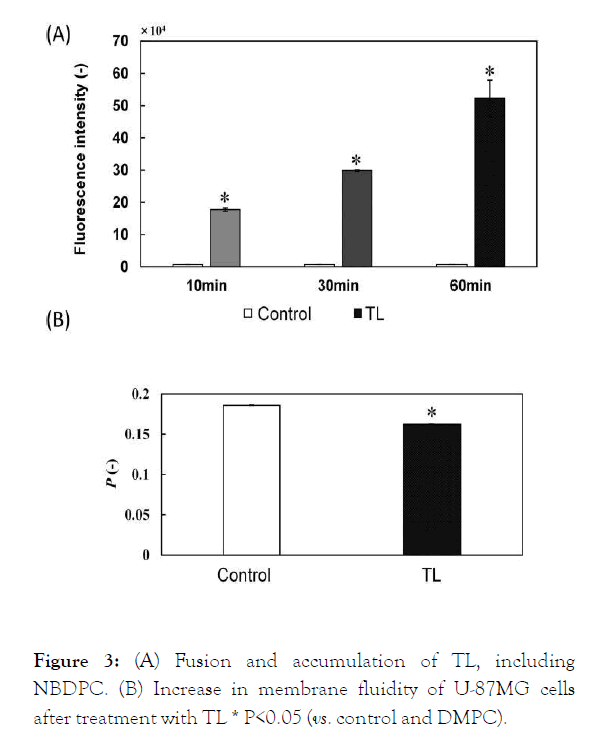
Figure 3: (A) Fusion and accumulation of TL, including NBDPC. (B) Increase in membrane fluidity of U-87MG cells after treatment with TL * P<0.05 (vs. control and DMPC).
Reduction in tumor area in brains of orthotopic graft mouse model of glioblastoma after treatmentwith TL
We examined the therapeutic effects of intravenous TL treatment on tumor growth in the orthotopic graft mouse model of glioblastoma after the inoculation of U-87MG cells. Analysis of autopsy photographs of tumors in the mice revealed the reduction in tumor growth (Figure 4A).
Remarkable inhibition of tumor enlargement (vs. control, P<0.05) was observed in the mice treated with TL based on relative brain weight (Figure 4B).
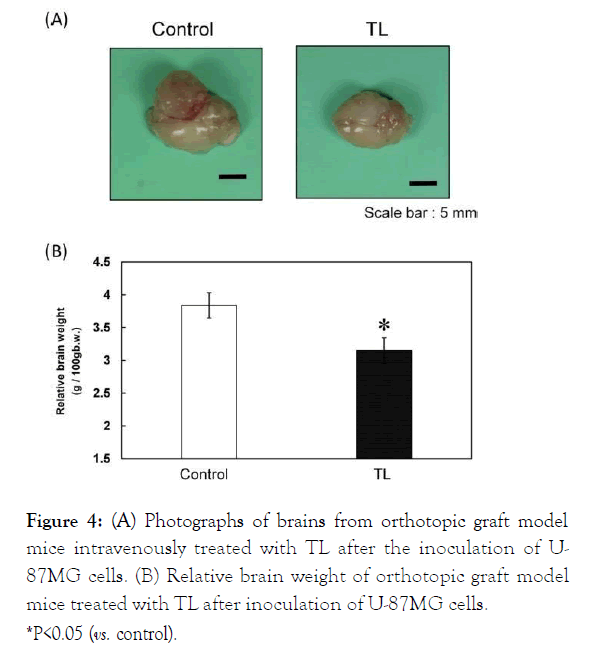
Figure 4: (A) Photographs of brains from orthotopic graft model mice intravenously treated with TL after the inoculation of U-87MG cells. (B) Relative brain weight of orthotopic graft model mice treated with TL after inoculation of U-87MG cells. *P<0.05 (vs. control).
These results suggest that TL is remarkably effective for inhibiting the growth of U-87MG cells and can cross the blood- brain barrier.
Discussion
The findings are the first evidence of the therapeutic effect of TL in an orthotopic graft mouse model involving U-87MG human glioblastoma cells. There are five noteworthy findings. First, TL with a hydrodynamic diameter <100 nm persisted for 4 weeks. Second, TL inhibited the growth of U-87MG cells. This inhibition was driven by increased apoptosis. Third, TL induced apoptosis in U-87MG cells via the release of AIF in a caspase- independent pathway manner. Fourth, TL fused and accumulated in U-87MG cell membranes, increasing their fluidity. Fifth, TL also inhibited tumor enlargement in an orthotopic graft mouse model of glioblastoma.
Conclusion
The findings are the first evidence of the therapeutic effect of TL in an orthotopic graft mouse model involving U-87MG human glioblastoma cells. The results could be potentially advantageous for future clinical applications of TL therapy in patients with glioblastoma.
Conflict of Interest
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Mayuri Sonoda for her technical assistance. This work was supported in part by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 20K05239).
REFERENCES
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(3):987-996.
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(2):699-708.
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(2):709-722.
- Matsumoto Y, Cao E, Ueoka R. Novel liposomes composed of dimyristoylphosphatidylcholine and trehalose surfactants inhibit the growth of tumor cells along with apoptosis. Biol Pharm Bull. 2013;36(8):1258-1262.
- Matsumoto Y, Cao E, Ueoka R, Growth inhibition by novel liposomes including trehalose surfactant against hepatocarcinoma cells along with apoptosis. Anticancer Res. 2013;36(11):4727-4740.
- Matsumoto Y, Kuwabara K, Ichihara H, Kuwano M. Therapeutic effects of trehalose liposomes against lymphoblastic leukemia leading to apoptosis in vitro and in vivo. Bioorg Med Chem Lett. 2016;26(2):301-305.
- Ichihara H, Kuwabara K, Matsumoto Y. Trehalose liposomes induce apoptosis of breast tumor cells in vitro and in vivo. Biochem Biophys Res Comm. 2020;532(4):505-512.
- Ichihara H, Kuwabara K, Matsumoto Y. Trehalose liposomes suppress the growth of tumor on human lung carcinoma bearing mice by induction of apoptosis in vivo. Anticancer Res. 2017;37(11):6133-6139
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory Effects and Anti-Invasive Activities of Trehalose Liposomes on the Proliferation of Lung Carcinoma Cells. J Carcinog Mutagen. 2017;8 (1):1000283.
- Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, et al. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1996;36(2):47-50.
- Ichihara H, Okumura M, Matsumoto Y. Theranostics with hybrid liposomes in an orthotopic graft model mice of breast cancer. Anticancer Res. 2018;38(10):5645-5654.
- Ueoka R, Matsumoto Y, Goto K, Ichihara H, Komizu Y. Membrane targeted chemotherapy with hybrid liposomes for tumor cells leading to apoptosis. Curr Pharm Des. 2011;17(17):1709-1719.
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into Balb/c and C57BL/6 strain of Rag-2/Jak3 double-deficient mice. J Biomed Biotechnol. 2011;2011(1):1-6.
- Allen TH, Hansen C, Martin F, Redemann C, Yau-Young A, Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29-36.
Citation: Kuwabara K, Ichihara H, Matsumoto Y (2021) Trehalose Liposomes Inhibit the Growth of Glioblastoma Cell In vitro and In vivo. JCarcinog Mutagen. 12:364.
Copyright: © 2021 Kuwabara K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited


