Indexed In
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 0, Issue 0
Tooth Discoloration in the Young Dentition: A Literature Review
Elbahary Shlomo1*, Weissman Gina1 and Azem Hanna22Department of Pediatric Dentistry, Tel Aviv University, Tel Aviv, Israel
Received: 04-Nov-2021 Published: 25-Nov-2021
Abstract
Tooth discoloration is one of the most frequent reasons why patients seek dental care and is usually esthetically displeasing and psychologically traumatizing. Understanding the etiology of tooth discoloration is vital to a dentist in order to make the correct diagnosis and treatment. To carry out a review of the literature on tooth discoloration with particular regard to endodontic aspects, more recent literature on the mechanisms of tooth staining and management.
Keywords
Traumatizing; Tooth discoloration; Enamel; Young dentition; Necrosis
Introduction
It is widely recognized that today’s youth and beauty oriented culture prizes an attractive smile and white teeth. It is accepted that the anterior teeth appearance have psychological and social impacts on children and elderly [1-3]. Teeth discoloration is one of the most frequent reasons why patients seek dental care. Tooth discoloration is usually esthetically displeasing and psychologically traumatizing [4]. Understanding the etiology of tooth discoloration is vital to a dentist in order to make the correct diagnosis and to explain the exact nature of the condition to the patient. In some instances, the staining mechanism might influence the treatment options offered by the dentist, and may affect the treatment outcome [5].
Classification
The causes for tooth discoloration can be classified according to the location of the stains, either as extrinsic or intrinsic [6,7]. Extrinsic discoloration lies on the tooth surface or in the acquired pellicle. The intrinsic discoloration occurs when the chromogens are deposited within the bulk of the tooth, which may be of local or systemic origin [8]. There causes of intrinsic tooth discoloration have either an endogenous or exogenous origin. These changes may occur during or after odontogenesis. During odontogenesis (pre-eruption), teeth may become discolored from the changes in the quality or quantity of enamel or dentin, or from the incorporation of discoloring agent into the hard tissues. Genetic disorder, medication, metabolic and environmental causes also have been suggested. Post-eruption discoloration occur when the discoloring agent incorporate the hard tissues. They may originate from the pulp or the tooth surface [9].
Dental Conditions and Caries
Tooth wear
Tooth wear is the progressive loss of enamel and dentin due to attrition, abrasion and erosion. As the enamel weakens the tooth becomes darker as the color of the dentin becomes more apparent. Once the dentin is exposed the potential for chromogens to enter the body of the tooth increases.
Caries
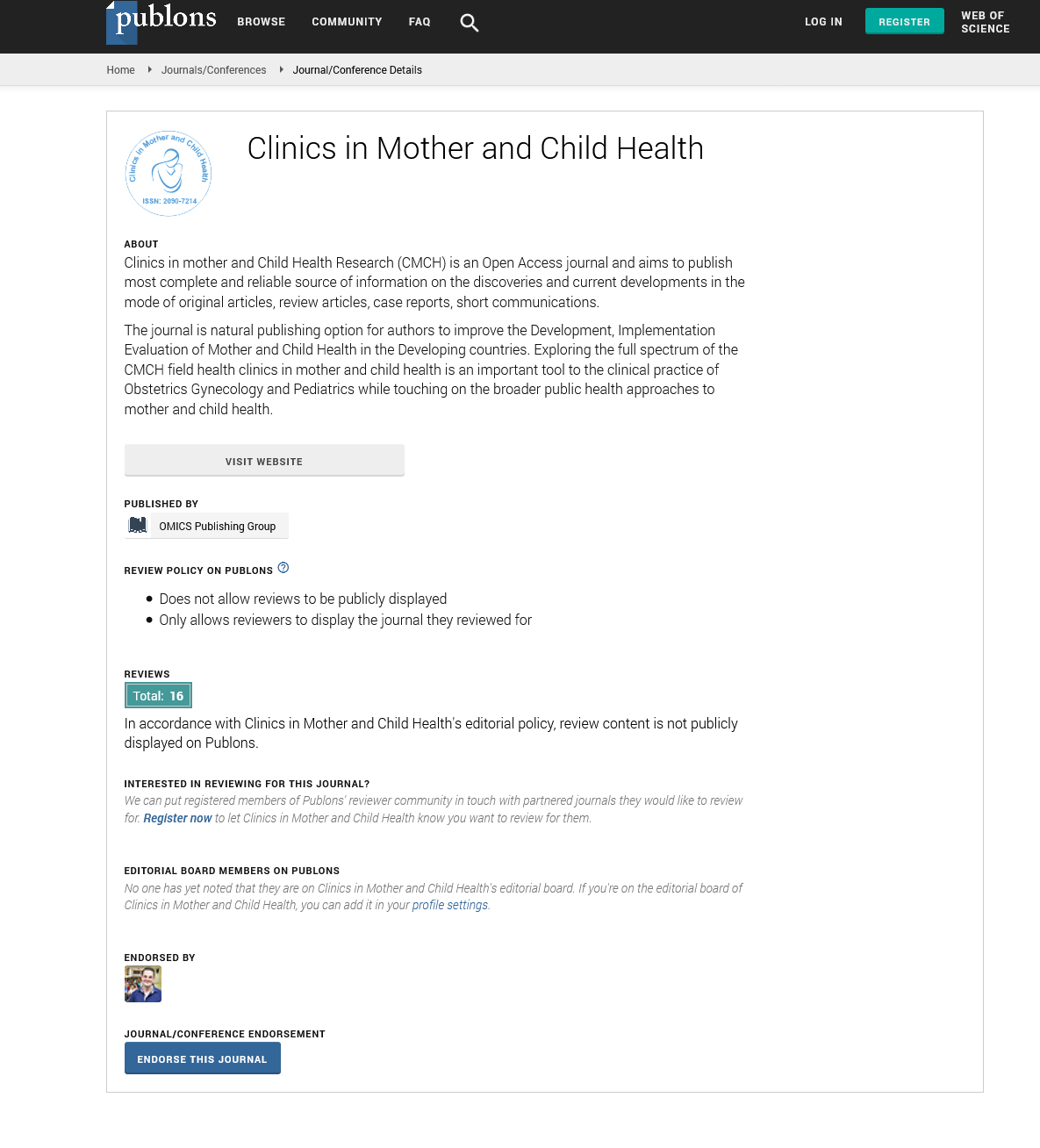
The various stages of carious process can be recognized by changes in color as the disease progresses. The pathogenesis of dental caries begins with an incipient lesion confined to the enamel layer. Incipient carious lesions are associated with plaque accumulation and manifest as chalky white areas of discoloration secondary to demineralization. As caries progresses into the dentin, the overlying translucent enamel reveals the color of the underlying caries and appears yellowish brown. Extensive caries that involve destruction of both enamel and dentin produce a color that ranges from light brown, to dark brown or almost black as shown in Figure 1 [10].
Figure 1: A 19 years old male, black-grey discoloration in central incisor due to secondary caries in old buccal composite restoration.
Aging
The natural darkening and the yellowing of the teeth and the change in their light transmission properties that occur with age can be due to the combination of the factors involving both enamel and dentin. The enamel undergoes both thinning and textural change, while the deposition of secondary and tertiary dentin and pulp stones all contribute to the darkening process of ageing.
Pulpal pathology
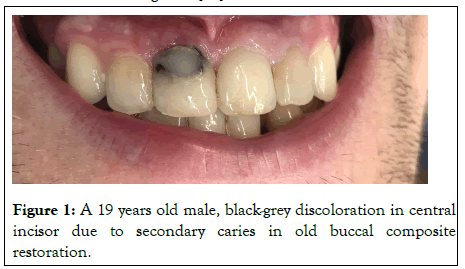
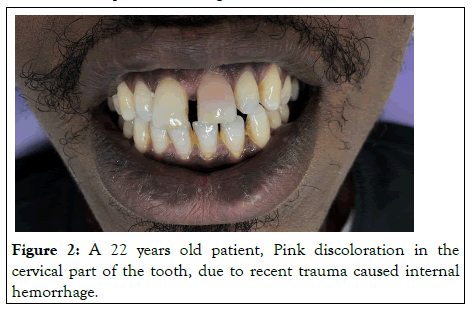
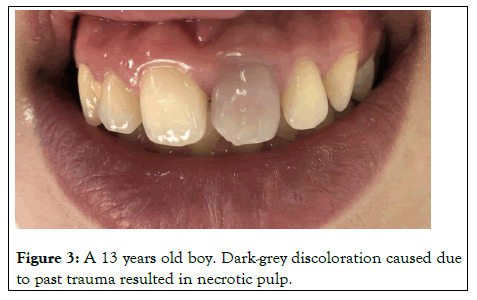
Bacterial, mechanical or chemical irritation to the pulp may result in tissue necrosis and the release of disintegration byproducts that might penetrate the tubules and discolor the surrounding dentin. Acute trauma to an erupted tooth can cause intrapulpal hemorrhage giving it a reddish tinge as shown in Figure 2. This discoloration can change to gray-brown in a matter of days as the pulp becomes necrotic. Hemolysis of the red blood cells would follow and release the heme group to combine with the putrefying pulp tissue to form black iron sulfide as shown in Figure 3. Excessive formation of irregular dentin in the pulp chamber and along the canal walls may occur following certain traumatic injuries. This is known as calcific metamorphosis. As a result of this, the translucency of the crown gradually decreases, giving rise to yellowish or yellowish brown discoloration. Root resorption following trauma often presents as a pink spot lesion at the cemento-enamel junction in an otherwise symptomless tooth, known as the 'Pink tooth of Mummery'. The resorption may be internal, being of pulp origin or external of periodontal origin.
Figure 2: A 22 years old patient, Pink discoloration in the cervical part of the tooth, due to recent trauma caused internal hemorrhage.
Figure 3: A 13 years old boy. Dark-grey discoloration caused due to past trauma resulted in necrotic pulp.
Inappropriate access cavity design and/or preparation
This type of coronal discolouration occurs because of inadequate removal of coronal pulp tissue especially when the cavity does not include the mesial and distal pulp horns, the erythrocytes, either in the remaining pulp tissue or in dentinal tubules regardless of the presence of a smear layer , will degrade into haemosiderin, haemin, haematin and haematoidin, which release iron during haemolysis. The iron can be converted to black ferric sulphide with hydrogen sulphide produced by bacteria, and this may cause grey discolouration of the tooth crown. Apart from blood degradation, other degrading proteins of necrotic pulp tissue may also cause staining. In addition, an inadequate access cavity may complicate the clinician’s ability to remove the root canal cement material from the pulp chamber while completing the root filling. Marin et al. observed the ability of blood components to penetrate dentine and induce discolouration of enamel, although it was not as pronounced as the discolouration of the coronal and radicular dentine. The authors commented that the discolouration of enamel by blood components possibly becomes more pronounced with longer exposure times. Although enamel has no tubular morphology, its organic structural features at the dentino-enamel junction, may play a role in the discolouration process.
Dental materials
Dental restorations most commonly cause intrinsic discoloration. Amalgam restorations can generate corrosion byproducts, leaving a blue-gray color in the tooth, especially in large cavity preparations with undermined enamel known as amalgam blue. Open margins around composite or glass ionomer restoration may allow chemicals to enter between the restoration and the tooth structure and discolor the underlying dentine.
Endodontic medicaments and root filling materials
A variety of endodontic medications and filling materials may be responsible for discolorations. A progressive discoloration is suggested to be primarily a result of materials penetrates into dentinal tubules. However, it has been shown that a visible crown discoloration may not necessarily be associated with tubule penetration and may be caused by material remnants in the pulp chamber, which get darker over time and transmit through the hard tissues.
Ledermix
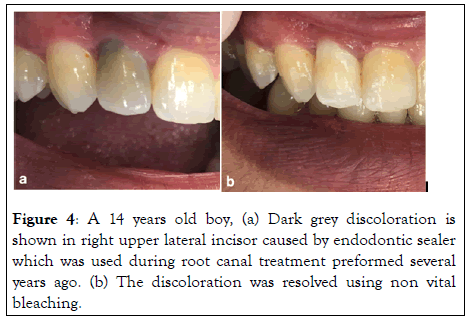
It is a glucocorticoid antibiotic compound. It was developed by Schroeder and Triadon in 1960. It is used to control pain and inflammation. Today the main therapeutic components are triamcinolone acetonide (1%-a corticosteroid which is used for its anti-inflammatory effects), and demethylchlortetracycline (3.021%-also known as demeclocyline. A tetracycline antibiotic which is used for its antibacterial action). Ledermix has regained attention in the field of dental traumatology, Placed as an intracanal medication after severe luxation injuries, it has the potential to inhibit inflammatory root resorption. Ledermix may be retained within the root canal as a medicament for periods varying from 2 to 12 weeks depending on the pathological condition being treated. Some clinicians have noted that Ledermix paste may cause discolouration as shown in Figure 4 of teeth when used for long periods, such discolouration is theoretically possible due to the presence of the tetracycline antibiotic because tetracyclines are known to be able to produce colour changes in hard tissues, including teeth. It was originally thought that tetracycline uptake into hard tissues would only occur in regions of active calcification but there is evidence in the literature demonstrating tetracycline uptake may occur topically in a fully mineralised tooth. In a study by Kim et al. color change due to the application of Ledermix as an intracanal medicament was evaluated. The results showed that its application led to tooth discoloration, and greater discoloration was found in the group where the medicament was placed in the pulp chamber than in the group where the medicament was placed only in the root canals. The reason for this may be that coronal dentin is more permeable than root dentin. However, in Lenherr et al. study, severe and increasing discoloration occurred in the Ledermix specimens especially after the specimens had been exposed to indirect sunlight. This is in accordance with other laboratory and clinical studies. From a clinical aspect, it may not be possible to completely avoid contamination of the coronal dentine with the medicament or to meticulously clean the surfaces after placement. Thus, alternative nonstaining alternatives are needed. This may be Odontopaste (Australian Dental Manufacturing, Kenmore Hills, Australia), a recently released alternative with substitution of the tetracycline component by clindamycin. Furthermore, as the inhibiting effect of Ledermix on external root resorption after severe dislocation injuries is primarily attributed to the corticosteroid component, a corticosteroid dressing may be a reasonable alternative in dental traumatology.
Figure 4: A 14 years old boy, (a) Dark grey discoloration is shown in right upper lateral incisor caused by endodontic sealer which was used during root canal treatment preformed several years ago. (b) The discoloration was resolved using non vital bleaching.
Triple Antibiotic Paste (TPA)
Regenerative Endodontic Therapy (RET) have been defined as biologically based procedures designed to replace damaged structures such as dentin, root structures, and cells of the pulpdentin complex. This new treatment modality has emerged as an alternative that, in addition to healing apical periodontitis, aims to promote normal pulpal physiologic functions. These include continued root development, immune competency, and normal nociception, as seen in some published cases. The treatment procedure begins with chemical disinfection by copious irrigation of the root canal space with NaOCl, followed by placement of an intracanal medicament at the first visit. Several medicaments like triple antibiotic mixture (3 mix) (metronidazole, ciprofloxacin, and minocycline) or calcium hydroxide have been used successfully. At the next visit, which should be at least 1 week after the initial session or more, in the absence of clinical signs of inflammation, the clinician removes the intracanal medicament and induces bleeding inside the root canal space by irritating the periradicular tissue. After clot formation, the clinician seals the root canal space by placing an MTA plug over the blood clot. There are several case studies that demonstrate successful clinical and radiographic outcome for this treatment approach in single- rooted and molar teeth.
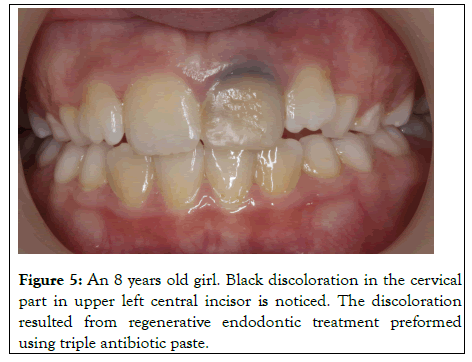
Discoloration of the tooth after regenerative endodontic treatments (Figure 5), as revealed by Kim et al is a problem mostly related to the use of minocycline in the triple antibiotic paste. Minocycline is a semisynthetic derivative of tetracycline and is effective against gram-positive and gram-negative bacteria. It binds to calcium ions via chelation to form an insoluble complex. Hence, the minocycline incorporated into the tooth matrix causes the discoloration. Discoloration starts in the first week and gradually increases with further penetration of the medicament until the tooth becomes completely dark. Similarly, Lenherr et al. showed that the greatest color change occurred at 12 weeks. In another study, Kim et al. demonstrated that 3Mix containing minocycline showed a greater color change than other 3Mix formulations, in which minocycline had been replaced with another antibiotic.
Figure 5: An 8 years old girl. Black discoloration in the cervical part in upper left central incisor is noticed. The discoloration resulted from regenerative endodontic treatment preformed using triple antibiotic paste.
Moreover, TAP can directly affect dentin including demineralization (possibly because of its very low pH of~3), and reduced microhardness and fracture resistance. Together, these data indicate that TAP has both beneficial antimicrobial efficacy and several potential adverse effects on the microenvironment of the root canal system. Therefore, it is imperative that the TAP be adequately removed from the canal space once it has served the antimicrobial purpose. It was shown that TAP is not adequately removed during regenerative procedures despite the use of different irrigation techniques. It was shown in Berkhoff et al study that more than 80% of the antibiotic paste was localized within dentin despite attempts to remove it with copious irrigation using different methods. This very high diffusion and affinity for dentin may explain why this drug combination leads to the minocycline-mediated staining often seen extending to the cementum layer. Indeed, most of the labeled TAP was found in depths greater than 350 mm. A recent study suggested sealing dentinal walls of the access cavity by using dentin bonding agent and composite resin before placement of triple antibiotic paste inside the canal. On the other hand, Kim et al examined the performance of this prevention technique for tooth discoloration. In this study teeth treated with dentin bonding were evaluated with naked eye and then with colorimeter. In the eye assessment teeth did not have any change in color, but in the colorimeter assessment they had they concluded that using dentin bonding agents before placement of the triple antibiotic paste might not completely prevent tooth discoloration.
A practical way to prevent discoloration is replacing minocycline with an antibiotic that does not stain. Thibodeau and Trope reported a successful regenerative endodontic treatment of a maxillary central incisor by using cefaclor instead of minocycline in the antibiotic mixture. Another promising alternative is the use of Calcium hydroxide (CaOH) as intracanal medication.
Calcium hydroxide (CaOH)
This material has several favorable biological properties, such as antimicrobial activity, tissue-dissolving ability, prevention of tooth resorption, and possible release of growth factors and biomolecules from dentin. Some studies have reported tooth discoloration, although insignificant, due to the application of CH. Some additional materials have been incorporated into the formulation of CH to confer antibacterial properties or radiopacity, it has been suggested that these materials may result in tooth discoloration following the application of CH. The different formulations of CH with variation in bismuth carbonate as part of a chemical composition, which can affect its discoloration potential. It has been proposed that the penetration of calcium ions into the dentinal tubules and their reaction with the hydroxyapatite crystals in the tubules result in lightening and yellowness of teeth medicated with CH.
Chlorhexidine (CHX)
Chlorhexidine (CHX) has long been suggested for use as an intracanal medicament due to its broad-spectrum antimicrobial activity and substantivity. Presence of a red tint in specimens over time was noticed due to the pink color of the CHX medicament, which shifts the color change towards redness in the red-green axis.
Root canal irrigants
While sodium hypochlorite (NaOCl) is the most common irrigant, other solutions have also been advocated. Some of these are used alone but most are used in combination with NaOCl, or as a final rinse to enhance the antimicrobial activity and substantivity against some resistant bacteria, to decrease the caustic effect or to aid in removing the smear layer. Although sodium hypochlorite is a bleaching agent and is not usually considered to cause tooth discolouration, it should be noted that NaOCl has been reported to cause dentine discolouration as a result of its contact with erythrocytes and its high tendency to crystallize on the root dentine, which may mean that it is difficult to completely remove from the canal, in addition combination of NaOCl with other adjunct irrigating solutions has been found to cause marked tooth discolourations.
Vivacqua-Gomes et al. observed a in dark brown precipitate when NaOCl combination was with chlorhexidine (CHX) results in dark brown discoloration.
Apart from this, NaOCl has been shown to react with MTAD (a mixture of a tetracycline isomer, an acid (citric acid) and a detergent) (Dentsply Tulsa Dental, Tulsa, OK, USA), in the presence of light, causing brown discolouration. This reaction may be caused by the dentinal absorption and release of the doxycycline, present in MTAD, which will be exposed to NaOCl if it is used as a final rinse after MTAD.
Calcium silicate-based materials
Mineral Trioxide Aggregate (MTA) both gray and white have been associated with discoloration. Materials based on industrial Portland cement have been introduced in dentistry. The first generation material was Mineral Trioxide Aggregate (MTA) which is composed of Portland cement and bismuth oxide. The Portland cement component when mixed with water results in the formation of calcium silicate hydrate, calcium hydroxide, and ettringite. The bismuth oxide is added to enhance the material radiopacity. After contamination of the specimens with blood, all Portland cement-based materials showed an increased discoloration. Bismuth oxide, is a possible factor responsible for the discoloration of teeth treated with MTA. It has been stated that oxidation of bismuth oxide material destabilizes the oxygen in its formulation, which reacts with carbon dioxide and produces bismuth carbonate that causes discoloration. Another theory is the interference of bismuth oxide with dentin collagen. Namazikhah et al. demonstrated that the microstructure of the materials shows pH-dependent porosities. These porosities may uptake blood components and may be responsible for the observed discoloration. This is of clinical relevance because Portland cement-based materials are usually placed in direct contact to vital, vascularized tissue. Thus, the development of biocompatible materials with a reduced porosity level may be beneficial. Although Bismuth oxide, the radiopacifier of MTA, has been suggested as the cause of the discoloration, other metal oxides such as iron, aluminum, and magnesium oxides present in MTA have also been implicated as the source of the discoloration. One possible mechanism of tooth discoloration by white MTA is related to the oxidation of the iron content remaining in the set material, which belongs to the calcium aluminoferrite phase of the powder.
Newer bioceramics have been developed to address the drawbacks of gray and white MTA. Biodentin (BD) calcium silicate-based product, which became commercially available in 2009 (Septodont, France), it has indications similar to MTA and according to manufacturers do not cause tooth discoloration. The powder of Biodentine contains tricalcium silicate, calcium carbonate, and zirconium oxide as the radiopacifier. The liquid contains calcium chloride to accelerate the setting reaction. When compare to MTA, BD showed non to minor discoloration.
Preventive guidelines
A well-designed and appropriately extended access cavity is essential. Successful detection, and removal of any ‘catch’ from the roof of the pulp chamber will ensure complete removal of the pulp tissues, particularly from the mesial and distal pulp horns. Thorough irrigation of the access cavity will also help to ensure that all pulp tissue has been removed from the pulp chamber. Practitioners should choose irrigating solutions carefully to suit the clinical condition that is being treated. Intracanal medicaments should be confined to the root portion of the root canal system below the gingival margin. They should not be placed in the crown portion of the tooth or in the pulp chamber to avoid coronal discolouration particularly because they have no therapeutic effect in the crown. Most medicaments are paste materials and they should be placed in the root canal in a manner that does not leave remnants in the pulp chamber. Similar to intra-canal medicaments, keeping the root canal filling materials in the root portion and apical to the gingival margin of the tooth is essential.
Management guidelines
Proper evaluation and preparation: A thorough clinical examination, augmented by an appropriate radiographic interpretation, is mandatory for proper evaluation of a discoloured tooth caused by endodontic procedures. Improper adaptation and/or discoloured margins of coronal fillings, the presence of carious lesions and extrinsic stains, as well as the quality and coronal extension of the root filling should all be identified initially. Prior to selecting a treatment approach, it is essential to treat caries, remove extrinsic stains if present, and to polish the external crown surface to facilitate the proper identification of the final tooth shade. When replacing defective/discoloured restorations as well as treating caries, the tooth should only be restored temporarily, unless the existing restorations or caries are the only causes of discolouration and no bleaching is required (post-endodontic procedures). Definitive restoration of the tooth should be deferred until after the normal tooth colour has been re-established via bleaching. Selection of the appropriate treatment approach
Removal of the cause: Adequate extension of the access cavity and removal of the cause of the discolouration (exampleremaining pulp tissue, medicament, root canal filling material or defective coronal restorations) is required before internal bleaching. The tooth should then be re-evaluated because the colour may become satisfactory once the cause has been removed. This is typically the case when the discolouring agent only acts as a dark background and has not yet penetrated into the dentinal tubules.
Internal bleaching (Walking bleach): Internal bleaching is a simple, inexpensive and reliable treatment approach for most coronal discolourations caused by endodontic procedures as shown in Figure 4. Hydrogen peroxide (H2O2) and hydrogen peroxide releasing agents such as sodium perborate (NaBO3.n- H2O-‘n’ represents the available formulations in monohydrate, trihydrate and tetrahydrate) and carbamide peroxide (CH6N2O3) are the most commonly used bleaching agents. Different concentrations, formulations (liquid or gel), combinations (sodium perborate/hydrogen peroxide and sodium perborate/carbamide peroxide) and application of heat or light have been suggested in an attempt to accelerate and optimize the bleaching process. However, it should be noted that the use of bleaching agents at high concentrations (such as 30% of hydrogen peroxide) with the aid of heat (thermos-catalytic technique) increases the risk for external invasive root resorption, especially in traumatized or infected teeth. Generally, the short- and long-term prognosis of internal bleaching is favourable and acceptable to the patient, as long as the coronal restoration is maintained with no marginal breakdown that could lead to further discolouration.
Bleaching in primary teeth: Discoloration, which is one of the most common reasons for poor aesthetics in primary teeth, is often a result of traumatic injury. Intrinsic discoloration may be caused by pulpal hemorrhaging that occurs following the traumatic injury. Although tooth bleaching has been performed over the years in children as young as age 4, it is rarely done in children younger than 6. It may be indicated for those children who report (or parents who report) that tooth discoloration “is bringing negative attention”. Similarly, some researchers advocate whitening for young permanent teeth, even when they are only partially erupted with incomplete root formation, because “even young patients can be highly concerned over discoloration of their anterior teeth”. Bleaching techniques may be performed extracoronally, intracoronally or via a combination of both techniques. In endodontically treated primary teeth, both techniques can be used. However, in vital primary teeth, bleaching is only done by external approach. Internal bleaching technique, which has advantages over external bleaching in that it, avoids gingival irritation and ingestion of the bleaching material. Arikan et al. describes the treatment of a darkened primary tooth of a 4 years old boy with sodium perborate using the walking bleach technique and its 1 year clinical and radiographical follow-up, Shaheen et al. assess, in vitro, the efficacy of 10 percent carbamide peroxide gel as an intracoronal bleaching agent of nonvital discolored primary teeth due to blood decomposition. In both studies the bleach technique was found to be successful in whitening primary teeth. The American Academy of Pediatric Dentistry (AAPD) supports the use of bleaching for vital and nonvital primary teeth. Due to the concern of the hydroxyl free radical and the potential side effects from internal bleaching, the AAPD recommends using the lowest effective concentration of the bleaching agents.
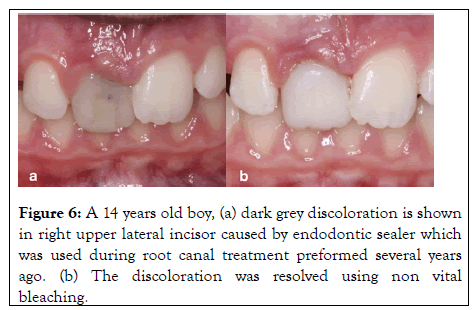
Other treatment options: Although internal bleaching is considered as a conservative treatment compared with other treatment approaches, in some resistant cases, it does not provide satisfactory outcomes. Hence, in such cases, more invasive aesthetic treatment such as the placement of labial porcelain or composite (Figure 6) veneer or a full coverage ceramic crown may be indicated.
Figure 6: A 14 years old boy, (a) dark grey discoloration is shown in right upper lateral incisor caused by endodontic sealer which was used during root canal treatment preformed several years ago. (b) The discoloration was resolved using non vital bleaching.
Conclusion
Knowledge of the aetiology of tooth discoloration is important to dental practitioners in order to establish correct diagnosis and treatment when encountering a discolored tooth. Many colour changes can be encountered when dealing with tooth discoloration. In some instances, the mechanism of staining may have an effect on the outcome of treatment and influence the treatment options the dentist will be able to offer to patients. Therefore it is essential that health care professionals be familiar with the causes of primary teeth staining and pigmentation. A better knowledge will guide the clinician to a proper diagnosis and facilitate the referral to a specialized pediatric dentist that will provide proper dental care.
REFERENCES
- Helm S, Kreiborg S, Solow B. Psychosocial implications of malocclusion: A 15-year follow-up study in 30-year-old Danes. Am J Orthod. 1985;87(2):110-118.
- Vallittu PK, Vallittu AS, Lassila VP. Dental aesthetics a survey of attitudes in different groups of patients. J Dent. 1996;24(5):335-338.
- Baldwin DC. Appearance and aesthetics in oral health. Community Dent Oral Epidemiol. 1980;8(5):244-256.
- Manuel ST, Abhishek P, Kundabala M. Etiology of tooth discoloration: A review. Nig Dent J. 2010;18(2):56-63.
- Watts AM, Addy M. Tooth discolouration and staining: A review of the literature. Br Dent J. 2001;190(6):309-316.
- Hattab FN, Qudeimat MA, Al-Rimawi HS. Dental discoloration: An overview. J Esthet Restor Dent. 1999 Nov;11(6):291-310.
- Sulieman M. An overview of tooth discoloration: Extrinsic, intrinsic and internalized stains. Dent Update. 2005;32(8):463-471.
- Vogel RI. Intrinsic and extrinsic discoloration of the dentition: A literature review. J Oral Med. 1975;30(4):99-104.
- Hayes PA, Full C, Pinkham J. The etiology and treatment of intrinsic discolorations. J Can Dent Assoc. 1986;52(3):217-220.
- Kleter GA. Discoloration of dental carious lesions: A review. Arch Oral Biol. 1998;43(8):629-632.
Citation: Shlomo E, Gina W, Hanna A (2021) Tooth Discoloration in the Young Dentition: A Literature Review. Clinics Mother Child Health. S11.004.
Copyright: © 2021 Shlomo E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.