Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2024) Volume 0, Issue 0
Therapeutic Potential of Combretum mossambicense Extracts Against P. Falciparum Parasite
Viness Milumbe Chikambwe1, Patrick Mubambe2, Kakoma K. Maseka2 and Lameck Banda2*2Department of Mathematics and Natural Sciences, Copperbelt University, Kitwe, Zambia
Received: 18-Jun-2024, Manuscript No. JBP-24-26034; Editor assigned: 20-Jun-2024, Pre QC No. JBP-24-26034 (PQ); Reviewed: 04-Jul-2024, QC No. JBP-24-26034; Revised: 11-Jul-2024, Manuscript No. JBP-24-26034 (R); Published: 18-Jul-2024, DOI: 0.35248/2155-9597.24.S27.099
Abstract
With the increase in the resistance of P. Falciparum (the deadliest malaria-causing Plasmodium) to antimalarial alkaloidbased drugs, there has been intense research on new drugs that can combat malaria. Plants provide an unlimited source of bioactive compounds that can be used to treat various diseases. In addition, plant host endophytes, such as bacteria and fungi, are regarded as ideal sources of bioactive constituents. The Combretum mossambicense plant is a medicinal plant traditionally used as an effective herbal remedy for malaria infection. However, very little research has been conducted to investigate the phytochemical composition of this plant. In this study, the phytochemistry of the extracts of this plant was investigated and referenced to the chemistry of the commercial drugs used to treat malaria.
The results showed that Combretum mossambicense extracts contained alkaloids. However, the alkaloids found in the plant extracts are not directly linked to those reported for the treatment of malaria. A literature review of other compounds found in the plant showed that other nonalkaloid compounds had a positive effect on P. Falciparum. According to the literature, antimicrobial compounds can be used to treat malaria. The profiles of all the plant parts revealed the presence of numerous compounds with reported biological importance, including antifungal, antibacterial, anti-inflammatory, anticancer, and antioxidant activities. Furthermore, some of these samples contained compounds like those reported for conventional nonalkaloid antimalarial drugs. It has been shown that Combretum mossambicense contains nonalkaloid but antiplasmodial compounds such as 9,12-octadecadienoic acid methyl ester (linoleic acid), 17 octadecynoic acid, bis (2-ethylhexyl) phthalate, and beta-sitosterol. These compounds are present as modern non-alkaloid-based antimalarial drugs that fight P. Falciparum resistance. Given the reported increase in the resistance of P. Falciparum to alkaloid-based antimalaria drugs, the efficacy of this nonalkaloid herbal remedy for malaria treatment is important.
Keywords
Malaria, Anti-plasmodial compounds, Alkaloids, Bioactive, P. Falciparum
Introduction
Malaria is a life-threatening disease caused by Plasmodium parasites that are transmitted to people through bites of malaria vector-infected female mosquitoes. A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are usually arthropods such as mosquitoes, ticks, flies, fleas, and lice.
Malaria is the most lethal disease in Africa [1]. In Benin and Zambia, up to 40% of all outpatient visits are due to malaria [2]. In 2015, the World Bank provided funding worth US $ 470 million to African countries to fight malaria. The World Health Organization (WHO) estimates that more than one million people in Africa, including 3,000 children, die from malaria every year [3].
Most of the infected populations in endemic countries use antimalarial medicinal plants to treat malaria. However, very little scientific data exist to validate the antimalarial properties of most medicinal plants. Studies to establish the identity, purity, and quality of natural products include macroscopic and microscopic evaluations, physicochemical and chemical characteristics of crude plant extracts, and alkaloids contents [4].
Alkaloids are a class of naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes compounds with neutral or weakly acidic properties. Some synthetic compounds with similar structures can also be termed alkaloids. Alkaloids in their pure form are usually colorless, odorless crystalline solids but can sometimes be yellowish liquids. They often have bitter tastes. More than 3,000 alkaloids are known to be present in more than 4,000 plants. All of these compounds are secondary compounds and are a collection of various elements and biomolecules derived from amino acids or transamination. There are three types of alkaloids: True alkaloids, protoalkaloids, and pseudoalkaloids. True alkaloids and protoalkaloids are produced from amino acids, whereas pseudoalkaloids are not derived from these compounds [5].
True alkaloids
This alkaloid is obtained from amino acids and contains a nitrogencontaining heterocyclic ring. They are highly reactive and exhibit potent biological activity. They form water-soluble salts, many of which are crystalline. They then conjugate with acids to form salts. Almost all true alkaloids are bitter in taste and solid, except nicotine, which is a brown liquid [6].
Their occurrence in plants occurs in three forms: (a) In the free state, (b) as N-oxides, or (c) as salts. Various amino acids, such as l-phenylalanine/l-tyrosine, l-ornithine, l-histidine, and l-lysine, are the main sources of alkaloids [7]. Cocaine, morphine, and quinine are common alkaloids found in nature (Figures 1-3).
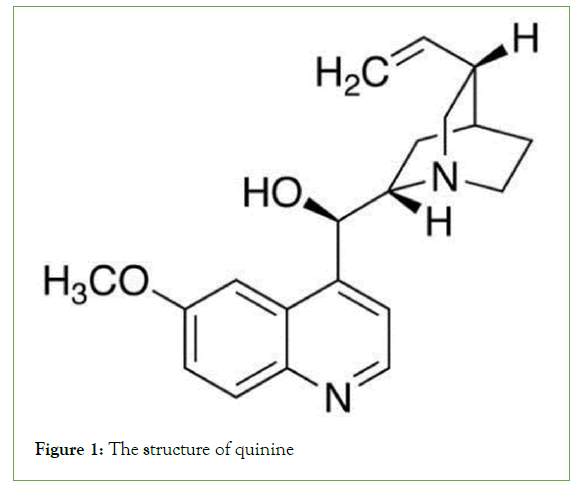
Figure 1: The structure of quinine
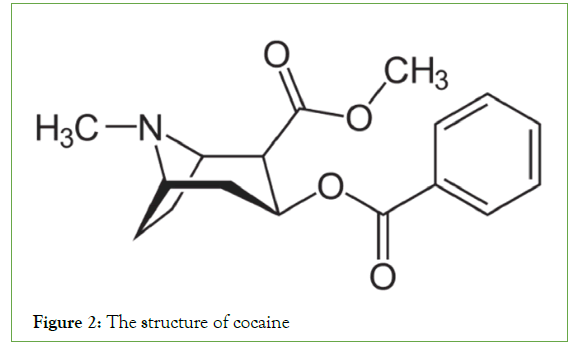
Figure 2: The structure of cocaine
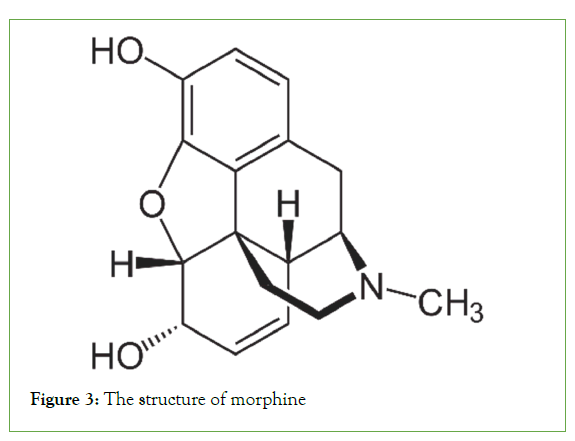
Figure 3: The structure of morphine
The malaria problem in Zambia
Zambia remains an endemic malaria country, with the entire population at risk of contracting malaria. The risk of contracting malaria is highest in the wetter, rural, and low-income provinces of Luapula, Northern Muchinga, and North-Western, and lowest in Lusaka and Southern provinces. The increase in malaria cases in Zambia has led to a high demand for antimalarial drugs. In addition to side effects, most modern medicines are too expensive for poor rural people. Some possible side effects of antimalarial drugs include dizziness, headache, sleep disturbances (insomnia and vivid dreams), and psychiatric reactions (anxiety, depression, panic attacks, and hallucinations). For many years, local people have used herbs to treat malaria and other ailments. Despite recent efforts to study these herbal remedies, little is known about the medicinal contents of most herbs. Several studies have been conducted in Zambia on the treatment of malaria using herbal remedies, although very little literature is available. This study is intended to add to the body of knowledge on well-utilized antimalarial herbal remedies. This study aimed to determine the presence of alkaloids in selected plants from the Chikankata District, which are known to treat malaria and other malaria-related diseases in the local population for many generations.
Malaria Disease
Malaria is a disease caused by Plasmodium parasites, which are transmitted to humans through the bites of infected female Anopheles mosquitoes [8]. In biology, a vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods, such as mosquitoes, ticks, flies, fleas, and lice. These four parasitic species are known to cause malaria in humans. These include Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax, but the two most common pathogens are P. Falciparum and P. vivax [9].
Transmission of Malaria
Malaria is transmitted through the bites of female Anopheles mosquitoes [10]. There are more than 400 different species of Anopheles mosquitoes, of which only 30 are vectors of malaria. An important vector species bites between dusks and dawns [11]. The intensity of transmission depends on factors related to the parasite, namely, the vector, human host, and environment [12]. Anopheles mosquitoes lay eggs in water, hatch into larvae, and eventually emerge as adult mosquitoes. The female mosquito uses the blood to nurture eggs. During a blood meal, the fungus sucks gametocytes, which develop into sporozoites in female mosquitoes. The sporozoites were injected into another human at the next blood meal [13].
Transmission is more intense in places where the mosquito lifespan is longer, as this increases the chance of the parasite fully developing inside the mosquito. These mosquitoes prefer to bite humans more than other animals. Approximately 90% of African malaria cases occur because of their long lifespan and human preferences [14]. Transmission also depends on climatic conditions, such as rainfall patterns, temperature, and humidity, which may affect the number of mosquitoes and their survival. Seasonal transmission peaks occur during and after the rainy season because of the large number of mosquito breeding sites [15]. Immunity is another factor that increases malaria transmission, especially in adults. Those with partial immunity, which develops over the years of exposure to the disease, provide partial protection [16].
Incubation of the parasite
P. Falciparum replicates repeatedly within erythrocytes over the course of 48 h, resulting in exponential growth and rapid disease progression. Following an infective bite by an Anopheles mosquito, the parasite grows and multiplies first in liver cells and then in red blood cells. The “incubation period” refers to the period before the first symptoms appear. The incubation period in most cases varies from seven to 30 days [17]. The incubation period of each parasite is nine to 14 days for Plasmodium falciparum, 12 to 17 days for Plasmodium vivax, and 18 to 40 days for Plasmodium malariae. Shorter periods were observed most frequently for P. Falciparum, and longer periods were observed for P. malariae.
Symptoms
Symptoms of malaria can develop as quickly as seven days after the infected mosquito is infected. Typically, the time between infection and symptom onset is 7-18 days, depending on the specific parasite that is infected. However, in some cases, symptoms can take up to a year to develop depending on the victim’s immunity [18].
The initial symptoms of malaria are flu-like. These included a high temperature of 38°C or above, heat and shivering, headache, vomiting, muscle pain, diarrhoea, and generally feeling unwell, just to mention a few. These symptoms are often mild and can sometimes be difficult to associate with malaria infection. In some types of malaria, symptoms occur in 48-hour cycles. During these cycles, one feels cold at first, with shivering, and then develops a high temperature, accompanied by severe sweating and fatigue. These symptoms usually persist for between 6 hours and 12 hours (Centers for Disease Control and Prevention, 2010). Without prompt treatment, this type of pregnancy can lead to the rapid development of severe and life-threatening complications, such as breathing problems and organ failure. As the symptoms are similar to those of influenza, malaria infection can be confirmed using only a malaria test.
The fatality rate
Malaria is among the leading causes of mortality and morbidity in Zambia [19]. Efforts to control, prevent, and eliminate COVID-19 have intensified over the past two decades. These efforts have contributed to a reduction in the incidence of malaria and fewer than five deaths. However, the incidence of malaria increased by 21% between 2010 and 2015. According to the World Malaria Report, there were an estimated 241 million malaria cases and 627 000 malaria deaths worldwide in 2020. These numbers represent approximately 14 million more cases in 2020 than in 2019 and 69,000 more deaths. Approximately two-thirds of these additional deaths (47,000) were linked to disruptions in malaria prevention, diagnosis, and treatment during the pandemic [20-22].
Since 2015, 24 countries have registered an increase in malaria deaths, the baseline year for the WHO’s global malaria strategy. Among the 11 countries that carry the highest burden of malaria worldwide, the number of cases increased from 150 million in 2015 to 163 million in 2020, and the number of malaria deaths increased from 390,000 to 444,600 over the same period.
Alkaloids
Classes of alkaloids: Alkaloids can be classified according to their biological system. The principal classes of alkaloids are pyrrolidines, pyridines, tropanes, pyrrolizidines, isoquinolines, indoles, quinolines, terpenoids, and steroids [23]. Alkaloids are natural plant compounds with basic characteristics that contain at least one nitrogen atom in a heterocyclic ring and exhibit biological activities. These compounds are mostly toxic and have strong physiological effects. The bioactive plant secondary metabolites include those with antimalarial, anticancer, anti-inflammatory, antimicrobial, and analgesic properties [24]. Uzor provides a very good review of the various types of alkaloids [25].
Other compounds that treat malaria: Owing to the resistance of P. Falciparum to alkaloid treatment, many compounds that treat malaria have been discovered. Some of these compounds are phenols, carboxylic acid esters, carboxylic acids, flavonoids, etc. Polyunsaturated fatty acids such as hexadecanoic acid, methyl ester, 9,12-octadecadienoic acid methyl ester (linoleic acid), 9,12,15-octadecatrienoic acid, methyl ester (linoleic acid), 9-octadecenoic acid (Z)-2-hydroxyethyl ester, eicosanoic acid, and 2-(acetyloxy)-1-((acetyloxy) methyl) ethyl ester have been found in active antiplasmodial fractions [26,27]. Butanedioic acid, mono ((3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12-Hpyrano (4,3-j)-1,2-benzodioxepin-10-yl) ester, common name Artesunate, and Artemether, with the chemical formula C16H26O5, are also used to treat malaria.
Materials and Methods
Sample size
In this study, we evaluated C. mossambicense extracts from many parts of the plant. The plant was selected because it is commonly used by locals in the area. Three extractions were conducted for each sample. The roots, stems, and leaves of each plant were extracted. Traditionally, roots have been used to prepare antimalarial herbal remedies from these plants. In this study, leaves and stems were included to investigate whether they also contained antimalarial remedies.
Collection of samples
Samples were collected from the Chikankata District in June 2021. The roots were removed from the ground using a hoe. Leaves and stems were obtained from the plants. The samples were subsequently transported from the source to Kitwe in airtight plastic bags. An image of Combretum mossambicense is shown in Figure 4.
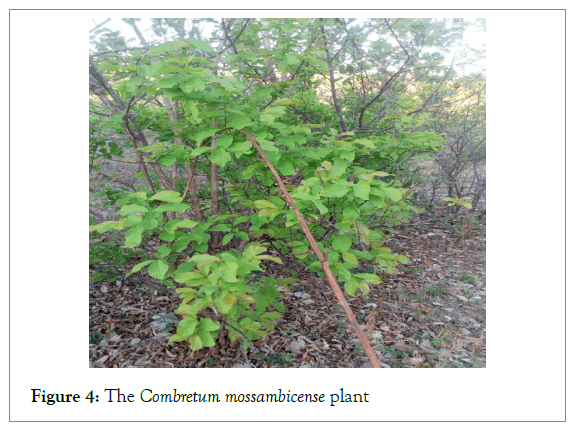
Figure 4: The Combretum mossambicense plant
Coding of Combretum mossambicense samples
Codes were developed and assigned for each part of the plant using the first letters of the names and sample numbers for easy identification of the samples. The sample codes used are listed in Tables 1 and 2.
| Code | Sample | Name |
|---|---|---|
| MZ | Plant | Botanical name: Combretum mossambicense |
| MZR 02 | Roots | |
| MZS 02 | Stems | |
| MZL 02 | Leaves |
Table 1: Sample Codes were developed and assigned for each part of the plant sample.
| Reference samples codes | |
|---|---|
| Lum | Lumartem (Coaterm) |
| Quin | Quinine |
| Sulp | Sulphadar (Fansidar) |
Table 2: Codes used for samples below listed.
Sample preparation for extraction
The roots, leaves, and stems of Combretum mossambicense plants were dried under shade. After approximately one month of drying, the samples were ground to a moderately coarse powder using a mortar and pestle. The powder was sieved and extracted. The samples were weighed into 10 g packets using a balance. The samples were steeped in 6 g of calcium hydroxide and 15 ml of sodium hydroxide. A measuring cylinder was used to measure 200 ml of ethanol, which was then transferred to a round-bottomed flask following the procedure described by Nafiah [28,29].
Extraction of alkaloids using a Soxhlet apparatus
Many methods are used for the extraction of alkaloids from herbs, and Soxhlet extraction is more effective for herb extraction. A 10- gram sample was placed in a 33 mm × 100 mm cellulose thimble, which was subsequently placed in the extraction chamber of a 200 ml Soxhlet apparatus. To prevent the sample particles from being transported to the distillation flask, cotton wool was inserted into the cellulose thimble. The Soxhlet apparatus was set up in a 500 ml distillation flask containing 200 ml of solvent. The extraction was performed at 80°C for 8 h.
Extracted samples
The extracted samples were vacuum filtered. The samples were then concentrated using a rotary thin-film evaporator. Most alkaloids are sensitive to light; therefore, the samples were packed into amber bottles. Because the decomposition of alkaloids occurs only above 70°C, the samples were stored under ambient conditions [30].
Preparation of reference samples
The conventional anti-malaria medicines used as references were purchased from milestone pharmacy in Kabulonga, Lusaka, in tablet form. The samples were analyzed at the Zambia Agriculture Research Institute (ZARI) Chemistry Laboratories in Lusaka. The following drugs were purchased for reference: Lumartem, also known as Coaterm; Sulphadar, commonly known as Fansidar; and Quinine.
Preparation of quinine ts for analysis by Gas Chromatography (GC) and High-Performance Liquid Chromatography (HPLC)
Using a crusher and pestle, a 300 mg quinine sulphate tablet was ground to powder. A 50 ml volumetric flask was filled with fifty milligrams (50 mg) of quinine sulphate. The powder was dissolved in methanol and used as a stock solution of quinine sulphate. Approximately 5 ml of the stock solution was transferred to a 50 ml volumetric flask and sonicated for 10 min before diluting with methanol to the desired concentration. Finally, the sample solution was filtered into a vial using a 0.45 mm membrane filter. As indicated in the packaging, the only active component in the tablet was quinine (248 mg), which contained 52 mg of sulphate. Using a micropipette, 1 ml of the filtered solution was transferred to and diluted in 2 ml of methanol for GC and HPLC analyses.
Preparation of Lumartem (Coartem) for GC and HPLC analysis
The lumarate pills were weighed and ground into a powder. A preparation comprising lumefantrine at a concentration of approximately 1.2 mg/ml (artemether at a concentration of approximately 0.2 mg/ml) was made using 0.2 g of powder. Methanol was acidified using acetic acid (0.5%) as a dilution solvent. Then, 2 ml of the stock solution was diluted to 10 ml. For the GC and HPLC analyses, using a micropipette, 1 μL (0.001 ml) of the sample was transferred and diluted to 2 ml. The active ingredients in the tablets were artemether (20 mg) and lumartem (120 mg), as indicated on the packet.
Preparation of Sulphadar (Fansidar) for GC and HPLC analysis
A 0.646 g tablet of Sulfadar was weighed and finely ground. The active ingredients in the tablets were sulfadoxine (500 mg) and pyrimethamine (25 mg). Approximately 0.100 g of tablet powder was transferred to a 50 ml volumetric flask and dissolved in methanol, followed by 0.002 ml of acetic acid. The mixture was sonicated for 15 min to disperse the contents completely. The volume was adjusted to the mark with acidified methanol. The sample was filtered through Whatman filter paper to obtain a stock solution. From this stock solution, 1 ml was transferred to a 10 ml volumetric test tube and diluted to the mark as a working sample. From the working sample, 1 μL was transferred to 2 ml and diluted with methanol for GC and HPLC analyses.
Instrumentation for analysis
High-performance liquid chromatography: An AT-20 highperformance liquid chromatograph with a dual solvent pump highpressure gradient system, an SPD-20A photodiode array detector, and an autosampler was used for the first-dimensional separation of alkaloids from the extract lid [31]. Chromatographic elution at pH 10.5 was conducted with a binary mobile phase gradient consisting of methanol (A) and water containing 0.2% phosphoric acid (B). The initial gradient conditions were set at 5% B at a flow rate of 1.0 ml/min before incorporating a linear gradient. HPLC was coupled with a UV and fluorescence detector. The parameters used for HPLC analysis of the samples are summarized in Table 3.
| Parameters | Values |
|---|---|
| Methanol | Mobile phase A |
| Acidified water | Mobile phase B |
| Injection volume | 8 µL |
| Location | 21 |
| Pump limit | 30 |
| Flow rate | 0.8 ml/min |
| Column | C18 (4.6 × 250 mm) |
| Column temperature | 25°C |
| Wave length | 190 to 400 nm |
| Gradient elution | From 10/90 to 100/0, V, V |
| Retention time | 30 min |
Table 3: Parameters used for HPLC for sample analysis.
All reagents were of analytical grade or similar grade, and the samples were prepared for HPLC analysis without further purification. The first sample was run for 30 min as a test sample once the apparatus was set.
Gas chromatography: Gas chromatography is an analytical technique used to separate the chemical components of a sample mixture to determine their presence or absence and how much is present. These chemical components are typically organic molecules or gases. For GC to be successful in analysis, these components need to be volatile, usually with a molecular weight less than 1250 Da, and thermally stable, so they do not degrade in the GC system [32].
GC-MS analysis: A Scion GC‒MS SQ system with a gas chromatograph interfaced with a mass spectrometer (GC-MS) equipped with an Elite-I fused silica capillary column (30 mm × 0.25 mm 1D × 1Mdf, consisting of 100% dimethyl polysiloxane) was used to analyze leaf, root, and stem samples. An electron ionization device with 70 eV ionizing energy was used for GCMS detection. The carrier gas was helium gas (99.999%) with a continuous flow rate of 1 mL/min and an injection volume of 2 L (split ratio of 10:1). The injector temperature was 25°C, and the ionization temperature was 280°C. Mass spectra were collected at 70 eV with a 0.5-second scan interval with fragments ranging from 45 to 450 Da. The GC run required 30 min to complete. A Turbo mass spectrometer was used to handle mass spectra and chromatograms, and the relative % amount of each component was computed by comparing its average peak area to the total area.
The National Institute of Standards and Technology (NIST) database, which contains more than 62,000 patterns, was used to interpret the GC-MS mass spectra [33]. The spectra of the unknown components were compared with the spectra of the known components contained in the NIST collection. The components of the test materials were identified based on their name, molecular weight, and structure.
Results
Three samples extracted from the Combretum mossambicense tree were analyzed using GC-MS and HPLC. The results and their interpretations are presented in the following sections.
Combretum mossambicense leaf
GC-MS analysis revealed 37 components in the ethanol extract of Combretum mossambicense leaves. Table 4 shows the active principles, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT).
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 5.147 | 120 | Propanoicacid, 2-mercapto-methyl ester | C4H8O2S |
| 2 | 4.97 | 76 | Propane, 2-fluoro-2-methyl- | C4H9F |
| 3 | 6.329 | 130 | 2 (3H) Furanane, dihydro-3-hydroxy-4,4-di | C6H10O3 |
| 4 | 6.251 | 130 | 2 (3H) Furanane, dihydro-3-hydroxy-4,4-di | C6H10O3 |
| 5 | 8.744 | 170 | Dodecane | C12H26 |
| 6 | 10.107 | 444 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 |
| 7 | 10.241 | 604 | Tetracontane,3,5,24-trimethyl- | C43H88 |
| 8 | 11.224 | 126 | 1,2,3-benzenetriol | C6H6O3 |
| 9 | 11.618 | 212 | Pentadecane | C15H32 |
| 10 | 12.332 | 576 | 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5- | C18H52O7Si7 |
| 11 | 12.902 | 212 | Pentadecane | C15H32 |
| 12 | 13.527 | 240 | Heptadecane | C17H36 |
| 13 | 13.765 | 282 | 2,2-dimethylloctadecane | C20H42 |
| 14 | 14.111 | 282 | Eicosane | C20H42 |
| 15 | 14.384 | 194 | Methyl-beta-D-thiogalactoside | C7H14O6 |
| 16 | 15.175 | 198 | Naphthalene,1,6-dimethyleth… | C15H18 |
| 17 | 15.954 | 88 | Silane, tetramethyl- | C4H12Si |
| 18 | 16.024 | 436 | Hentriacontane | C31H64 |
| 19 | 16.335 | 282 | Eicosane | C20H42 |
| 20 | 16.49 | 350 | Cyclohexane, nonadecyl- | C25H50 |
| 21 | 16.542 | 178 | Phenanthrene | C14H10 |
| 22 | 17.044 | 278 | 1,2-benzenedicarboxylic acid, bis (2-methyl | C16H22O4 |
| 23 | 17.441 | 652 | 2-(2’,4’,4’,6’,6’,8’,8’)-heptamethyltetrasiloxane | C16H48O10Si9 |
| 24 | 17.858 | 188 | Dodecane, 1-fluoro- | C12H25F |
| 25 | 17.958 | 256 | n- Hexadecanoic acid | C16H32O2 |
| 26 | 18.348 | 338 | Tetracosane | C24H50 |
| 27 | 18.542 | 350 | Cyclohexane, nonadecyl- | C25H50 |
| 28 | 19.175 | 340 | 1-heneicosyl formate | C22H44O2 |
| 29 | 19.647 | 254 | Cis-7-hexadecenoic acid | C16H30O2 |
| 30 | 19.846 | 282 | Oleic acid | C18H34O2 |
| 31 | 20.185 | 436 | Hentriacontane | C31H64 |
| 32 | 20.414 | 350 | Cyclohexane, nonadecyl- | C25H50 |
| 33 | 21.131 | 304 | Malonic acid, bis (2-trimethylsilyethyl ester | C13H28O4Si2 |
| 34 | 22.188 | 676 | 1,4,10-trihydroxy-5-(hydroxyethyl)-8 methyl | C30H44O10Si4 |
| 35 | 22.983 | 502 | Dodecyl phthalate | C32H54O4 |
| 36 | 24.373 | 358 | Octadecanoic acid,2,3-dihydroxypropropyl ester | C21H42O4 |
| 37 | 27.484 | 490 | 17-pentatriacontene | C35H70 |
Table 4: Compounds detected in the leaf ethanol extract of Combretum mossambicense.
Combretum mossambicense root
GC-Ms analysis revealed 45 components in the ethanol extract of Combretum mossambicense roots. Table 5 shows the active principles, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT).
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 4.978 | 120 | Propanoicacid, 2-mercapto-methyl ester | C4H8O2S |
| 2 | 6.252 | 130 | 2(3H)-Furanone, dihydro-3-hydroxy-4,4-di | C6H10O3 |
| 3 | 6.741 | 219 | 4-Methyl-Piperidine-1-Carboxylic acid | C13H17NO2 |
| 4 | 8.746 | 170 | Dodecane | C12H26 |
| 5 | 8.962 | 184 | (3H)-Furanane,5-heptydihydro | C11H20O2 |
| 6 | 9.174 | 126 | 5-hydroxymethylfurfural | C6H6O3 |
| 7 | 10.106 | 444 | Cyclohexasiloxane, Dodecamethyl | C12H36O6Si6 |
| 8 | 11.233 | 126 | 1,2,3-benzenetriol | C6H6O3 |
| 9 | 11.315 | 216 | Nonane,1,1-diethoxyl | C13H28O2 |
| 10 | 11.618 | 212 | Pentadecane | C15H32 |
| 11 | 12.329 | 576 | 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5-dioxa-2-silabicyclo(2,2,1)heptane | C18H52O7Si7 |
| 12 | 13.527 | 436 | Hentricontane | C31H64 |
| 13 | 14.108 | 240 | Heptadecane | C17H36 |
| 14 | 14.211 | 168 | Cyclopentane, 1-Hexyl-3-methylcyclopentane | C12H24 |
| 15 | 14.407 | 180 | d-mannose | C6H12O6 |
| 16 | 15.874 | 150 | 1,2,3,4,5-cyclopentanepentol | C5H10O5 |
| 17 | 15.957 | 88 | Silane, tetramethyl | C4H12Si |
| 18 | 16.333 | 296 | Heneicosane | C21H44 |
| 19 | 16.49 | 336 | Cyclohexane, Octadecylcyclohexane | C24H48 |
| 20 | 16.541 | 178 | Diphenylacetylene | C14H10 |
| 21 | 17.04 | 278 | 1,2-benzenedicarboxylic acid, bisdimethyl phthalate | C16H22O4 |
| 22 | 17.857 | 436 | Hentriacontane | C31H64 |
| 23 | 17.965 | 256 | n-Hexadecanoic acid | C16H32O2 |
| 24 | 18.28 | 340 | Eicosanoic acid, ethyl ester | C22H44O2 |
| 25 | 18.345 | 338 | Tetracosane | C24H50 |
| 26 | 18.541 | 182 | Heptycyclohexane | C13H26 |
| 27 | 19.177 | 364 | 1-hexacosene | C26H52 |
| 28 | 19.601 | 280 | 9,12-octadecadienoic acid (Z, Z)- | C18H32O2 |
| 29 | 19.653 | 280 | 17-octadecynoic acid | C18H32O2 |
| 30 | 19.731 | 238 | 7- hexadecenal, (Z)- | C16H30O |
| 31 | 19.848 | 284 | Octadecanoic acid | C18H36O2 |
| 32 | 19.913 | 282 | Oleic acid | C18H34O2 |
| 33 | 20.185 | 604 | Tritetracontane | C43H88 |
| 34 | 21.133 | 652 | 2,2,4,4,6,6,8,8-heptamethyl-2,4,6,8-tetrasiloxane | C16H48O10Si9 |
| 35 | 21.869 | 436 | Hentriacontane | C31H64 |
| 36 | 22.462 | 322 | Benzene, 1,1’-(1,2-ethanediyl) bis (1,1’-(1,2-Ethanediyl)bis(benzene) | C24H34 |
| 37 | 22.518 | 266 | Conocarpan | C18H18O2 |
| 38 | 22.816 | 358 | Octadecanoic acid,2,3-dihydroxypropyl trioleate | C21H42O4 |
| 39 | 22.985 | 390 | Phthalic acid, di(2-proylpentyl) ester | C24H38O4 |
| 40 | 24.186 | 266 | Z, E-3,13-octadecadin-1-ol | C18H34O |
| 41 | 24.373 | 358 | Octadecanoic acid, 2,3-dihydroxypropyl. | C21H42O4 |
| 42 | 24.975 | 236 | Acetic acid, Bis (trimethylsilyl) sulfide oxide, trimethylsilyl ((trimethylsilyl) thio)acetate |
C8H20O2SSi2 |
| 43 | 27.483 | 254 | 13-tetradecen-1-ol | C16H30O2 |
| 44 | 30.023 | 410 | Butyl tetracosyl ether | C28H58O |
| 45 | 30.624 | 414 | Beta-sitosterol | C29H50O |
Table 5: Compounds detected in the root ethanol extract of Combretum mossambisense.
Combretum mossambicense stem
GC-MS analysis revealed 39 components in the ethanol extract of Combretum mossambicense stems. Table 6 shows the active principle, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT) of the TSLP.
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 8.746 | 170 | Dodecane | C12H26 |
| 2 | 10.105 | 444 | Cyclohexasiloxane, dodecamethyl | C12H36O6Si6 |
| 3 | 10.969 | 254 | 9-methylheptadecane | C18H38 |
| 4 | 11.619 | 212 | Pentadecane | C15H32 |
| 5 | 12.33 | 576 | 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3- | C18H52O7Si7 |
| 6 | 12.901 | 198 | Tridecane, 6-methyl- | C14H30 |
| 7 | 13.529 | 436 | Hentriacontane | C31H64 |
| 8 | 13.767 | 436 | Hentriacontane | C31H64 |
| 9 | 14.112 | 240 | Heptadecane | C17H36 |
| 10 | 14.212 | 182 | Heptylclohexane | C13H26 |
| 11 | 14.632 | 150 | 1,2,3,4,5-cyclopentanepentol | C4H22O4 |
| 12 | 15.176 | 198 | Naphthalene,1,6-dimethyl-4-(1-methylet) | C15H18 |
| 13 | 15.721 | 436 | Hentriacontane | C31H64 |
| 14 | 15.955 | 88 | Silane, tetramethyl | C4H12Si |
| 15 | 16.025 | 436 | Hentriacontane | C31H64 |
| 16 | 16.333 | 282 | Eicosane | C20H42 |
| 17 | 16.489 | 350 | Cyclohexane, nonadecyl | C25H50 |
| 18 | 16.541 | 178 | Phenanthrene | C14H10 |
| 19 | 17.043 | 278 | 1,2-benzenedicarboxylic acid, bis(2-met) | C16H22O4 |
| 20 | 17.441 | 652 | 2-(2’,4’,4’,6’,6’,8’,8)-heptamethytetrasiloxane | C16H48O10Si9 |
| 21 | 17.856 | 436 | Hentriacontane | C31H64 |
| 22 | 17.952 | 256 | n-hexadecanoic acid | C16H32O2 |
| 23 | 18.004 | 278 | Dibutyl phthalates | C16H22O4 |
| 24 | 18.073 | 436 | Hentriacontane | C31H64 |
| 25 | 18.284 | 340 | Eicosanoic acid, ethyl ester | C22H44O2 |
| 26 | 18.349 | 338 | Tetracosane | C24H50 |
| 27 | 18.544 | 242 | 1-hexadecanol | C16H34O |
| 28 | 19.175 | 256 | n-heptadecanol-1 | C17H36O |
| 29 | 19.591 | 280 | 9,12-octadecadienoic acid (Z, Z)- | C18H32O2 |
| 30 | 19.643 | 310 | 9-Eicosenoic acid, (Z)- | C20H38O2 |
| 31 | 19.73 | 310 | Heneicosane,5-methyl- | C22H46 |
| 32 | 19.843 | 284 | Octadecanoic acid | C18H36O2 |
| 33 | 20.185 | 436 | Hentriacontane | C31H64 |
| 34 | 20.411 | 350 | Cyclohexane, nonadecyl | C25H50 |
| 35 | 21.133 | 234 | Oxalic acid, 2TMS derivative | C8H20O2SSi2 |
| 36 | 22.981 | 390 | bis (2-ethylhexyl) phthalate | C24H38O4 |
| 37 | 24.978 | 236 | Mercaptoacetic acid, 2TMS derivative | C8H20O2SSi2 |
| 38 | 24.978 | 236 | Mercaptoacetic acid, 2TMS derivative | C8H20O2SSI2 |
| 39 | 25.598 | 436 | Hentriacontane | C31H64 |
Table 6: Compounds detected in the stem ethanol extract of Combretum mossambicense.
GC-MS results for reference samples
Coartem: The ethanol extract of Coartem was analyzed by GC-MS, and eleven components were detected. Table 7 shows the active principle, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT).
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 10.110 | 444 | Cyclohexasiloxane, Dodecamethylcyclohexasiloxane | C12H36O6Si6 |
| 2 | 12.330 | 576 | 3-isopropoxyl-1,1,1,7,7,7-hexamethyl-3, 5-dioxatetrasilabicyclo(3.3.0)octane | C18H52O7Si7 |
| 3 | 13.039 | 206 | 2,4-Di-tert-butylphenol | C14H22O |
| 4 | 15.170 | 238 | 1,4-Dihydroxy-1,2,3,3a,4,5,6,8a-octahydroazulene |
C15H26O2 |
| 5 | 16.300 | 180 | 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propen-1-ol | C12H20O |
| 6 | 17.524 | 205 | (5R, 8R,8aS)-8-Methyl-5- (pent-4-yn-1-yl) tetradecanamine | C14H23N |
| 7 | 18.002 | 256 | n-hexadecanoic acid | C16H32O2 |
| 8 | 18.054 | 222 | 5-mthoxy-10,10-dimethyl-6-methylenebic | C14H22O2 |
| 9 | 18.930 | 236 | 5-mthoxy-10,10-dimethyl-6-methylenebic | C15H24O2 |
| 10 | 19.536 | 186 | Methyl-8-methyl-nonaoate | C11H22O2 |
| 11 | 19.901 | 264 | Octadecanoic acid | C18H36O2 |
Table 7: Compounds detected in the ethanol extract of Coartem.
Sulphadar: By GC‒MS analysis, 10 components in the Sulphadar ethanol extract were detected. Table 8 shows the active principle, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT) of the TSLP.
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 5.839 | 93 | Aniline | C6H7N |
| 2 | 8.674 | 182 | 6-tridecane, Z)- | C13H36 |
| 3 | 12.384 | 152 | Benzoic acid, 4- hydroxyl- hydrazide | C7H8N2O2 |
| 4 | 12.604 | 328 | Carbonic acid, ethyl heptedecyl ester | C20H40O3 |
| 5 | 14.197 | 228 | Lauryl acetate | C14H28O2 |
| 6 | 15.041 | 260 | Hexadecane,1- chlorohexadecane. | C16H33Cl |
| 7 | 17.970 | 256 | n-Hexadecanoic acid | C16H32O2 |
| 8 | 19.965 | 284 | Octadecanoic acid | C18H36O2 |
| 9 | 20.328 | 248 | Pyrimethamine | C12H13ClN4 |
| 10 | 25.742 | 310 | Sulfadoxine | C12H14N44O4S |
Table 8: Compounds detected in the ethanol extract of Sulphadar.
Quinine: A GC‒MS study of Quinine’s ethanol extract revealed the presence of twelve components. Table 9 shows the active principle, Molecular Formula (MF), Molecular Weight (MW), and Retention Time (RT).
| Index | Retention time | Molecular weight | Name | Formula |
|---|---|---|---|---|
| 1 | 10.110 | 444 | Cyclohexasiloxane, Dodecamethylcyclohexasiloxane. | C12H36O6Si6 |
| 2 | 4.032 | 151 | Oxime, methoxy-phenyl-oxime | C8H9NO2 |
| 3 | 12.374 | 152 | Benzoic acid,4-hydroxy-hydrazide | C7H8N2O2 |
| 4 | 12.374 | 152 | Benzoic acid, 4-hydroxy-hydraide | C7H8N2O2 |
| 5 | 14.066 | 222 | Diethyl phthalate | C12H14O4 |
| 6 | 17.625 | 228 | Methyl 11-methyl-dodecanoate | C14H28O2 |
| 7 | 17.963 | 256 | n-hexadecanoic acid | C16H32O2 |
| 8 | 19.848 | 284 | Octadecanoic acid | C18H36O2 |
| 9 | 20.246 | 248 | Pyrimethamine | C12H13ClN4 |
| 10 | 25.580 | 356 | Quinine 1,1’-dioxide, (9S)- ibogaine | C20H24N2O2 |
| 11 | 25.874 | 324 | Quinine | C20H24N2O2 |
| 12 | 26.286 | 324 | 4- (5-ethylquinulidine-2-carbonyl)-6- ergotamine | C20H24N2O2 |
Table 9: Compounds detected in the ethanol extract of quinine.
Analysis of Results
The data were organized into a Table 9, with color codes used to designate substances of interest. All common components were combined for analysis. Alkaloids are indicated in green, active chemicals for malaria treatment are indicated in yellow, and common molecules found in reference medications are indicated in red. Table 10 shows the results for the Combretum mossambicense plant.
| Compounds | Formula | Retention time | Molecular weight | Activity |
|---|---|---|---|---|
| Propanoic acid, 2-mecapto- methyl ester | C4H8O2S | 5.147 | 120 | Used as a solvent in pharmaceuticals |
| Propane-2-fluoro-2-methyl | C4H9F | 4.970 | 76 | insecticide |
| 2(3H)-Furanone, dihydro-3-hydroxy-4-4-di |
C4H10O3 | 6.329 | 130 | Causing relaxation. Increasing mental clarity Relieving depression and stress. |
| Dodecane | C12H26 | 8.744 | 170 | Antibacterial activity and antifungal activity. |
| Cyclohexasiloxane, dodecamethyl | C12H36O6Si6 | 10.107 | 444 | Medical devices, blood-handling equipment, as a blood defoaming agents, protective barriers, lubricants, and surface treatment of wound dressings |
| Tetracontane,3,5,24-trimethyl | C43H88 | 10.241 | 604 | Anti-inflammatory |
| 1,2,3- Benzenetriol | C6H6O3 | 11.224 | 126 | Antimicrobial, Anti-inflammatory, antioxidant, Analysis, insecticide, anticancer, cytoxic |
| Pentadecane | C15H32 | 11.618 | 212 | Used in organic synthesis and as a solvent. |
| 3-isopropoxyl-1,1,1,7,7,7-hexamethyl-3-5 | C18H52O7Si7 | 12.332 | 576 | Antimicrobial |
| Heptadecane | C17H36 | 12.902 | 212 | Antifungal |
| 2,2-dimethyoctadecane | C20H42 | 13.527 | 282 | Antimicrobial |
| Eicosane | C20H42 | 13.765 | 282 | Antibacterial, antifungal, antitumor, antimicrobial, larvicidal |
| Methyl-beta-d-thiogalactoside (maaliol) |
C7H14O6 | 14.111 | 194 | Antinociceptive Anticancer |
| Naphthalene,1,6-dimethyl-4-(1- methyllethane | C15H18 | 14.384 | 198 | Antioxidant, Antibacterial |
| Silane, tetramethyl | C4H12Si | 15.175 | 88 | used as a starting material for synthesizing more complex organosilanes, |
| Hentriacontane | C31H64 | 15.954 | 436 | Used to treat diseases such as skin diseases, ulcers, diabetes, piles, dysentery, asthma, gonorrhea, gleets, leucorrhoea, and urinary diseases. |
| Cyclohexane, nonadecyl | C25H50 | 16.024 | 350 | |
| Phenanthrene | C14H10 | 16.335 | 178 | Used to make dyes, plastic, pesticides, explosives and drugs |
| 1,2-benzenedicarboxylic acid, bis (2- methyl propyl ester) | C16H22O4 | 17.044 | 278 | antibacterial |
| 2-(2’,4’,4’,6’,6’,8’,8’, -heptamethyltetrasiloxane | C16H48O10Si9 | 17,441 | 652 | Antifungal |
| Dodecane, 1-fluoro-dodecane | C12H25F | 17.854 | 188 | |
| n-Hexadecanoic acid | C16H32O2 | 17.958 | 256 | Antioxidant, anti-inflammatory, hypochglestero lenic, nematicide, pesticide, lubricant, antiandrogenic, flavor |
| Tetracosane | C24H50 | 18.384 | 338 | Treatment of nervous debility, insomnia, fatigue, low energy level, and brain tonic for memory functions. |
| Cyclohexane, nonadecyl | C25H50 | 18.542 | 350 | |
| Hexanedioic, bis (2-ethylhexyl) ester | C22H44O2 | Antifungal | ||
| Cis-7-hexadecenoic acid | C16H30O2 | 19.647 | 254 | antibacterial |
| Oleic acid | C18H34O2 | 19.846 | 282 | Anti-inflammatory, anti-androgenetic cancer preventive |
| Malonic acid, bis (2-trimethy silyl ethyl ester) | C13H38O4Si2 | 21.131 | 304 | anti-inflammatory effect, bactericidal |
| 1,4,10-trihydroxy-5-(hydroxymethyl) -8-methyl | C30H44O10Si4 | 22.188 | 676 | antimicrobial |
| Didode cyl phthalate | C32H54O4 | 22.983 | 502 | Used as a solvent and vehicle for fragrance and cosmetic ingredients, as well as an alcohol denaturant – that is, an additive to alcohol to make it unfit to drink. |
| Octadecanoic acid,2,3-dihydroxypropyl | C21H42O4 | 24.373 | 358 | Anticancer, antimicrobial |
| 17-pentatriacontene | C35H70 | 27.484 | 490 | anti-inflammatory, anticancer, antibacterial, and ant-arthritic properties |
| 4-methylpiperidine-1-carboxylic acid, | C13H17NO2 | 6.741 | 219 | Anti-inflammatory, and rheumatic disorders used in ophthalmological eyedrops to enlarge pupils. |
| 2(2H)-Furanone,5-heptydihydro | C11H20O2 | 8.962 | 184 | Antifungal, antibacterial |
| 5-hydroxymethylfurfural | C6H6O3 | 9.174 | 128 | antioxidants |
| Nonane,1,1-dethoxy | C13H28O2 | 11.315 | 216 | Give a strong fruity aroma |
| Cyclopentane,1-hexyl-3-methyl | C12H24 | 14.211 | 168 | It has a role as a human metabolite and a mammalian metabolite. |
| d-mannose | C6H12O6 | 14.407 | 180 | Use to treat a rare disease called carbohydrate-deficient glycol protein syndrome type 1b |
| 1,2,3,4,5- cyclopentol | C5H10O5 | 15.874 | 150 | Used for pharmaceuticals, dyes, and spices production, it is also used as a solvent for drugs and spices. |
| Cyclohexane, octadecyl | C24H48 | 16.490 | 336 | Used for organic synthesis |
| biphenyl acetylene | C24H10 | 16.541 | 178 | It is used as a building block in organic synthesis and as a ligand in organometallic chemistry. |
| 1,2-benzenedicarboxylic acid, bis (2-methyl ester) | C16H22O4 | 17.040 | 278 | Antimicrobial, antifouling |
| Eisosanoic acid, ethyl ester | C22H44O2 | 18.280 | 340 | Helps to store the skin's natural oils |
| Tetracosane | C24H50 | 18.345 | 338 | Used for organic synthesis |
| heptylcyclohexane | C13H26 | 18.541 | 182 | |
| 1-hexacosane | C26H52 | 19.177 | 364 | antimicrobial activity |
| 9,12-octadecadienoic acid (z, z) methyl ester | C18H32O2 | 19.601 | 280 | Antibacterial, antiplasmodial activity |
| 17-octaecynoic acid | C18H32O2 | 19.653 | 280 | Antibacterial, anti-inflammatory Antiplasmodial |
| 7-hexadecenal, (z) | C16H30O | 19.731 | 238 | |
| Octadecanoic acid | C16H36O2 | 19.848 | 284 | Antibacterial |
| Benezene,1,1-(1,2-ethane diyl) bis (2,3,4,5---- | C24H34 | 22.462 | 322 | Antinociceptive anti-Inflammatory |
| Conocarpan | C18H18O2 | 22.518 | 266 | Anticancer, antimicrobial |
| Octadecanoic acid, 2,3-dihydroxy propxyl | C21H42O4 | 22.816 | 358 | Antimicrobial antifouling, antibacterial activity |
| Phthalic acid, di(2-propylpentyl) ester | C24H38O4 | 22.985 | 390 | Antibacterial used to treat TB, anti-malarial |
| Z, E-3,13-octadecadien-1-ol | C18H34O | 24.186 | 266 | Helps to lose weight |
| 13-tetradecen-1-ol acetate | C16H30O2 | 27.483 | 254 | Used for treatment of Parkinson's disease |
| Butyl tetra cosyl ether | C28H58O | 30.023 | 410 | Used to lower the level of lipids in the blood |
| Beta-sitosterol | C29H50O | 30.624 | 414 | Anti-inflammatory, antipyretic, anti-ulcer, and arthritic antiplasmodial |
Table 10: The results for the Combretum mossambisense plant.
Discussion
The samples collected from the leaf stems and roots presented in Table 7 show the results for the Combretum mossambicense plant used in this study. Due to space constraints, some of the GC MS data did not provide a complete name, but the chemical formula helped identify what they were.
Results for an extract of parts of Combretum mossambisense
Leaf extract results and analysis (MZL02): Table 7 shows the results of the GC‒MS investigation, which revealed a total of 37 chemicals. The peaks were visible in the GC‒MS chromatogram. Some of the chemicals found were propanoic acid, 2-mercaptomethyl ester, 3-isopropoxyl-1, 1, 1, 7, 7,7-xxamethyl-3, 5-, malonic acid, bis(2-trimethylsilyyl ester), Octadecanoic acid, and 2,3-dihydroxypropropyl ester. There were no alkaloids found. Table 3 shows the results of the leaf extracts of Combretum mossambisense, and none of the substances found had antiplasmodial activity according to the available literature.
Root extract results and analysis (MZR02): GC‒MS analysis revealed 45 compounds (Table 4). Only one alkaloid was detected, and it is used to treat anti-inflammatory and rheumatic illnesses, as well as to widen pupils in ophthalmological eye drops.
MZR02 contained many compounds, including n-hexadecanoic acid, eicosanoic acid, ethyl ester, 9,12-octadecadienoic acid (z,z), 17-octadecynoic acid, octadecanoic acid, and beta-sitosterol. The roots contained the greatest number of antimalarial chemicals, four of which were identified: 9,12-octadecadienoic acid (Z,Z)-methyl ester, 17-octadecynoic acid, phthalic acid, di (2-propyl pentyl) ester (also known as bis (2-ethylhexyl) phthalate), and beta-sitosterol [34-36].
Stem extract results and analysis (MZS02): The ethanol extract of MZS02 (Table 5) yielded a total of 39 compounds. Some of the detected compounds were heptadecane, cyclopentane, 1-hexyl-3-methyl, hentriacontane, tetradecane, 4-ethyl, silane, tetramethyl, 1,2-benzenedicarboxylic acid, bis (2-met., eicosane, 2-(2’,4’,4’,6’,6’,8’,8)-heptamethyletrasiloxane, phthalic acid, and 2-chloropropyl isobutyl ester.
No alkaloids were found, but two antiplasmodial or antimalarial compounds were detected. These include 9,12-octadecadienoic acid (Z,Z)-methyl ester, , phthalic acid, and di (2-propyl pentyl) ester, also known as bis (2-ethylhexyl) phthalate.
Analysis of conventional malaria drugs
Many diseases, including malaria, have been treated using single component medications in recent decades. Combination therapy, a new technique that is effective against other multidrug-resistant illnesses, such as Human Immunodeficiency Virus (HIV) and tuberculosis, is now widely suggested for malaria treatment [37]. As a result of the rapid increase in drug resistance among Plasmodium parasites worldwide, combination therapy has gradually supplanted single-drug treatment of malaria [38]. Combination therapy, particularly quinine, which has been linked to Plasmodium parasite resistance, was used in combination with traditional drugs [39]. Researchers are working on novel medications to combat malaria, in addition to combination therapy with alkaloid drugs.
Use of other compounds to treat malaria
There is advanced research on other compounds in the treatment of malaria, apart from alkaloids, as the resistance of the parasite to drugs increases. Esters, ethers, and phenols are some of these compounds. Stigmasterol, p-hydroxycinnamic acid ethyl ester, docosanoic acid ethyl ester, octadecanoic acid methyl ester, and 9-octadecenoic acid (Z)-ethyl ester were obtained. Hexadecanoic acid, methyl ester, 9,12-octadecadienoic acid, methyl ester (linoleic acid), 9,12,15-octadecatrienoic acid, methyl ester (linoleic acid), 9-octadecenoic acid (Z) eicosanoic acid, 2-(acetyloxy)-1-((acetyloxy) methyl) ethyl ester and 2-hydroxyethyl ester are polyunsaturated fatty acids that exhibit anti-plasmodial action. This activity is said to increase as the degree of unsaturation increases. According to previous research on bis(2-ethylhexyl) phthalate, it has a similar effect on malaria parasites as artesunate, scientifically called ((3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-3,6,9-trimethyl- 3,12-epoxy-12 Hpyrano ((4,3-j)-1,2-benzodioxepin-10-yl) ester, an effective conventional malaria drug.
Conclusion
In this work, a phytochemical investigation of Combretum mossambisense, a plant whose herbal extract is utilized as a potent herbal anti-malaria remedy, was performed to establish whether the plant contains alkaloids found in anti-malaria drugs. However, it was established that the plant does not contain any antimalarial alkaloids. However, it was observed that the plant contains chemicals similar to those found in conventional malaria medicines. Compounds such as octadecanoic acid, n-hexadecanoic acid, cyclohexasiloxane, tetrasiloxane 3-isopropoxyl-1, 1, 1, 7, 7, and 7-xamethyl-3, 5, and 5 TIS (trimethylsiloxyl) were detected in the herb, as were conventional medications. Combretum mossambisense, as well as Coartem and quinine, contains n-hexadecanoic acid. Quinine and Combretum mossambicense contain cyclohexasiloxane. The tetrasiloxane 3-isopropoxyl-1, 1, 1, 7, 7,7-hexamethyl-3, 5, 5 TIS (trimethylsiloxyl) was discovered in Combretum mossambicense and Coartem. Several comparable chemicals were also observed.
Other forms of alkaloids were found, but the data indicated that they could be useful for treating other conditions but not malaria. Norepinephrine (R), a 4TMS derivative, is an example of such an alkaloid. It is used to treat life-threatening low blood pressure (hypotension), which can arise because of certain medical conditions or surgical procedures.
It was observed from the data gathered in this study that malaria is treated by more than just alkaloids. Other chemicals are also effective. The following compounds are reported to have favorable effects on the malaria parasite P. Falciparum: 9,12-octadecadienoic acid (Z, Z), methyl ester, and bis (2-ethylhexyl) phthalate, which were found in the Combretum mossambicense extract. The Combretum mossambicense also contained 17-octadecynoic acid and betasitosterol. According to the literature, 17-octadecynoic acid inhibits both Plasmodium infections and plasmodial FAS-II enzymes, while beta-sitosterol in combination with other compounds shows potential antiplasmodial activity. Because of these properties, it would be safe to conclude that the Combretum mossambicense extract is an effective non-alkaloid-based antimalarial herbal remedy. These results are important because of the observed resistance of P. Falciparum to alkaloid-based antimalaria drugs. This could also help to explain why some modern conventional anti-malaria drugs are nonalkaloid based. Further studies on Combretum mossambicense investigating the efficacy and toxicity of this herbal remedy will be reported in a subsequent publication.
References
- CDC-Centers for disease control and prevention. 2010.
- Intensifying the fight against malaria. 2008.
- Bank W. Bioactive alkaloids from medicinal plants of Lombok.2002.
- Vivekraj A, Vijayan A, Anandgideon V, Muthuselvam D. Phyto-chemical Profiling of Abutilon hirtum (lam.) Sweet. Leaf Extracts Using GC-MS analysis. World J Pharm Res. 2015; 4(3):1270-12755
- Dey P, Kundu A, Kumar A, Gupta M, Lee BM, Bhakta T, et al. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Rec Adv in Nat Prod Anal 2020; 505-567
- Aniszewski T. Alkaloids-secrets of life: Aklaloid chemistry, biological significance, applications and ecological role. Elsevier. 2007.
- Alamgir AN. Therapeutic use of medicinal plants and their extracts: Volume 1. Springer Int Pub AG. 2017.
- Hermans M, Akoègninou A, van der Maesen J. Medicinal plants used to treat malaria in southern Benin. Econ Bot. 2004; 58(1):239-252.
- Bikash D, Jashim U, Prasenjit P, Manik D, Debasish MC, Kuntal. Estimation of alkaloids and phenolics of five edible cucurbitaceous plants and their antibacterial activity’. Int J Pharm Sci. 2005; 7(12): 223-227.
- World Health Organization. (2020). World malaria report: 20 years of global progress and challenges.
- Ross I.A. Medicinal plants of the world. 2001.
[Crossref]
- Kabula B, Tungu P, Matowo J, Kitau J, Mweya C, Emidi B, et al. Susceptibility status of malaria vectors to insecticides commonly used for malaria control in Tanzania. Trop Med Int. Health 2012;17(6):742-750.
- Mosquito Life Cycle | US EPA. 2021.
- Rasmussen C, Alonso P, Ringwald P. Current and emerging strategies to combat antimalarial resistance. Expert Rev Anti Infect Ther. 2022; 20(3):353-372.
- Bilia AR, Lapenna S, Bergonzi MC, Vincieri FF. NPC Natural product communications 2008. NPC Nat Prod Com. 2008.
- World Malaria Report: 20 years of global progress and challenges. 2020.
- CDC - Malaria - malaria worldwide - Impact of Malaria. 2015.
- Blenkinsopp A, Duerden M, Blenkinsopp J. Symptoms in the pharmacy: A guide to the management of common illnesses. John Wiley Sons. 2022 .
- Nawa M, Hangoma P, Morse AP, Michelo C. Investigating the upsurge of malaria prevalence in Zambia between 2010 and 2015: A decomposition of determinants. Malaria J. 2019;18:1-0.
- World Malaria Report: 20 years of global progress and challenges. 2020.
- World Malaria Report. 2021.
- World Malaria Report 2015-World Health organization-google books. In WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland. 2015.
- Kurek J. Alkaloids: Their importance in nature and human life. BoD–Books on Demand. 2019.
- Nchabeleng EK. Determination of biological activity of Celtis africana extracts and its endophytic microflora and mycoflora.2017.
- Uzor PF. Alkaloids from plants with antimalarial activity: A review of recent studies. Evid Based Complement Alternat Med. 2020; 2020(1):8749083.
- Mustofa SE, Wahyuono S. In vitro and in vivo antiplasmodial activity and cytotoxicity of extracts of Phyllanthus niruri L. herbs traditionally used to treat malaria in Indonesia. Southeast Asian J Trop Med Public Health. 2007;38(4):609-15.
- Okokon JE, Augustine NB, Mohanakrishnan D. Antimalarial, antiplasmodial and analgesic activities of root extract of Alchornea laxiflora. Pharm Biol. 2017; 55(1):1022-31.
- Okokon JE, Antia BS, Mohanakrishnan D, Sahal D. Antimalarial and antiplasmodial activity of husk extract and fractions of Zea mays. Pharm Biol. 2017 ;55(1):1394-400.
- Nafiah MA, Khoo J, Chen H, Mohammad SN, Awang K, Hadi AH, et al. Extraction and isolation of alkaloids from the leaves of Alseodaphne corneri Kosterm. Malaysian J Chem. 2013;15(1):27-32.
- Rotary Evaporation-an overview.2020.
- Li L, Long W, Wan X, Ding Q, Zhang F, Wan D. Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “Sankezhen”. J Chrom Sci. 2015 ;53(2):307-11.
- Banakar P, Jayaraj M. GC-MS analysis of bioactive compounds from ethanolic leaf extract of Waltheria indica Linn. and their pharmacological activities. Int J Pharm Sci Res. 2018;9(5):2005-10.
- Rajadurai MV, Maithili RA, Yogesh V. Phytochemical profiling of medically significant crude extract using GC-MS analysis. Int J Curr Pharm. Res. 2021;10:16-20.
- Tajuddeen N, Van Heerden FR. Antiplasmodial natural products: An update. Malaria J. 2019;18:1-62.
- Enenebeaku UE, Duru CE, Mgbemena IC, Ukwandu NC, Nwigwe HC, Enenebeaku CK, et al. Phytochemical evaluation and molecular docking of bioactive compounds from the roots of Dictyandra arborescens (Welw.) against Plasmodium berghei protein targets. CABI Database. 2021; 5(2): 370-381.
- Gakunju DM, Mberu EK, Dossaji SF, Gray AI, Waigh RD, Waterman PG, et al. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob Agents Chemother. 1995;39(12):2606-9.
- Marimani M. Combination therapy against multidrug resistance. Comb Ther Ag Multid Res. 2020; 39-64.
- Hunt P, Martinelli A, Modrzynska K, Borges S, Creasey A, Rodrigues L, et al. Experimental evolution, genetic analysis and genome re-sequencing reveal the mutation conferring artemisinin resistance in an isogenic lineage of malaria parasites. BMC Genomics. 2010;11:1-3.
- Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg. 2004;71(2):179-86.
Citation: Chikambwe MV, Mubambe P, Maseka KK, Banda L (2024) Therapeutic Potential of Combretum mossambicense Extracts Against P. Falciparum Parasite. J Bacteriol Parasitol.S27:99.
Copyright: © 2024 Chikambwe MV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

