Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Paper - (2019) Volume 7, Issue 6
Therapeutic Efficacy of Coartemî in Treatment of Simple Plasmodium falciparum Malaria in Woreta, South Gonder Zone, Ethiopia
Sintayehu Tsegaye Tseha*Received: 06-Jul-2019 Published: 14-Aug-2019
Abstract
Background: The aim of the study was to investigate the therapeutic efficacy of Coartem® in the treatment of simple P. falciparum malaria in Woreta Town, South Gonder Zone, Ethiopia.
Methods: 2240 febrile patients attending the health center were screened and capillary blood was obtained by finger prick. Giemsa stained thick and thin blood smears were prepared and used for parasite density and species identification. Of the 2240 patients tested, 88 with confirmed falciparum malaria were enrolled and treated with Coartem®. Haemoglobin concentration of the study participants was measured on day 0, 14 and 28. Of the 88 patients enrolled, five were lost to follow and five were excluded from treatment response analysis due to protocol violation. As a result, 78 patients were evaluated for treatment outcomes.
Results: The adequate clinical and parasitological response was 100% at day 28. Fever clearance was fast with only 1.7% being febrile at day three. Parasite clearance was rapid with almost all patients (98.9%) being free of parasitmia on day 2. A significant increase in haemoglobin level was observed on day 28.
Conclusion: Thus, these findings further support the use of Coartem as an effective treatment of simple P. falciparum malaria in the infectious disease area.
Keywords
Coartem®; Cure rate; Fever clearance; Parasite clearance; P. falciparum
Introduction
Malaria remains one of the most widespread parasitic diseases in tropical and subtropical regions of the world. The estimated number of cases of malaria in 2011 was 216 million. There were an estimated 655,000 deaths each year in the world, of which 91% were in Africa [1,2].
In Ethiopia, 75% of the area is malarious; making the disease major public health problem in the country. One of the challenges of controlling malaria is the evolution of resistant strains of Plasmodium against antimalarial drugs [3]. Coartem® has used as first-line therapy of simple Plasmodium falciparum malaria in Ethiopia since it replaced sulfadoxine-pyrimethamine in 2004. Studies done in different parts of Asia showed P. falciparum resistance against artemisinin-based therapies [4]. Furthermore, a report from Ethiopia indicated low-level resistance of P. falciparum to Coartem® treatment [5]. Therefore, the aim of this study was to investigate the therapeutic efficacy of Coartem® in the treatment of simple P. falciparum malaria in Woreta Town, South Gonder Zone, Ethiopia.
Materials and Methods
Study area
The study was conducted between November 2010 and January 2011 in Woreta Town, South Gonder Zone, Ethiopia. It is located in the Debub Gondar Zone of the Amhara Region, east of Lake Tana and south of Addis Zemen, with an elevation of 1828 meters above sea level [6].
Sample size determination
The study was conducted based on the revised WHO recommendation for the assessment and monitoring of antimalarial drug efficacy [7]. According to the WHO protocol for estimating the population proportion, a minimum sample size of 73 patients are required. This calculation is based on expected clinical failures of 5%, 95% confidence interval, and 5% precision.
The formula N=(z/d)2 p(1-p) was used for the calculation.
N=(1.96/0.05)20.05(1-0.05)=73
Therefore, once the minimum number of the sample obtained, by adding 20% contingency, (73+15), 88 patients enrolled for the study.
Malaria diagnosis and quantification of parasite density
Blood films were taken at least eight times for each study participants during the study period (day 0, 1, 2, 3, 7, 14, 21 and 28) and on any unexpected visit. Giemsa stained thick and thin blood smears were prepared on the same slide from each study participants in each follow-up day. Thin smears were used for species identification and thick smear used for determination of parasite density. The slides were examined under a light microscope using 100x oil immersion objective lens.
The number of parasites per microliter of blood was calculated using the formula:
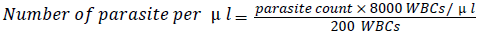
Treatment and follow-up
Enrolled patients were treated with the standard six dose regimen of Coartem, given twice daily for three consecutive days. The tablets were administered at 0, 8, 24, 36, 48 and 60 hours. Dosing was administered according to the WHO weightbased guideline [7].
Coartem was given based on the weight on days 0, 1, and 2. Follow up was done on days; 1, 2, 3, 7, 14 21 and 28. At all visits, their health condition was assessed and axillary temperature and the blood sample was taken for parasitological assessment. Haemoglobin level was measured on days 0, 14 and 28.
Measurement of body temperature
Axillary temperature was measured at all visits using a digital thermometer. Any measured temperature below 36°C was repeated.
Haemoglobin measurement
Finger-prick blood samples were collected from each patient on day 0 for determination of ANAEMIA among the study participants. Furthermore, haemoglobin level was measured on day 14 and 28 to assess the recovery of ANAEMIA, as follows. The fingertip was cleaned with alcohol and prinked with sterile lancet wiped away the first drop. Then, the next drop of blood was used to fill the microcuvette. Finally, the micro cuvette was pushed into haemocue analyzer and the displayed value was recorded in g/dl.
Classification of treatment responses
Early Treatment Failure (ETF): Presence of any of the following:
Development of danger signs or severe malaria on day 1, 2, or 3 in the presence of parasitemia; Parasitaemia on day 2 higher than day 0 count irrespective of axillary temperature; Parasitaemia on day 3 with axillary temperature ≥ 37.5°C; Parasitaemia on day 3, ≥ 25% of count on day 0.
Late Clinical Failure (LCF): The presence of any of the following:
Development of danger signs or severe malaria after day 3 in the presence of parasitemia without previously meeting any of the criteria of ETF; the presence of parasitemia and axillary temperature ≥ 37.5°C or history of fever on any day from day 4 to day 28 without previously meeting any of the criteria of ETF.
Late Parasitological Failure (LPF ): Presence of any of the following:
The presence of parasitemia on any day from day 7 to day 28 and irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical failure.
Adequate Clinical and Parasitological Response (ACPR): Presence of any of the following:-The absence of parasitemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure, late clinical failure, or late parasitological failure.
Ethical clearance
Ethical clearance was obtained from Addis Ababa University and written informed consent was obtained from each participant or guardians of those under 18 years old.
Data analysis
Data of patients excluded from the study due to protocol violation and lost to follow up were excluded from treatment outcome analysis. Data were double entered in the WHO excel spreadsheet designed for therapeutic efficacy data. Paired T-test was used to compare haemoglobin changes from baseline to day 14 and day 28 and a two-sided p-value <0.05 was considered statistically significant. Microsoft Excel was used to show parasite and fever clearance following Coartem treatment.
Results
Baseline characteristics of the study participants
A baseline characteristic of the study participants was shown in Table 1. During the enrollment period (between November 2010 and January 2011), a total of 2240 blood films of patients suspected to have malaria were screened for eligibility in Woreta health center and of this, 88 with confirmed falciparum malaria were enrolled and treated with Coartem®. Age stratification showed a large number of the study population (65%, n=57) were adults (>15 years), 19% were between 5 and 15 years and the remaining 12% were children under five years.
| Characteristics | Age category in yeas | ||||
|---|---|---|---|---|---|
| <5 years (n=12) | 5-15years (n=19) | >15 years (n=57) | Total | ||
| Sex | Male | 7 | 8 | 44 | 59 |
| Female | 5 | 11 | 13 | 29 | |
| Average age in years | 2.6 (0.83-4) | 8.4(5-14) | 49.4 (30-50) | 17.5 | |
| Average weight in kg | 10.5(8-15) | 19.8 (10-56) | 49.4 (30-70) | 26.6 | |
| Average parasitemia/µl | 23, 788 (500-95,000) | 11, 981 (800-40,000) | 13, 840 (1,640-95, 000) | 14, 362 (500-85,000) | |
| % febrile patients | 91.7% | 84.2% | 56.1% | 68.2% | |
| Average axillary temperature in °C | 38.8°C (37.2-403) | 38.9°C (36.7-40.3) | 38.1°C (36-40.8) | 38.4°C (36-40.8) | |
| Average haemoglobin concentration (in g/dl) | 11.1 (8.1-13.6) | 12.3 (6.7-16.5) | 13.6 (8.8-18.7) | 13 (6.7-18.7) | |
| % anaemic cases | 58.3% | 26.3% | 26.3% | 30.7% | |
Table 1: Baseline characteristics of simple P. falciparum malaria patients enrolled in the in vivo efficacy study of court in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
The average parasite count was 14, 362/μl (500-85,000) and the highest mean parasite count (23, 788) was observed in children under five years as compared with other age groups. The mean haemoglobin concentration was 13 g/dl (6.7-18.7). Of the 88 study participants, 60 were febrile at day 0 and 30.7% were anaemic. About 58% of children under five years, 29.4% of children between 5 and 15 years and 33.3% of adults were anaemic at the day of admission. The average axillary temperature was 38.4°C. The average weight of the study participants was 26.6 Kg (10.5 Kg-70 Kg).
Parasite clearance
Treatment resulted in rapid clearance of parasites. Parasites were cleared from 72.7% patients by day 1 and only one patient remained positive till day 3 (Figure 1).
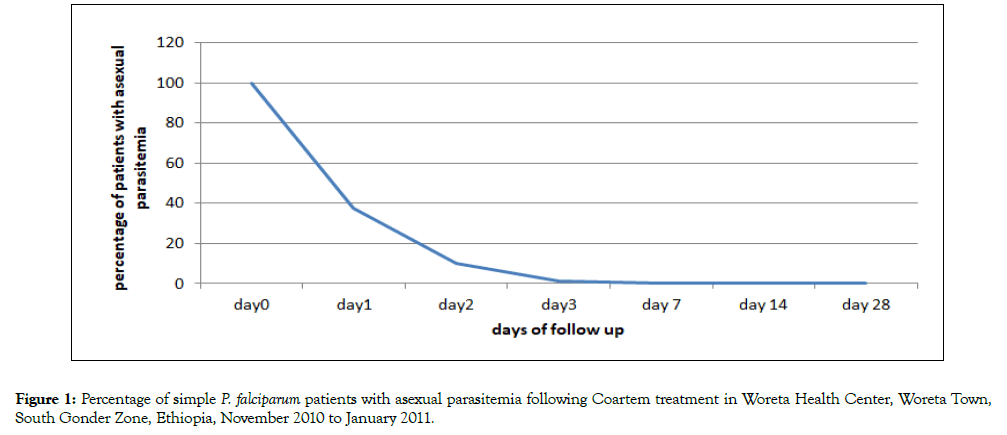
Figure 1. Percentage of simple P. falciparum patients with asexual parasitemia following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
Fever clearance
Fever clearance was also rapid. Fever was cleared from almost all patients on day 3, (Figure 2).
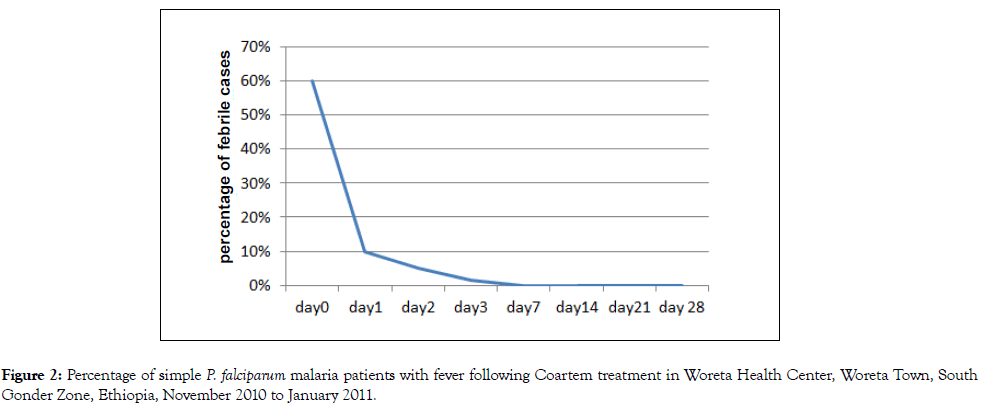
Figure 2. Percentage of simple P. falciparum malaria patients with fever following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
The cure rate of coartem
Of the 88 study participants, ten were excluded from treatment outcome analysis due to protocol violation and lost to follow up. As a result, 78 subjects were included in the treatment outcome analysis. Coartem showed 100% adequate clinical and parasitological response (Table 2).
| Treatment outcomes | Number (Percent) |
|---|---|
| Early treatment failure | 0 (0) |
| Late clinical failure | 0 (0) |
| Late parasitological failure | 0 (0) |
| Adequate clinical and parasitological response | 78 (100) |
Table 2: Cure rate of coartem against simple P. falciparum patients in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
Improvement in haemoglobin level
A significant decrease in haemoglobin concentration (from 13g/dl to 12.6% g/dl) was observed on day 14 following treatment, with the resultant increase in the proportion of anaemic cases from 30.7% on day zero to 36.1% on day 14. However, a significant increase in haemoglobin concentration observed at day 28 (from 13.0 g/dl in day 0 to 13.4 in day 28), with the resultant decline in anaemia prevalence (Table 3).
| Day 0 | Day 14 | Day 28 | |
|---|---|---|---|
| Haemoglobin concentration | 13.0 g/dl | 12.6 g/dl | 13.4 g/dl |
| Percentage of anaemic cases | 30.7% | 36.1% | 19% |
Table 3: Change in haemoglobin concentration in Coartem treated simple P. falciparum patients in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
Discussion
The 100% adequate clinical and parasitological response observed in the present study is consistent with the result of studies conducted in different parts of Ethiopia [8,9]. However, it is contrary to findings from Asia and other studies carried out in two regions of Ethiopia, which reported a low level of Coartem treatment failure [4,5,10]. The low level of treatment failure in these areas might be due to the evolution of Coartem resistant strains of P. falciparum.
The artemisinin derivatives are fast-acting on different blood stages of the parasite [11], which explains the short time required for clearance of parasitemia. In agreement with this study, studies from different parts of Ethiopia [5,8,9,10], demonstrated rapid clearance of parasitemia in court treated uncomplicated P. falciparum malaria patients.
The rapid clearance of fever noted in this study is consistent with findings of other studies in Ethiopia [5,8,9,10]. The fact that fever is attributed to various cytokines released in response to malaria toxins and various metabolic end products released into the bloodstream following the breakdown of infected RBCs at the completion of schizogony [12], could explain the strong association between fever clearance and parasite clearance following Coartem treatment.
The fact that anaemia is a result of haemolysis of RBCs following erythrocytic-schizogony [13], may explain the strong relationship between clearance of asexual parasitemia and recovery of anaemia following Coartem treatment. The significant improvement in haemoglobin level following Coartem treatment noted in this study is an agreement with findings of studies done in different parts of Ethiopia [8,9].
Conclusion
From this study, we may conclude that Coartem® results in rapid clearance of fever and parasitemia; it achieves a high cure rate and increases haemoglobin level on day 28 post-treatment.
Recommendations
As the parasite may develop resistance for the drug, further study of therapeutic efficacy of Coartem® in Wortea town will be important.
Funding
This work was supported by the Ethiopian Health and Nutrition Research Institute.
Conflict of Interest
There is no conflict of interest.
Data Availability Statement
Data used to support the conclusions of this study are included in the article.
Acknowledgement
I am grateful for Professor Beyene Petrose, Mr. Moges Kassa, Mr. Ashenafi Assefa, and Mr. Hussen; Mr. Abrheham Fasil; Miss Kassanesh for their cooperation during the fieldwork. I would like also to acknowledge the study participants and staff members of Wortea health center for their contribution during screening and follow up the study participants. Finally, I would like to express my gratitude to the 10th Ethiopian malaria research network symposium organizers for they gave me the opportunity to present this study in St. Paul Millennium Medical College from December 19-20, 2018, Addis Ababa, Ethiopia.
REFERENCES
- WHO. World Malaria Report. Geneva 27, Switzerland. 2008.
- WHO. World Malaria Report. Geneva 27, Switzerland. 2011.
- Federal Ministry of Health. National malaria guidelines. Addis Ababa, Ethiopia. 2012:155.
- Baykika-Kibwika P, Lamorde M, Mayanja-Kizza H, Merry C, Colebunders B, Van Geertruyden JP. Update on the efficacy, effectiveness and safety of artemether-lumefantrine combination therapy for treatment of uncomplicated malaria. Ther Clin Risk Manag. 2011;6:11.
- Assefa A, Kassa M, Tadese G, Mohamed H, Animut A, Mengesha T. Therapeutic efficacy of (Coartem®) against Plasmodium falciparum in Kersa, South West Ethiopia. Parasit Vectors. 2010;3:1.
- https://en.wikipedia.org/wiki/Wereta
- Assessment and monitoring of antimalarial drug efficacy for treatment of uncomplicated P. falciparum malaria. WHO. 2003.
- Lemma M. Assessment of therapeutic efficacy of Coartem® in patients with uncomplicated P. falciparum Malaria in Halaba Special Woreda. Malaria J. 2015;14:236.
- Besufikad B. Therapeutic efficacy of Artemether-Lumefantrine (Coartem®) for the treatment of uncomplicated Falciparum Malaria in Wondogenet Woreda, Sidama Zone, Ethiopia. J Pharma Care Health Sys. 2017;4:2.
- Tekiemariam M, Assefa A, Kassa M, Mohhamed H, Mamo H. Therapeutic efficacy Arthmeter-lumefantrineagaist uncomplicated Plasmodium falciparum malaria in a high transmission area in northwest Ethiopia. PloS One. 2017;12: 4.
- White J. Monitoring and survilance. Trend Parasitol. 2002;18:1471-4922.
- Clark A, Budd C, Alleva M, Cowden B. Human malaria disease: A consequence of inflammatory cytokine release. Malar J. 2001;5:85.
- Ekvall H, Arese P, Turrini F, Ayi K, Mannu F, Premji Z, et al. Acute HAEMOLYSIS in childhood P. falciparum malaria. Trans R Soc Trop Med Hyg. 2001; 95:611-617.
Citation: Tseha ST (2019) Therapeutic Efficacy of Coartem® in Treatment of Simple Plasmodium falciparum Malaria in Woreta, South Gonder Zone, Ethiopia. J Trop Dis 7:330. doi: 10.35248/2329-891X.19.7.330
Copyright: © 2019 Tseha ST. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

