Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 7, Issue 4
The University of North Carolina Heart-Lung Transplant Experience: Historical Perspective and Notes on Surveillance for Very Long-Term Survivors
Audrey L. Khoury1, Eric G. Jernigan2, Jennifer S. Nelson3, Paula D. Strassle1,4, Vincent J. Gonzalez5, Luma Essaid6, Muntasir H. Chowdhury7, Jason M. Long1 and Mahesh S. Sharma1*2Lenox Hill Hospital, Department of Surgery, New York, NY, USA
3Nemours Children’s Hospital, Department of Cardiovascular Services, Orlando, FL, USA
4University of North Carolina Department of Epidemiology, Chapel Hill, NC, USA
5Baylor College of Medicine & Texas Children’s Hospital, Department of Pediatrics, Section of Pediatric Cardiology, Houston, TX, USA
6University of North Carolina School of Medicine, Department of Pediatrics, Chapel Hill, NC, USA
7Marshall University Joan C. Edwards School of Medicine, Huntington, WV, USA
Received: 15-Jun-2021 Published: 05-Jul-2021, DOI: 10.35248/2573-4598.21.7.161
Abstract
Background: The University of North Carolina (UNC) pioneered heart-lung transplant (HLT) in the state of North Carolina in 1991. Specific guidelines for surveillance of very long-term survivors of HLT are non-existent. We report historical context for the UNC 30-year experience with HLT, complexity of subsequent medical care, and a standardized approach to follow-up.
Methods: The medical and UNOS records for all patients who underwent HLT at UNC were reviewed. Demographics, perioperative details, and post-transplant medication regimens were abstracted. Early (30 day) and late (>30 days post HLT) morbidity was described, and Kaplan-Meier curves estimated long-term survival.
Results: Overall, 15 patients (67% male, 73% adults) underwent HLT, and 80% had congenital heart disease. Five-, twenty-, and twenty-five-year survival was 40% (n=6), 27% (n=4), and 20% (n=3), respectively. All 15-year survivors (n=5) experienced late complications (infections-100%; chronic kidney disease-60%; malignancies-40%; and pulmonary allograft rejection-60%). None had cardiac graft rejection.
Dedicated transplant cardiologists and pulmonologists directed long-term care, and survivors were followed every 6-12 months with non-invasive cardiopulmonary testing. Invasive testing with cardiac catheterization and/or bronchoscopy was performed every 2-3 years.
Limitations: Limitations of the study include small sample size typical of a single-center study. However, this historicallysignificant series represents the entire HLT experience at UNC.
Conclusion: UNC pioneered HLT in the state of North Carolina in 1991. HLT remains a rarely-performed, but viable option for end-staged cardiopulmonary failure as evidenced by favorable long-term survival. Late complications are common and warrant close surveillance and ongoing coordinated care by a specialized multi-disciplinary team.
Introduction
After the first successful heart-lung transplant (HLT) was performed by Shumway, Reitz, and Wallwork in 1981 [1], there was an initial increase in the number of HLT cases performed per year until case volume peaked at 226 cases in 1989 [2,3]. In 1991, a former student of Dr. Shumway, Drs. Michael Mill and Thomas Egan performed the first HLT in North Carolina with Drs. Frank Detterbeck, C. Jake Lambert Jr., and Karl S. Ulicny at University of North Carolina (UNC) Hospitals (see Figure 1 for operative findings from original operative report). In subsequent decades, there was a progressive decline in yearly HLT cases reported to the International Society for Heart and Lung Transplantation (ISHLT) [2,4,5]. The reasons for this trend were multifactorial.

Figure 1: Operative findings from original operative report of first heart-lung transplant in North Carolina.
Between 1989 and 1999, HLT allocation was a separate category, allocated after Status 1 heart. In 1999, organ allocation guidelines changed, limiting the number of heart-lung blocks and lengthening waitlist times. At that time, HLT candidates were required to be listed on both heart and lung transplant waitlists as multi-organ candidates, and they competed with United Network for Organ Sharing (UNOS) status 1A heart-only candidates for hearts. Lungs were allocated with hearts, and hearts were allocated with lungs if there was no Status 1A candidate available. The HLT candidates rarely fulfilled criteria for status 1A heart listing, which limited their access to organs. UNOS guidelines regarding utilitarian distribution of these limited donor organs resulted in a shift from HLT to isolated lung transplantation in patients with structurally normal hearts with preserved function [6,7].
Improvements in medical and surgical care also contributed to the decrease in HLT cases after 1989. Advances in medical therapy for pulmonary arterial hypertension (PAH) revolutionized care for a large patient population that once looked to HLT for treatment of end-stage disease. Double lung transplant gained favor over HLT in cystic fibrosis (CF) patients, as pioneering centers reported shorter cardiopulmonary bypass times and decreased morbidity in lung transplantation alone versus HLT [6,8-10]. Despite diminishing indications, HLT remained a life-saving option for a small subset of patients in the 1990s and 2000s.
HLT is an infrequently performed surgical procedure with significant morbidity and mortality; however, it remains the preferred surgical option for a select population of patients with cardiopulmonary failure. Since the first HLT was performed in the early 1980s, there has been a progressive improvement in both early and late mortality rates with each subsequent era. According to the ISHLT Registry, initial operative mortality was 25% and survival rates at 1, 2, 5, and 10 years were 56%, 49%, 37%, and 26%, respectively [9,11,12]. Later, the 2016 ISHLT Registry report evaluated HLT patients from 1982-2014 and described improved survival rates at 3 months, 1, 3, 5, and 10 years of 71%, 63%, 52%, 45%, and 32%, respectively [13].
HLT remains the only curative option for a select population of patients with cardiopulmonary failure. The leading indications for HLT in the United States include congenital heart disease (CHD) with Eisenmenger physiology, PAH, and CF [2,4]. While early mortality remains high, late mortality in HLT is low for patients with CHD surviving one year after transplant [14].
There are no clinical management guidelines for long-term HLT survivors. Also, the care of very long-term transplant survivors becomes increasingly complex over time, and patients on chronic immunosuppression regimens may face novel and rare complications. Large national registries lack the necessary granularity to study rare complications and surveillance practice patterns, but single center studies from experienced transplant centers can offer detailed clinical information and valuable management recommendations. As such, the aims of this study were to report the historical context and complete 30-year experience of HLT at UNC and to provide details on long-term sequelae, surveillance, and longitudinal outcomes.
Methods
Study Design and Setting
The first HLT in the state of North Carolina was performed at UNC Hospitals in 1991, and this retrospective cohort study includes all patients who underwent HLT at UNC Hospitals between 1991 and 2005. HLT has not been performed at UNC since 2005. No UNC HLT patients were excluded from the analysis. This study was approved by the UNC School of Medicine Institutional Review Board (IRB# 20-0571).
Data Collection
Patient characteristics, operative details, donor characteristics, immunosuppressive regimens, and complications were recorded. Donor details were collected from UNOS records, and recipient details were extracted from the UNC medical record. Long-term care plans and surveillance algorithms were recorded based on progress notes written by transplant surgeons, cardiologists, and pulmonologists following these patients.
Data Analysis
Univariateanalyses were used to describe the recipient and donor characteristics. Kaplan-Meier survival curves were used to estimate the 5, 10, 15, 20, and 25-year survival.
Results
A total of 15 patients (67% male) underwent HLT at UNC between 1991 and 2005 (Table 1). Ten (67%) study patients were male, and median age at HLT was 30 (range 3-49). 67% of the patients were white. The most common indication for HLT was cardiopulmonary failure secondary to complex CHD (80%). Of the patients with CHD, the most common underlying diagnosis was transposition of the great arteries (33%), followed by tetralogy of Fallot (20%) and truncus arteriosus (20%) (Table 1). Although the majority of patients (73%) were young adults (≥18 years old) at the time of transplant, patients with CHD were younger (median age 28 years), compared to patients without CHD (median age 32 years).
| Variable | n (%)a |
|---|---|
| Male | 10 (67) |
| Age, years, median (IQR) | 30 (18-36) |
| Weight, kg, median (IQR) | 46 (32-64) |
| Race | |
| White | 10 (67) |
| Black | 4 (27) |
| Native American | 1 (7) |
| Congenital heart disease | 12 (80) |
| Primary diagnosis | |
| Transposition of the great arteries | 5 (33) |
| Tetralogy of Fallot (TOF)c | 3 (20) |
| Truncus arteriosus | 3 (20) |
| Sarcoidosis | 2 (13) |
| Partial anomalous pulmonary | 1 (7) |
| venous return | |
| Primary pulmonary hypertension | 1 (7) |
| Smoker | |
| Yes, before transplant | 2 (15) |
| Never | 11 (85) |
| Unknown | 2 |
| Days on waitlist, median (IQR) | 414 (127-621) |
| On home oxygen | 13 (93) |
| FEV1d, %, median (I)QR | 59 (35-80) |
| FVCc, L, median (IQR) | 3 (1-4) |
| Pulse O2 saturation, %, median (IQR) | 88 (85-98) |
Abbreviations: IQR, interquartile range; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; O2, oxygen
a Values are n (%), unless otherwise stated.
b At transplant; weight missing for 6 subjects.
c TOF includes pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries.
dFEV1 and FVC missing for 4 subjects.
Table 1: Patient demographics and pre-transplant characteristics, n=15.
Survival
Early outcomes
Four patients (27%) died within 30 days of HLT, including two patients who died secondary to post-operative hemorrhage. One patient died intra-operatively due to primary graft dysfunction. In-hospital mortality after HLT was 47%. One-year survival was 47%.
Long-term outcomes
Five-year survival was 40% (n=6), and ten- and fifteen-year survival was 33% (n=5) (Figure 2). Twenty-year and twenty-five-year survival were 27% (n=4) and 20% (n=3), respectively. The most common causes of late death were sepsis (27%, n=3) and bronchiolitis obliterans (18%, n=2) (Table 2). Among patients who survived the first year (n=7), 5-year survival was 86% (n=6), 10- and 15-year survival was71% (n=5), 20-year survival was 57% (n=4) and 25-year survival was 43% (n=3). Among patients who survived at least 5 years, the 20- and 25-year survival rates were 67% (n=4) and 50% (n=3), respectively.
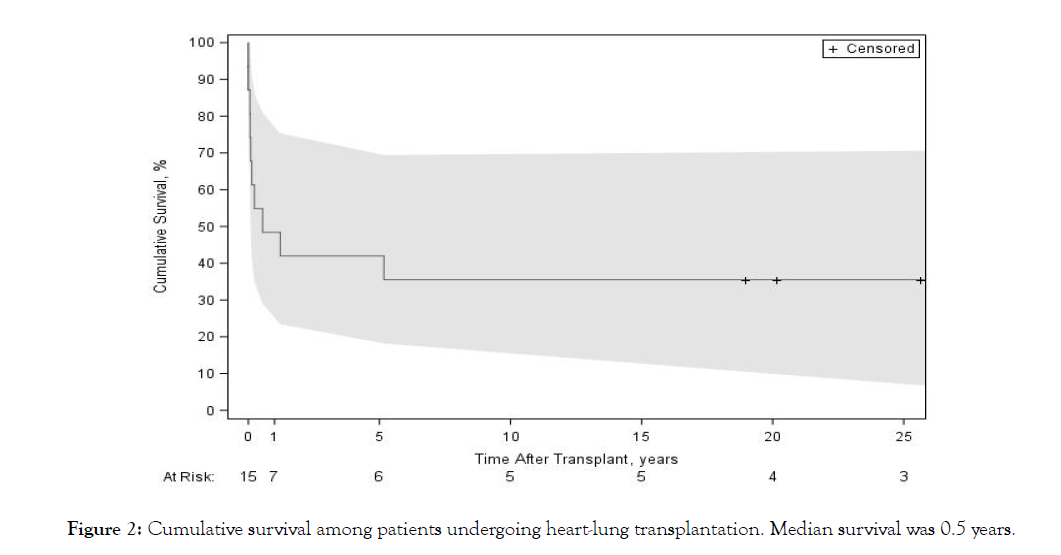
Figure 2: Cumulative survival among patients undergoing heart-lung transplantation. Median survival was 0.5 years.
| Patient ID | Age at Transplant | Survival Time (years) | Cause of Death |
|---|---|---|---|
| 1 | 26 | 26 years | Sepsis |
| 2 | 3 | 27 years | - |
| 3 | 25 | 25 years | - |
| 4 | 10 | 20 years | - |
| 5 | 10 | 18 years | - |
| 6 | 30 | 5 years | Bronchiolitis obliteran |
| 7 | 49 | 1 years | Bronchiolitis obliterans |
| 8 | 39 | 6 months | Sepsis |
| 9 | 32 | 84 days | Multi-system organ failure |
| 10 | 21 | 47 days | Pulmonary graft failure |
| 11 | 35 | 32 days | Cerebral edema/herniation |
| 12 | 36 | 25 days | Post-operative hemorrhage |
| 13 | 33 | 22 days | Sepsis |
| 14 | 33 | 1 days | Post-operative hemorrhage |
| 15 | 17 | 0 days | Primary graft dysfunction |
arenal/bone marrow failure and chronic pulmonary insufficiency
bwith cytomegalovirus (CMV) pneumonitis
clower gastrointestinal bleed and aortic tear superior to aortic anastomosis on post-operative day 23
intraoperative death
Table 2: Patient survival and causes of death, n=15.
Complications
Five of the long-term survivors lived longer than 15 years after their HLT, and four of the five are still living. The longest surviving UNC patient is currently alive 27 years post-HLT. Related to chronic immunosuppression, all five long-term survivors suffered infectious complications (including Para influenza pneumonia, influenza, cytomegalovirus [CMV] esophagitis, hepatitis C, Pseudomonas sepsis, peritonitis from Candida-infected peritoneal dialysis catheter, and skin infections). Three patients (60%) developed chronic kidney disease secondary to calcineurin-inhibitor (CNI) toxicity (see Table 3 for immunosuppressive regimens), ultimately leading to two kidney transplants (KTx) for one patient and one kidney transplant for another. Two of them (40%) were diagnosed with malignancies (squamous cell carcinoma and melanoma). No patient had rejection of his/her cardiac graft. None of the patients have undergone heart or lung re-transplantation. However, three patients (60%) have experienced pulmonary graft rejection after HLT (one with bronchiolitis obliterans).
| Patient | Discharge Regimen | Year 1 Regimen | Year 15 Regimen | Year 20 Regimen | Year 25 Regimen |
|---|---|---|---|---|---|
| 1 | Azathioprine 125 mg daily Cyclosporine 300 mg twice daily - Prednisone 15 mg daily |
Azathioprine 50 mg daily Cyclosporine 125 mg daily Prednisone 10 mg twice daily |
Cyclosporine 125 mg twice daily Mycophenolate mofetil 500 mg twice daily - Prednisone 7.5 mg daily |
Cyclosporine 50 mg twice daily Mycophenolate mofetil 500 mg twice daily Prednisone 7.5 mg daily |
Cyclosporine 75 mg twice daily Mycophenolate mofetil 500 mg twice daily Prednisone 7.5 mg daily |
| 2 | Azathioprine 4.5 mg twice daily Cyclosporine 100 mg three times daily - Prednisolone 4.5 mg twice daily |
Azathioprine 20 mg daily Cyclosporine 120 mg three times daily |
Sirolimus 2 mg daily Tacrolimus 2 mg twice daily |
Mycophenolate mofetil 1000 mg twice daily Prednisone 5 mg daily Tacrolimus 3 mg twice daily |
Mycophenolate mofetil 1000 mg twice daily Prednisone 5 mg daily Tacrolimus 3 mg twice daily |
| 3 | Azathioprine 200 mg daily Cyclosporine A 350 mg twice daily Prednisone 20 mg daily |
Cyclosporine 150 mg twice daily - Prednisone 15 mg daily |
Cyclosporine 125 mg twice daily | Cyclosporine 175 mg twice daily | Azathioprine 25 mg daily Cyclosporine 150 mg AM and 125 mg PM Prednisone 5 mg daily |
| 4 | Mycophenolate mofetil 750 mg twice daily Prednisone 12 mg daily Tacrolimus 8 mg twice daily |
Tacrolimus 9 mg twice daily | Mycophenolate mofetil 500 mg twice daily Prednisone 10 mg daily Tacrolimus 4 mg twice daily |
Mycophenolate mofetil 500 mg twice daily Prednisone 10 mg daily Tacrolimus 4 mg twice daily |
NA |
| 5 | Mycophenolate mofetil 600 mg twice daily Prednisone 5 mg am, 10 mg pm Tacrolimus 4 mg twice daily |
Mycophenolate mofetil 600 mg twice daily Prednisone 5 mg daily Tacrolimus 2 mg twice daily |
Mycophenolate mofetil 500 mg three times daily Prednisone 7.5 mg daily Tacrolimus 4 mg twice daily |
NA | NA |
| 6 | Mycophenolate mofetil 1000 mg twice daily - Prednisone 20 mg twice daily - Tacrolimus 1 mg twice daily |
Mycophenolate mofetil 500 mg twice daily Prednisone 10 mg daily Tacrolimus 1 mg am, 2 mg pm |
NA | NA | NA |
| 7 | Mycophenolate mofetil 1000 mg twice daily Prednisone 10 mg twice daily Tacrolimus 6 mg twice daily |
Mycophenolate mofetil 1500 mg twice daily Prednisone 15 mg twice daily Tacrolimus 6 mg three times daily |
NA | NA | NA |
| 8 | Mycophenolate mofetil 1500 mg twice daily Prednisone 15 mg am, 12.5 mg pm |
NA | NA | NA | NA |
| 9 | NA | NA | NA | NA | NA |
| 10 | Azathioprine 50 mg daily Cyclosporine 200 mg twice daily Prednisone 24 mg daily |
NA | NA | NA | NA |
| 11 | NA | NA | NA | NA | NA |
| 12 | NA | NA | NA | NA | NA |
| 13 | NA | NA | NA | NA | NA |
| 14 | NA | NA | NA | NA | NA |
| 15 | NA | NA | NA | NA | NA |
Table 3: Immunosuppressive regimens over time.
One patient underwent HLT for Eisenmenger syndrome as a late complication of tetralogy of Fallot (psuedotruncus arteriosus) and was discharged with an immunosuppressive regimen consisting of prednisone, cyclosporine, and azathioprine. Given his end-stage renal disease requiring hemodialysis, he received a living-related KTx two years post-HLT, a cadaveric KTx 14 year’s post-HLT for biopsy-proven focal segmental glomerulosclerosis, and was re-listed for a third transplant. Chronic immunosuppression also led to other complications, such as multiple hospitalizations for infections (Para influenza pneumonia, cytomegalovirus [CMV] esophagitis, hepatitis C, and multiple skin infections [including multidermatomal shingles, erosion interdigitalis blastomycetica, Pseudomonas, tinea versicolor, molluscum contagious, condyloma acuminate, cellulitis, and abscesses]. He also developed squamous cell carcinoma of the right leg, osteoporosis leading to multiple a traumatic foot fractures, chronic pancytopenia, and severe chronic neuropathic leg pain. Over time, his heart and lung graft function remained normal without clinical history of cardiac allograft vasculopathy or rejection, as he was maintained on cyclosporine, mycophenolate, and prednisone. Twenty-six years after his HLT, he died of Enterococcus facials.
The longest surviving UNC patient is currently living 27 years post-HLT. Two years after undergoing angioplasty for aortic coarctation, he underwent HLT for complex cyanotic heart disease with anomalous pulmonary venous drainage of his right lung into the right atrium, congenital pulmonary venous stenosis with pulmonary hypertension, and atrial septal defect (ASD). He was initially discharged with cyclosporine, azathioprine, and prednisone. He underwent KTx 24 year’s post-HLT due to CNI toxicity. He was diagnosed with lung allograft rejection soon after HLT and also 18 years post-HLT. Infectious complications include pseudomonas sepsis, peritonitis from a Candida-infected peritoneal dialysis catheter, recurrent left pleural effusion (managed by antibiotics, thoracentesis, chest tube placement, and left video-assisted thoracoscopic decortication and complicated by cardiopulmonary arrest requiring chest compressions), and influenza B. He continues to do well on tacrolimus, prednisone, and mycophenolate.
Surveillance
Dedicated transplant teams direct the care of long-term survivors (Table 4). The type and severity of complications influences the frequency of recommended clinic visits, laboratory tests, and cardiopulmonary tests. In general, HLT recipients are followed every six months in clinic with laboratory studies and non-invasive cardiopulmonary testing. Laboratory testing includes: a complete blood count, basic metabolic panel, liver function tests, and viral serologies, (i.e. CMV, Ebstein-Barr virus [EBV]). Chest X-rays, and electrocardiograms are also routinely obtained. Pulmonary function testing (PFT) is performed every 3-12 months, depending on graft function and rate of disease progression. Echocardiograms are performed yearly and cardiac stress tests are performed every 1-3 years. Invasive monitoring with cardiac catheterization (with or without biopsy) is recommended for patients with cardiac allograft vasculopathy (CAV). In our cohort, cardiac catheterizations were performed every 3-6 years. We recommend bronchoscopy every 2-3 years or more frequently if indicated.
| Type of monitoring or test | Recommended interval |
|---|---|
| Multidisciplinary transplant team clinic visit | Every 6 months – 1 year |
| Laboratory testing, chest X-ray, electrocardiogram | Every 6 months – 1 year |
| Pulmonary function testing | Every 3-12 months |
| Echocardiograms | Yearly |
| Cardiac stress tests | Every 1-3 years |
| Cardiac catheterizations | Every 3-6 years |
| Bronchoscopy | Every 2-3 years |
Table 4: Surveillance algorithm and recommended intervals for monitoring and tests.
An extensive network of transplant physicians, nurses, pharmacists, social workers, nutritionists, and care managers who understand the needs of this unique patient population collaborate to ensure appropriate testing, follow-up, and access to care. Nurse calls with patients are scheduled within three days of being seen for an appointment or hospitalization. Transplant pharmacists are intimately involved with the patients and care managers to facilitate convenient laboratory testing for immunosuppressive drug monitoring. Nutritionists also work closely with the care teams on an inpatient and outpatient basis to ensure optimal patient nutrition, lifestyle, and long-term health. This multi-disciplinary team creates an ideal network for care and contributes to the long-term success of UNC transplant patients.
Discussion
The University of North Carolina pioneered HLT in the state during the early 1990s. In that era, most HLTs were performed for end-stage CHD with severe pulmonary hypertension. Early mortality of HLT was high, although long-term survival was favorable for patients living beyond the first post-transplant year. All of the long-term survivors of HLT in our cohort experienced a high burden of late complications from immunosuppressive therapy.
Out of a total experience of 15 patients, 80% had an underlying etiology of CHD, and one-third enjoyed very long-term survival with a median survival of 25 years (4 patients currently living). Overall, survival at 5, 10, 15, 20, and 25 years was 40%, 33%, 33%, 27%, and 20%, respectively. An experienced and dedicated cardiopulmonary transplant team followed long-term survivors and provided individualized care plans within a structured surveillance algorithm. Although 5-year survival was lower than that noted in the 2016 ISHLT Registry report (40% vs. 52%), 10-year survival was similar (33% vs. 32%) [13]. The 2019 ISHLT Registry report demonstrated that median HLT survival between 2010-2017 is 6.5 years, which is dramatically increased from median survival of 3.7 years between 1992-2001 [15]. Much of this mortality occurred early after HLT, which is consistent with our study results. This improvement in survival may be attributed to improvements in operative technique, patient selection, immunosuppressive regimens, and a better understanding of long-term predictors of poor outcome. Ali et al. recently analyzed the ISHLT Registry and showed that factors associated with prolonged (more than 15 years) survival after lung transplantation included younger recipient age, lower body mass index, female gender, bilateral lung transplant, and transplant center performing more than 25 transplants per year [16].
Immunosuppression regimens have evolved significantly over time. According to the 2019 International Thoracic Organ Transplant (TTX) Registry Report, the use of cyclosporine and azathioprine continues to gradually decline. This change in practice is related both to their adverse side effect profiles (including nephrotoxicity, leukopenia, thrombocytopenia, anemia, and hepatoxicity), and to the increasing number of patients receiving tacrolimus, mycophenolate mofetil, and prednisone for immunosuppression [17,18]. Despite modern immunosuppressive regimens with more favorable side-effect profiles, renal dysfunction, diabetes, malignancy, and chronic allograft rejection (allograft vasculopathy and bronchiolitis obliterans) remain common complications following HLT, as seen in our study results [17]. Close surveillance of HLT patients is therefore critical.
While there has been an improvement in survival after HLT, there has also been an overall decline in the number of HLT cases per year performed over recent decades [19]. Despite this trend, there could be a future demand for HLT, due to the growing population of adults with CHD and concurrent pulmonary disease. At our institution, almost a third of HLT recipients have survived greater than 20 years, but their care has been complex. Collaboration within a multi-disciplinary team of caregivers is paramount to the success of a transplant team.
UNC holds unique historical significance in its pioneering role in the development of cardiopulmonary transplantation in North Carolina. Herein, we described the 30-year experience of all patients who underwent HLT at UNC. We also recommend a surveillance strategy for both invasive and non-invasive cardiopulmonary testing as dedicated transplant cardiologists and pulmonologists work with a multidisciplinary team to direct long-term care for these patients. Limitations of the study include the small sample size and limited generalizability, common to other single-center reports of HLT. However, detailed longitudinal follow-up including documentation of surveillance practices and rare complications are strengths, as these data are not available from national registries. To identify factors associated with long-term survival, a statewide, multi-center study of HLT sites (e.g. collaboration between Duke and UNC) would be helpful.
Although infrequently applied in modern clinical practice, HLT remains a viable option for end-staged cardiopulmonary failure as evidenced by favorable long-term survival. Late complications are common and warrant close surveillance and ongoing coordinated care by a specialized multi-disciplinary team. With the burgeoning population of adults living with CHD, HLT should remain in the armamentarium of mature transplant centers.
REFERENCES
- Reitz BA. The first successful combined heart-lung transplantation. J Thorac Cardiovasc Surg. 2011;141(4):867-869.
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011;30(10):1104-1122.
- Le Pavec J, Hascoet S, and Fadel E. Heart-lung transplantation: current indications, prognosis and specific considerations. J Thorac Dis. 2018;10(10):5946-5952.
- Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):989-997.
- Benden C, Aurora P, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Fourteenth Pediatric Lung and Heart-Lung Transplantation Report--2011. J Heart Lung Transplant. 2011;30(10):1123-1132.
- Orr Y. Overview of paediatric heart-lung transplantation: a global perspective. J Thorac Dis. 2014;6(8):1159-1163.
- Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant. 2012;12(12):3213-3234.
- Spahr JE and West SC. Heart-lung transplantation: pediatric indications and outcomes. J Thorac Dis. 2014;6(8):1129-1137.
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31(10):1073-1086.
- Idrees JJ and Pettersson GB. State of the Art of Combined Heart-Lung Transplantation for Advanced Cardiac and Pulmonary Dysfunction. Curr Cardiol Rep. 2016;18(4):36.
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1009-1024.
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1-15.
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant.2016;35(10):1170-1184.
- McGlothlin D and De Marco T. Transplantation in adults with congenital heart disease. Prog Cardiovasc Dis. 2011;53(4):312-323.
- Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report – 2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042-1055.
- Ali HA, Neely M, Long A, et al. Factors Associated with Prolonged Survival (>15 Years) after Lung Transplantation: Analysis of the ISHLT Registry. J Heart Lung Transplant. 2019;38(4):S327-S328.
- Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042-1055.
- Scheffert JL and Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6(8):1039-1053.
- The International Society for Heart and Lung Transplantation. ISHLT Transplant Registry Quarterly Reports for Heart/Lung in North America. Available at: https://ishltregistries.org/registries/quarterlyDataReportResults.asp?organ=HL&rptType=all&continent=4. Accessed November 5, 2020.
Citation: Khoury AL, Jernigan EG, Nelson JS, Strassle PD, Gonzalez VJ, Essaid L, et al. (2021) The University of North Carolina Heart-Lung Transplant Experience: Historical Perspective and Notes on Surveillance for Very Long-Term Survivors. J Pat Care 7:160.
Copyright: © 2021 Khoury AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
