Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 2
The Significance of Immune and Non-immune Causes Associated with Platelet Refractoriness in Haemato-oncology Patients: A Systematic Review and Meta-Analysis
Xiangyu Long and Denise E. Jackson*Received: 22-Jan-2024, Manuscript No. JBDT-24-24627; Editor assigned: 24-Jan-2024, Pre QC No. JBDT-24-24627 (PQ); Reviewed: 14-Feb-2024, QC No. JBDT-24-24627; Revised: 21-Feb-2024, Manuscript No. JBDT-24-24627 (R); Published: 28-Feb-2024, DOI: 10.4172/2155-9864.24.15.579
Abstract
Platelet Transfusion Refractoriness (PTR) can be defined as failed to elevate the level of platelet after receiving repeated platelet transfusion. PTR can be seen in normal patients as well as haemato-oncology patients when treating thrombocytopenia and it’s related to increased morbidity and mortality rate. Various risk factors can lead to PTR development, including both immune and non-immune factors. There was no comprehensive review of risk factors associated with PTR in haemato-oncology patients previously. Therefore, this study aims to determine the significance of both immune and non-immune causes associated with PTR in patients with haematological malignancies. The systematic review was conducted with relevant articles searched from five databases from 2003 to 2023. The meta-analysis was conducted using Open Meta Analyst software with one-arm proportion (arcsine transformed proportion) under binary random effects model with maximum likelihood method. A total of nine studies were analysed. Statistically significant results were indicated in forest plots with levels of heterogeneity. Results demonstrated significant correlation between bleeding (44.33%; 35.04%), infection (68.04%; 17.95%), fever (67.01%), and PTR in haemato-oncology patients (39.34%; 8.26%). Small to moderate correlation between splenomegaly (28.21%), HLA alloantibody (17.40%) associated PTR in haemato-oncology patients. This study illustrated PTR was an important adverse condition, as well as bleeding, infection, and fever were strongly associated with PTR development in patients with haematological malignancies. Such conditions required monitoring throughout clinical management to ensure better outcomes for the patients.
Keywords
Platelet Transfusion Refractoriness (PTR); Haematological malignancies; Oncology; Bleeding; Infection; Fever; Splenomegaly; HLA alloantibody
Introduction
Platelet refractoriness
Individuals who failed to elevate the platelet count to expected level after platelet transfusion could have potentially developed Platelet Transfusion Refractoriness (PTR). Scientifically speaking, PTR can be defined as either the Corrected Count Increment (CCI) 1 hour is less than 7.5*10^9/L or CCI 24 hours is less than 4.5*10^9/L post transfusion [1,2]. Around 30%-70% of patients who received platelet transfusion because of various clinical conditions of Thrombocytopenia (TP) have developed PTR [3]. Evidence shows PTR associated bleeding and haemorrhage can be fatal as it increases both morbidity and mortality rate, which makes understanding the mechanisms behind PTR vital and essential for clinical management to improving patients outcome [4]. The causes of PTR can be categorised into Immune cause and non-immune cause. Studies have advised that over 70% of PTR cases are associated with non-immune cause and around 25% of cases are related to immune cause [5,6].
Immune causes of PTR
Human Leukocyte Antigen (HLA): The HLA system plays an important role in the blood product transfusion setting as it is able to stimulate immune responses upon identify self from foreign antigens through direct and indirect recognition by the immune system [7]. HLA Class I consist of three classical alleles: HLA-A, HLA-B and HLA-C, which HLA-A and HLA-B are predominantly involved in immune caused PTR as they expressed almost on all nucleated cells including platelet. HLA antigens are highly immunogenic due to the consist of high degree of polymorphism among all populations around the world, which increases the risk of developing allo-immune antibodies that targeting HLA antigens on the platelet surface as consequence of incompatible blood product transfusion [8]. Investigations have shown that HLA Class I allo-antibody contributes majority of the immune caused PTR cases, thus, transfusion using HLA matched platelet could potentially reduce the risk of inducing HLA allo-antibodies and further improve patient outcomes [9,10].
Human Platelet Antigen (HPA): Similar to HLA, HPA can also expressed on platelet surface and inducing immune response when encounter foreign platelets with different HPAs and producing alloantibodies to the non-self-platelet antigens, it attributes to around 8% of the alloimmunisation caused PTR [5]. To date, HPA system has 35 recognised antigens and the majority of them are on the platelet membrane glycoprotein GPIIb/IIIa complex on the platelet surface [11]. According to the previous study, HPA-1 is strongly associated with PTR and is the most immunogenic HPA antigen [12]. Investigations have represented that HPA alloantibody is not only be sufficient to elicit PTR by itself, but also can be found more frequently in combination with HLA alloantibody in development of PTR [13,14]. As HPA system is a newly discovered system and the clinical significance of developing immune-mediated PTR is high, platelet transfusion with HPA matched platelet has been introduced and can be successfully supported with HPA-alloimmunised patients with PTR [15].
Non-immune causes of Platelet Transfusion Refractoriness (PTR)
Bleeding and haemorrhage: Bleeding and haemorrhage are responsible for necessary platelet consumption, leading to inadequate platelet transfusion with low CCI [16]. Previous studies have described the consequent PTR is more likely following developing bleeding and haemorrhage, and the patients suffered from bleeding and haemorrhage related PTR reflected drastically decreased survival rate [4,17,18]. Thus, the clinical management is critical in treating the PTR along with the active bleeding and haemorrhage to improve the clinical outcomes for the patients.
Infection and sepsis: Infection and related sepsis associated PTR have been reported by numerous of studies [4,18,19]. The mechanisms are mainly based on platelets directly interact with exogenous pathogens, interactions between neutrophils to form extracellular traps, platelet desialylation, and CD169 positive macrophages-mediated PTR in sepsis, which increasing transfused platelet destruction and consumption significantly and resulting infection related PTR with low CCI [20-22].
Fever: Platelet is heat-dependent system and only works in a specific spectrum of temperatures. Either hypothermia or hyperthermia could effectively impact on both the platelet function and platelet counts [23,24]. Fever (body temperature greater than or equals 38ºC) related PTR is revealed by Kerkhoffs et al., Kumawat et al., and Comont et al., all three studies illustrated positive correlation between fever and PTR development [4,18,19]. It is known that one of the classical platelets clearance mechanisms is involving monocytes and macrophages [22]. Thus, in PTR patients with fever, the low CCI count would not only due to unbearable high temperature mediated by interleukin-1, but also because of monocyte and macrophage induced platelet phagocytosis stimulated by overproduction of pro-inflammatory cytokines (IL-1, IL6, Tumor Necrosis Factor (TNF) and Interferon-gamma (INF)) [25,26].
Splenomegaly: The spleen plays an important role in controlling levels of platelet circulation as it normally stores around 25% of platelet in a healthy individual [27]. It is the most vital organ that could impact the platelet CCI posttransfusion and elicit PTR. Study conducted by R H Aster has demonstrated the accumulation of platelets posttransfusion in the spleen was fast in about 10 mins [27]. When individuals who have splenomegaly, the proportion of transfused platelet accumulating in the spleen increased, resulting around 50%-90% of transfused platelet harboured in the spleen which decreased the circulated transfused-platelet [27]. R L Hill-Zoble et al. have also proved that more transfused platelets have been destroyed in splenomegaly patients compared to normal individuals, and splenomegaly patients have doubled the amount of transfused platelets sequestrated in the spleen compared to normal individuals [28].
Haemato-oncology patients with Platelet Transfusion Refractoriness (PTR): Around 28%-30% of haemato-oncology patients has developed severe TP and require platelet transfusion [29,30]. A study conducted in 2016 stated that around 67% of all platelet transfusion is related to the clinical management of haemato-oncology patients [31]. As the patients have huge demand in managing TP via platelet transfusion, the impact of PTR induced in haemato-oncology patients can be profound and life threatening.
Overall, HLA and HPA allo-immunity can be seen in the haemato- oncology patients with PTR [18,19]. Symptoms and complications such as bleeding, splenomegaly, fever, and infection are always related to haematological cancer, which could potentially correlate with the development of PTR [32,33].
In this systematic review and meta-analysis, we aim to investigate the significance of PTR in patients with haemato-oncology disorders including leukaemia, lymphoma, myeloma and their variants and subtypes. We are going to study the correlation between PTR and numerous of immune and/or non-immune causes in the haemato- oncology patients to elaborate the understanding of what to expect during the platelet transfusion for these patients. Therefore, better clinical management can be put in place with certain symptoms, providing better outcomes for the patients. We hypothesise that PTR in haemato-oncology patients is significant, and it has strong correlation with various causes including bleeding, splenomegaly, fever, infection and HLA/HPA alloimmunisation.
Materials and Methods
Study design
The Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) has been conducted to assist collecting the relevant studies in this systematic review [34]. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist has been assessed against the included studies to evaluate their quality and completeness [35].
Search strategies
The relevant articles are systematically researched from database PubMed, Scopus, ProQuest, Embase and Web of Science ranging from 2003 to 2023. Key search terms used across all databases including “Platelet refractoriness” and “Haemato-oncology”, “leukaemia”, “lymphoma”, “myeloma”; “alloimmunised”, and “HLA alloantibody”. A manual search was implemented from the reference list with three extra studies found and included.
Inclusion and exclusion criteria
The eligibility of the included studies was assessed based upon PRISMA flow chart started from duplicates removal, followed by titles and abstracts screening, and full text articles assessment against inclusion and exclusion criteria. Studies focused on the risk factors of PTR including either non-immune or immune causes in Haemato- oncology patients considered eligible for this review. Studies exclusions are due to: a) Articles didn’t look at platelet refractoriness; b) Articles didn’t look at Haemato-oncology patients; c) Case report articles/not primary study or irrelevant, d) Articles are not available in English; e) Abstract are not available or full-text is not available, f) Articles with insufficient data; g) Review articles, and h) PTR is not primary study.
Data extraction
Data extracted from eligible studies with their author, publication year, study design, country of the study conducted, population size of the study including total number of patients and number of patients developed PTR and their characteristics, parameters of the study including PTR definition, and risk factors (Bleeding, infection, fever, splenomegaly, and HLA alloantibody).
Statistical analysis
Open Meta Analyst software from Brown University website was used to conduct the meta-analysis [34]. Total of nine one-arm proportion analysis were performed using arcsine transformed proportion for overall proportion of PTR in haemato-oncology patients which defined by either CCI (Corrected Platelet Count Increment) or PPI (Posttransfusion Platelet Increment), bleeding related PTR, infection related PTR, fever related PTR, splenomegaly related PTR, and HLA alloantibody related PTR. All analysed under binary random effects model with maximum likelihood method and presented with six sets of forest plot. The results produced by the software contains overall P-value to elaborate statistically significant, 95% confidence intervals and I square value with P-value for heterogeneity to determine results variation between studies. P-value<0.05 was defined statistically significant for both overall and heterogeneity.
Results
Study selection
The study selection is represented in PRISMA (Figure 1). There are 6389 articles obtained initially from the five databases mentioned above. Total of 2358 duplicates were then removed, the remaining 4031 articles underwent titles and abstracts screening with 3909 articles excluded based on various reasons. There were 122 articles assessed for eligibility, 116 were excluded mainly based on PTR is not primary study and studies with insufficient data to analyse. Couple of meta-analysis studies were also excluded and that leaves six eligible studies along with three manual searched studies from reference included in the meta-analysis.
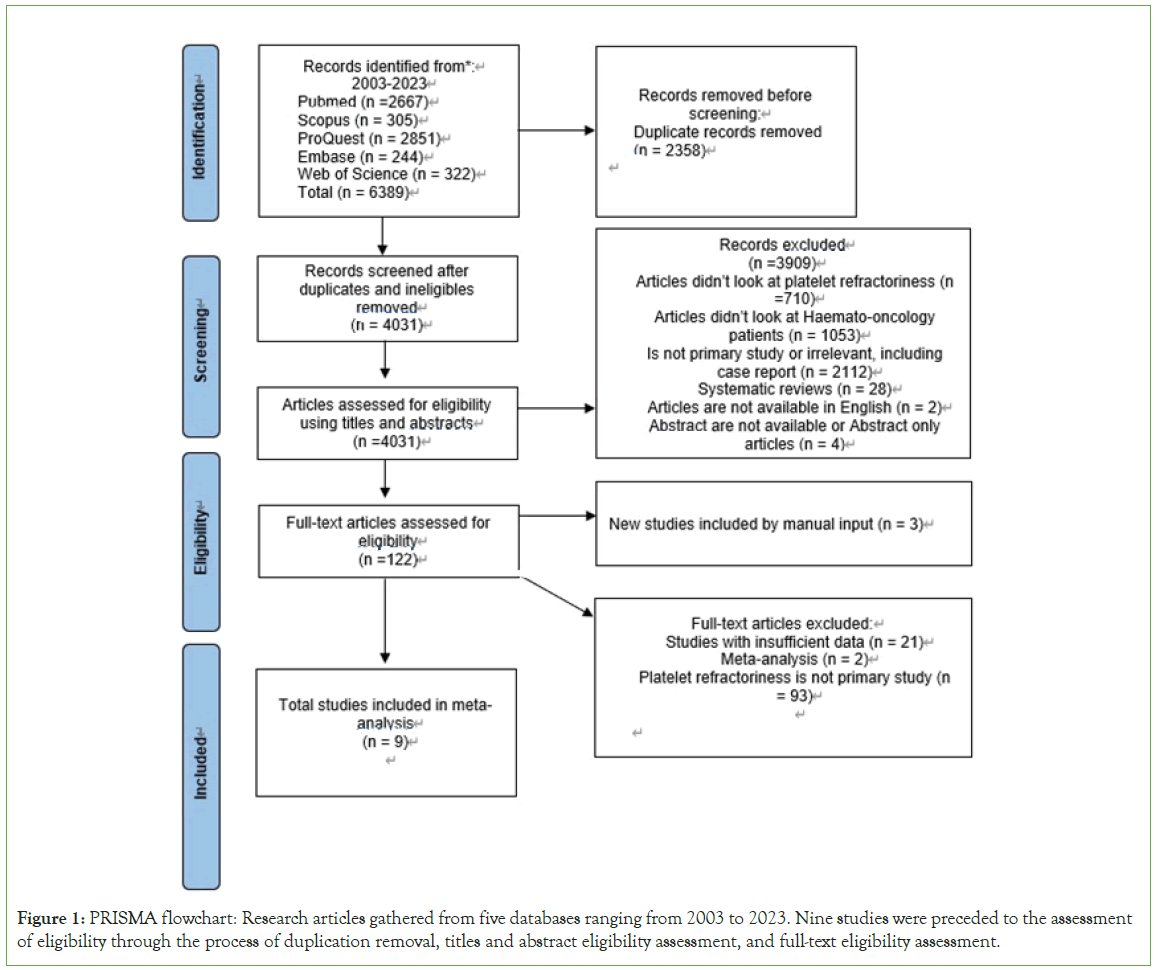
Figure 1: PRISMA flowchart: Research articles gathered from five databases ranging from 2003 to 2023. Nine studies were preceded to the assessment of eligibility through the process of duplication removal, titles and abstract eligibility assessment, and full-text eligibility assessment.
Study characteristics
The characteristics of all nine studies included in this study are demonstrated in Table 1. Studies separated into two groups by difference PTR definition: 24h CCI defined, and PPI defined. Four studies in the CCI group are mainly prospective ranging from 2008 to 2020 in four different countries. The sample size contained total number of haemato-oncology patients from 23 to 114, along with total number of PTR patients from 10 to 58. Five studies in the PPI group are mainly retrospective within the last five years across four countries, with one exception published in 1995 that is from manual search [35]. The sample size included total number of haemato-oncology patients from 87 to 897, the total number of PTR patients from 10 to 66. Most studies emphasised the risk of PTR related to bleeding, infection, fever, splenomegaly, or HLA alloantibody.
| Study | Study design | Country | Study period | Sample size* | PTR definition** | Risk factors |
|---|---|---|---|---|---|---|
| Kerkhoffs et al. [4] | Retrospective | Netherlands | 2003-2005 | 58/114 | 24h CCI | Bleeding, infection, and fever. |
| Kumawat et al. [18] | Prospective | India | 2015 | 17/30 | 24h CCI | Bleeding, infection, and fever. |
| Chen et al. [38] | Prospective | China | 2016-2018 | 22/105 | CCI | Bleeding, infection, and fever. |
| Murtasyidah et al. [32] | Prospective | Indonesia | 2019 | 10/23 | 24h CCI | / |
| Comont et al. [19] | Retrospective | France | 2001-2014 | 41/897 | PPI | Bleeding, infection. Fever, splenomegaly, and HLA alloantibody. |
| Lieberman et al. [33] | Retrospective | Canada | 2009-2013 | 10/87 | PPI | Bleeding, infection, and splenomegaly. |
| Hu et al. [36] | Retrospective | China | 2012-2018 | 66/560 10/133*** | PPI | Bleeding, infection, fever, splenomegaly, and HLA alloantibody |
| Cheok et al; [37] | Retrospective | Australia | 2003-2017 | 29/341 | PPI | HLA alloantibody |
| DeCoteau et al. [35] | Prospective | Canada | 1987-1990 | 23/162 | PPI | HLA alloantibody |
Note: *PTR/Total patients; **CCI: Corrected count increment; PPI: Post transfusion Platelet Increment; ***Numbers of patients with HLA-I antibody/Number of patients screened for HLA-I antibody.
Table 1: Characteristics of eligible investigations for the meta- analysis of immune and non-immune factors associated PTR in Haemato-oncology patients.
The eligible investigation data used in meta-analysis (Table 2). Sample size is the total number of haemato-oncology patients involved in the study. The 24 hours CCI, or PPI is the definition of PTR which reflects the overall PTR proportion presented by numbers of patients with PTR over the total numbers of patients? Fever, infection, bleeding, splenomegaly, and HLA alloantibody is calculated by the total PTR patients with certain risk factor over the total numbers of PTR patients. To be noted, HLA alloantibody data from study conducted by Hu et al is derived from a part of the total patient sample (133 out of 560) and 10 PTR patients tested positive for HLA alloantibody from that part of the sample [36]. This data is only analysed in line with other HLA alloantibody data, which cause no effects towards other risk factor group.
| Study | Sample size | 24 hours CCI, or PPI | Fever | Infection (sepsis) | Bleeding (haemorrhage) | Splenomegaly | HLA alloantibody | HPA alloantibody |
|---|---|---|---|---|---|---|---|---|
| Kerkhoffs et al. [4] | 114 | 58/114 | 43/58 | 41/58 | 17/58 | / | / | / |
| Kumawat et al. [18] | 30 | 17/30 | 13/17 | 12/17 | 15/17 | / | / | / |
| Chen et al. [38] | 105 | 22/105 | 9/22 | 13/22 | 11/22 | / | / | / |
| Murtasyidah et al. [32] | 23 | 10/23 | / | / | / | / | / | |
| Comont et al. [19] | 897 | 41/897 | 17/41 | 7/41 | 29/41 | 9/41 | 31/41 HLA | / |
| Lieberman et al. [33] | 87 | 10/87 | / | 7/10 | 7/10 | 6/10 | / | / |
| Hu et al. [36] | 560 (133)* | 66/560 10/133* | 40/66 | 7/66 | 5/66 | 18/66 | 10/66 HLA | / |
| Cheok et al. [37] | 341 | 29/341 | / | / | / | / | 29/341 HLA | / |
| DeCoteau et al. [35] | 162 | 23/162 | / | / | / | / | 12/23 HLA | / |
Note: *133 patients have screened for HLA-I antibody.
Table 2: Data of eligible investigations for the meta-analysis reporting immune and non-immune risk factors associated with PTR in haemato-oncology patients.
Study quality assessment
STROBE checklist was used to assess the quality and completeness for the included studies shown in Table 3. for the included studies shown in Table 3. All studies have provided sufficient details in title and abstract, introduction, and results section. Most of studies had a high-quality methods section with two studies failed to describe all statistical methods and one study only described a part of statistical methods [18,32,35]. Three studies failed to addressed exclusion criteria in eligibility criteria selection [32,35,37], and one study didn’t provide sufficient information for exclusion criteria [19]. A few studies provided detailed summary for key findings but failed to state any limitations [4,18,19,38]. Overall, the assessment of STROBE checklist has suggested that studies included for meta-analysis have relatively high quality which would minimise the risk of inaccuracy and unreliable of this meta-analysis.
| Study | Title and abstract | Introduction | Methods | Results | Discussion | |||
|---|---|---|---|---|---|---|---|---|
| Title or the abstract clearly demonstrated study design | Expressed the scientific background and the investigation purpose | Described the detailed study method | Indicated eligibility criteria selection | Described all statistical methods | Explained how the study size was arrived at | Stated characteristics of study participants | Summarised key findings and discussed study limitations | |
| Kerkhoffs et al. [4] | Y | Y | Y | Y | Y | Y | Y | Yd |
| Kumawat et al. [18] | Y | Y | Y | Y | N | Y | Y | Yd |
| Chen et al. [38] | Y | Y | Y | Y | Y | Y | Y | Yd |
| Murtasyidah et al. [32] | Y | Y | Y | Ya | N | Y | Y | Y |
| Comont et al. [19] | Y | Y | Y | Yb | Y | Y | Y | Yd |
| Lieberman et al. [33] | Y | Y | Y | Y | Y | Y | Y | Y |
| Hu et al. [36] | Y | Y | Y | Y | Y | Y | Y | Y |
| Cheok et al. [37] | Y | Y | Y | Ya | Y | Y | Y | Y |
| DeCoteau et al. [35] | Y | Y | Y | Ya | Yc | Y | Y | Y |
Note: Y-Criteria fulfilled; N-Criteria not fulfilled; a Inclusion criteria only, no exclusion criteria; b Only limited exclusion criteria mentioned; c Not all statistical methods have been described; d Summarised key findings without limitations.
Table 3: Evaluate the quality of investigations included in meta-analysis using Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
Meta-analysis of overall PTR proportion in haemato-oncology patients
Meta-analysis and six sets of forest plot are conducted on overall PTR incidence, bleeding related PTR, infection related PTR, fever related PTR, splenomegaly related PTR, and HLA alloantibody related PTR which all indicated in respectively using the eligible data in Table 2, Figures 2 and 3.
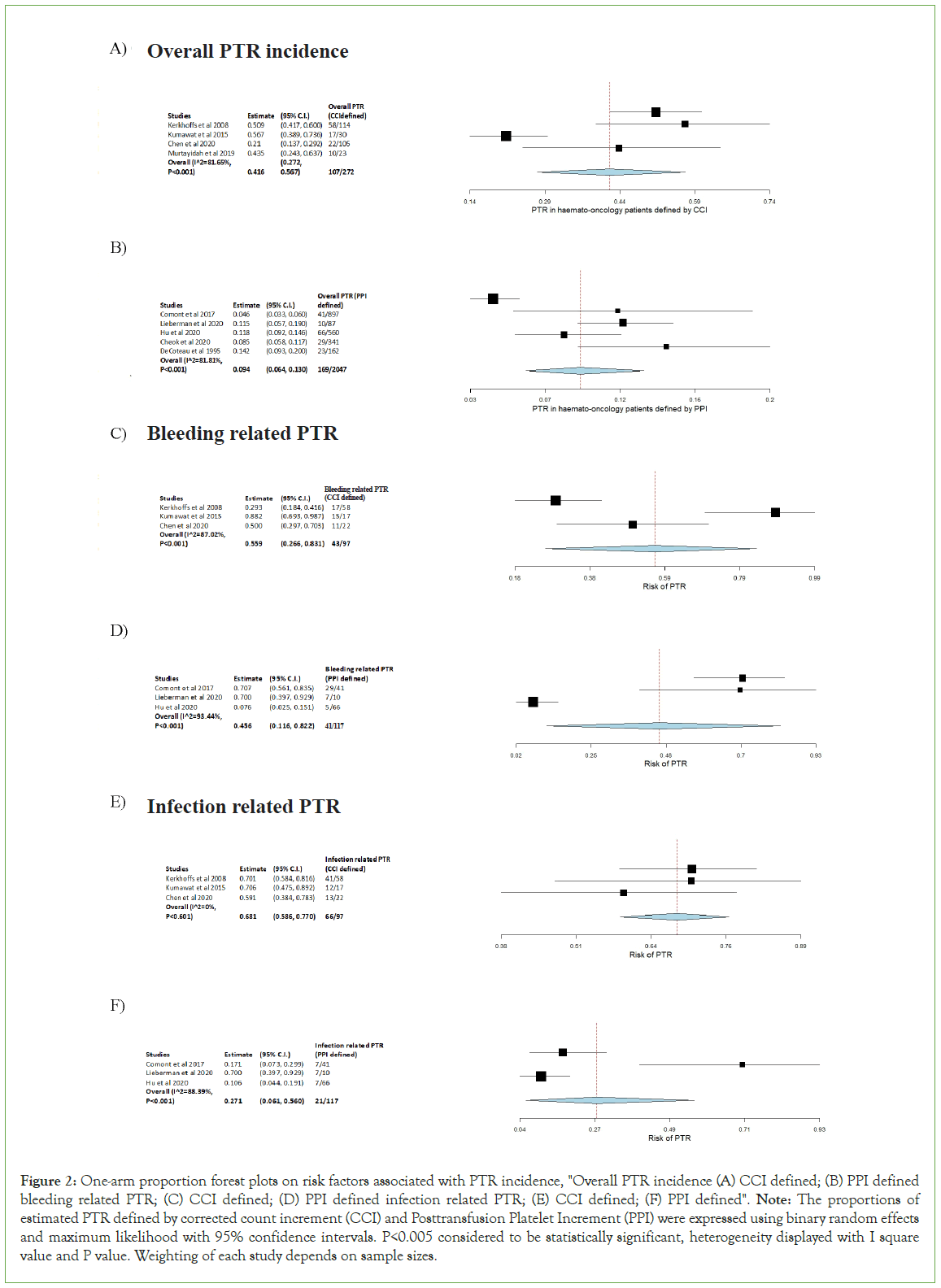
Figure 2: One-arm proportion forest plots on risk factors associated with PTR incidence, "Overall PTR incidence (A) CCI defined; (B) PPI defined bleeding related PTR; (C) CCI defined; (D) PPI defined infection related PTR; (E) CCI defined; (F) PPI defined". Note: The proportions of estimated PTR defined by corrected count increment (CCI) and Posttransfusion Platelet Increment (PPI) were expressed using binary random effects and maximum likelihood with 95% confidence intervals. P<0.005 considered to be statistically significant, heterogeneity displayed with I square value and P value. Weighting of each study depends on sample sizes.
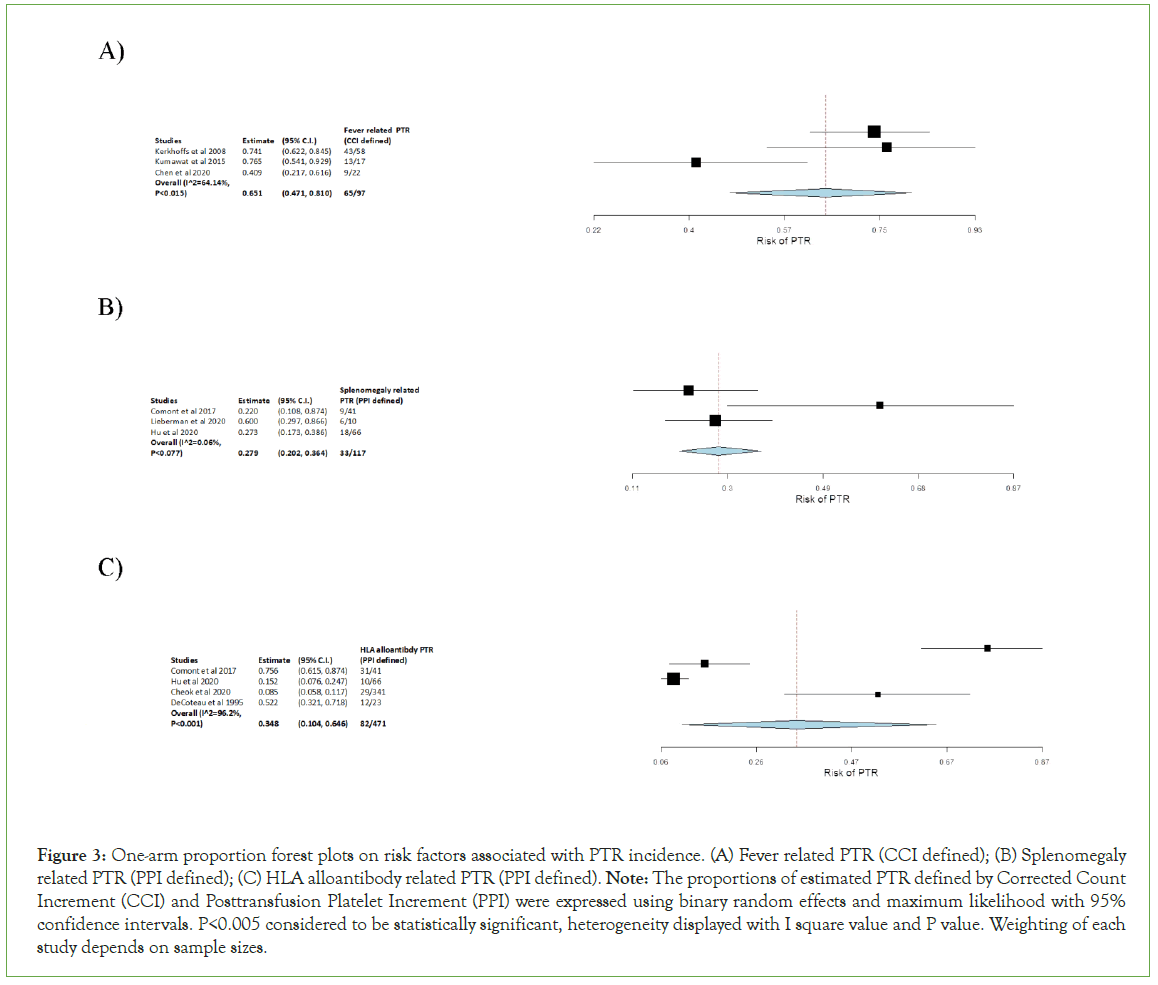
Figure 3: One-arm proportion forest plots on risk factors associated with PTR incidence. (A) Fever related PTR (CCI defined); (B) Splenomegaly related PTR (PPI defined); (C) HLA alloantibody related PTR (PPI defined). Note: The proportions of estimated PTR defined by Corrected Count Increment (CCI) and Posttransfusion Platelet Increment (PPI) were expressed using binary random effects and maximum likelihood with 95% confidence intervals. P<0.005 considered to be statistically significant, heterogeneity displayed with I square value and P value. Weighting of each study depends on sample sizes.
One arm proportion analyse performed on overall PTR defined by CCI and PPI, the PTR incidence for CCI was 39.34% (Figure 2A), whereas for PPI was 8.26% (Figure 2B). Both can be considered statistically significant with P-value<0.001. The I2 value for CCI was 81.65% with heterogeneity P-value<0.001, it demonstrates high heterogeneity which there are variations in the data (Figure 2A). Similar to CCI, I2 value for PPI was 81.81% with a heterogeneity P-value<0.001 (Figure 2B). It also reflects a relatively high heterogeneity and variability within the dataset. Both results illustrated PTR can be seen in 8-40% of haemato- oncology patients who require platelet transfusion. In comparison, CCI (95% C.I. 0.272, 0.567) defined PTR shows higher incidence than PPI (95% C.I. 0.064, 0.130).
Bleeding related PTR defined by CCI and PPI also analysed by one arm proportion. The incidence for bleeding related PTR was 44.33% for CCI defined (Figure 2C), and 35.04% for PPI defined (Figure 2D). They can all be considered statistically significant as their P-value<0.001. Bleeding related PTR defined by CCI had I2 value of 87.02%, P-value<0.001 (Figure 2C), whereas PPI had I2 value of 93.44%, P-value<0.001 (Figure 2D), which both indicated high heterogeneity and variations between these data. In comparison between CCI defined and PPI defined bleeding related PTR, CCI (95% C.I. 0.266, 0.831) had slightly higher risk than PPI (95% C.I. 0.116, 0.822).
CCI and PPI defined infection related PTR analysed by a one arm proportion was resulting both P-value<0.001 and they were statistics significant. Incidence of CCI-PTR was 68.04% (Figure 2E), compared to PPI-PTR of 17.95% (Figure 2F). Interestingly, for CCI, the I2 value is 0 with heterogeneity P-value of 0.604 which demonstrated fairly low variability in the data (Figure 2E). However, for PPI, the I2 value is 88.39% with P-value<0.001, it reflected high heterogeneity of the data (Figure 2F). The risk of infection related PTR defined CCI (95% C.I. 0.586, 0.770) is greater than the PPI (95% C.I. 0.061, 0.560) defined.
A one arm proportion analyse conducted for fever related PTR defined by CCI (Figure 3A). The incidence was 67.01% and it’s statistically significant with P-value<0.001. This dataset had I2 value of 64.14% with heterogeneity P-value=0.015 which illustrated high heterogeneity (Figure 3A). The results indicated a relatively high risk for fever related PTR (95% C.I. 0.471, 0.810).
Analysis of splenomegaly related PTR defined by PPI was also conducted by one arm proportion, which showed statistically significant with a P-value<0.001 (Figure 3B). The I2 value of 0.06% with heterogeneity P-value=0.077 suggested a slightly raised heterogeneity with some degrees of variation (Figure 3B). The incidence of splenomegaly related PTR was 28.21% which illustrated relatively high risk (95% C.I. 0.202, 0.364).
HLA alloantibody related PTR defined by PPI was also analysed by one arm proportion (Figure 3C). It had incidence of 17.4% along with P-value<0.001 which can be considered statistically significant. The I2 value of 96.2% with heterogeneity P-value<0.001 stated high heterogeneity with large variability in the data (Figure 3C). Overall, HLA alloantibody related PTR has the lowest risk among all other risk factor analysed above (95% C.I. 0.104, 0.646).
Discussion
PTR is often developed within the haemato-oncology patients as they have high platelet transfusion demand in treatment of thrombocytopenia secondary to malignancies [31,39]. Platelet transfusion failure could leads to tragic outcomes for the patients, Kerkhoffs et al, revealed that only 39.7% of PTR patients with haematological malignancies survived compared to 55.9% survival rate for non-PTR patients [4]. To define PTR, there are several measures have been used: CCI, PPI and Platelet Recovery Rate (PR) [5]. In this meta-analysis, studies with CCI and PPI defined PTR are included to discuss the importance and correlation between PTR and related immune, non-immune risk factors in haemato-oncology patients, who are PTR vulnerable groups rarely investigated by other systematic reviews.
The overall PTR incidence among patients with haematological malignancy explored in this study. In one hand, the CCI defined PTR proportion was statistically significant with overall 39.34% (107/272) patients developed PTR (95% C.I. 0.272, 0.567. P<0.001). I2 value=81.65% with P<0.001 represented a high heterogeneity-caused variability across four studies. Small numbers of studies included in this meta-analysis could be the cause, differences in sample size could also explained the variability of the data. Chen et al, had the lowest PTR incidence compared to other studies, this might be due to differences in definition of the PTR and small sample size among other studies [38]. In another hand, the PPI defined PTR had an overall 8.26% of incidence among haemato-oncology patients (169/2047) and was statistically significant (95% C.I. 0.064, 0.130. P<0.001). High heterogeneity also describes the variability of the data with I2=81.81% and P<0.001. This is mainly due to Comont et al, with the most sample size but had the lowest incidence [19]. It was found that in this study, they have implanted a stricter measure when describing PTR which was looking at the total post transfusion platelet count rather than increment only [19]. Overall, the results suggested that CCI defined PTR has higher incidence than PPI, that might be due to platelet count for PPI is normally recorded one-hour post transfusion which the platelet consumption or destruction has not initiated. Therefore, the impact on platelet count cannot be revealed by that time. It could be suspected either immune or non-immune cause based on the platelet count increment, the variation of the sample size could also contributes to the incidence difference (272 for CCI and 2047 for PPI) [1]. PTR incidence ranging from 8%-39% represented it is not to be neglected in haemato-oncology patients. Risk factors of PTR are needed to be understood for further clinical management.
Bleeding
Bleeding related PTR was analysed between CCI and PPI defined. Both were statistically significant (P<0.001) with high heterogeneity, CCI defined with I2=87.02%, P<0.001 and PPI defined with I2=93.44%, P<0.001.The numbers of studies included in the meta-analysis could explain the data variation caused high heterogeneity. CCI defined bleeding related PTR occurred in 44.33% patients (43/97) (95% C.I. 0.266, 0.831), which is slightly higher than PPI defined with 35.96% (41/117) (95% C.I. 0.116, 0.822). This analysis contained the studies indicated bleeding have positive correlation with the development of PTR [4,18,19], it would increase platelet consumption and decreases platelet count [16,33,36,38]. Kerkhoffs et al, suggested that PTR patients with bleeding had lower platelet count for a longer period of time than non-bleeding PTR patients, they have showed lower survival rate as well [4]. Their findings also align with results from Comont et al, 24.4% of PTR patients dead from bleeding (P<0.001) [19].
Infection
Infection plays an important role in developing PTR in haemato-oncology patients. This analysis reviewed infection related PTR for both CCI and PPI defined and indicated that infection is statistically significant with P<0.001. Interestingly, drastically different proportion explored when comparing two groups of patients, 68.04% (66/97) (95% C.I. 0.586, 0.770) CCI defined PTR patients were complicated to infection, whereas PPI defined PTR was 17.95% (21/117) (95% C.I. 0.061, 0.560). As two groups had similar numbers of sample size, the possible cause of this difference could be infection caused platelet destruction and consumption driven by neutrophils, macrophages and exogenous pathogen can only be observed in more than one hour after platelet transfusion [20-22]. Possible cause for low incidence in PPI group could also be studies included are all retrospective that some bias may potentially affect the statistics and sampling. Data from all three studies in CCI group had no heterogeneity with I2=0% P=0.604, which represented variability in this data are due to difference in sampling or chance. In infection related PPI defined PTR, a high heterogeneity demonstrated by I2=88.39% and P<0.001 explains the variability of the data is due to heterogeneity rather than chance or sampling error. It could be due to Lieberman et al, has smaller sample size than others which presented the highest proportion [33]. Even though there are significant PTR occurrences differences between CCI and PPI groups, averaging 40.65% of PTR patients along with previous study have suggested strong positive correlation between infection and PTR that can be induced by several mechanisms including pathogen interaction, phagocytosis, platelet desialylation and spleen sequestration that could lead to enhanced platelet clearance [21].
Fever
Three studies were included in the meta-analysis to evaluate the fever as an important factor for fever related PTR defined by CCI [4,18,38]. This analysis was statistically significant (95% C.I. 0.471, 0.810) and incidence of 67.01% (65/97) illustrated fever had positive correlation with the development of PTR among haemato-oncology patients, the result supported by Legler et al, finding [40]. Fever induced platelet clearance and destruction sometimes can be associated with infection, increasing levels of IL-1, IL-6 and such pro-inflammatory cytokines would activate the monocytes and macrophages, enhancing platelet clearance through phagocytosis and Fc gamma receptor mediated clearance [39]. High heterogeneity reflected by I2=64.14% P=0.015, which explains the variation of the data, and it could be due to the small numbers of articles included in the analysis.
Splenomegaly
Meta-analysis of splenomegaly related PTR defined by PPI consisted of three studies which was statistically significant. 28.21% (33/117) of PTR occurrences rate is relatively low compared to other risk factors (95% C.I. 0.202, 0.364). However, spleen is not only the most important organ that harboured platelet and controlled platelet sequestration, but splenomegaly also involves in haematological malignancies, which can potentially induce PTR in those patients who require platelet transfusion [27,41]. The data has no heterogeneity with I2=0.06% P=0.077, which represented data variation in this analysis is not due to heterogeneity but chance or difference sampling. Data from Lieberman et al with small sample size compared to others was mainly the cause [33].
HLA alloantibody
The immune-mediated PTR are normally contributing to a large portion of the PTR occurrence specially the HLA alloantibody which has drawn attention since they were discovered [9]. However, immune-mediated PTR are not predominantly found in haemato-oncology patients because they have impaired immune system and immunosuppressed due to the malignancy which reduces the ability of producing antibodies [42]. In this meta-analysis, four studies were included, and the results were statistically significant. Really high heterogeneity was observed with I2=96.2% P<0.001, which described the variation of the data. There were 17.41% (82/471) of PTR patients found to be related to mainly HLA class I alloantibody (95% C.I. 0.104, 0.646) which has the lowest incidence among all risk factors mentioned in this review. Comont et al, had the highest incidence than others, this could be due lack of identification of the HLA alloantibody as they could be induced by other underline conditions rather than PTR [19]. Although HLA alloantibody induced PTR had the lowest proportion, HLA matched platelet transfusions are still required for the best outcome of the patients [8,43].
Limitations
There are several limitations in relation to this review. Other than the risk factors analysed above, Disseminated Intravascular Coagulation (DIC), drug-related, HSCT (Hematopoietic Stem Cell Transplantation), ABO incompatible platelet transfusion, and other immune-mediated platelet destruction were not included in this review [39]. HPA was discovered recently to be a risk factor related to PTR, however, it was not discussed in this review due to lack of adequate articles and data [15,44]. Statistical heterogeneity overall in this study is high, this can be explained by limited number of studies included and small sample size in some of the studies. Some studies didn’t indicate all the selection criteria or expressed the limitations of the study. Also, some studies included are retrospective which could potentially contained bias and confounding factors that cannot be controlled. Lastly, PTR occurrence comparison between 1 hour and 24 hours platelet increment wasn’t included due to inadequate studies.
Conclusion
This systematic review and meta-analysis identified that PTR was an important adverse condition in haemato-oncology patients. Bleeding, infection, and fever was significantly correlated with PTR in haemato- oncology patients. Splenomegaly and HLA alloantibody was less significant, but precautions are still needed when encountering patients with these conditions. Given the high heterogeneity, limited number of studies and sample size consisted in this review, further investigation should be undertaken involving more adequate studies that analysed platelet increment in both 1 hour and 24 hours post transfusion with larger sample size, and studies analysed more risk factors such as DIC, and other immune mediated PTR.
Conflict of Interest
Authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Youk HJ, Hwang SH, Oh HB, Ko DH. Evaluation and management of platelet transfusion refractoriness. Blood Res. 2022;57(1):6-10.
[Crossref] [Google Scholar] [PubMed]
- Kerkhoffs J-LH, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, et al. A multi-centre randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108(9):3210-3215.
[Crossref] [Google Scholar] [PubMed]
- Hagino T, Sato T, Tsuno NH, Tasaki T. Incidence and management of non-immune platelet transfusion refractoriness: A narrative review. Annals of Blood. 2021;6:28.
- Kerkhoffs JL, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: Correlation with bleeding and survival. Transfusion. 2008;48(9):1959-1965.
[Crossref] [Google Scholar] [PubMed]
- Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–Practical approaches and ongoing dilemmas in patient management. Br J Haematol. 2015;171(3):297-305.
[Crossref] [Google Scholar] [PubMed]
- Cohn CS. Platelet transfusion refractoriness: How do I diagnose and manage? Hematology Am Soc Hematol Educ Program. 2020;2020(1):527-532.
[Crossref] [Google Scholar] [PubMed]
- Choo SY. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 2007;48(1):11-23.
[Crossref] [Google Scholar] [PubMed]
- Brown CJ, Navarrete CV. Clinical relevance of the HLA system in blood transfusion. Vox Sanguinis. 2011;101(2):93-105.
[Crossref] [Google Scholar] [PubMed]
- Kiefel V, Konig C, Kroll H, Santoso S. Platelet alloantibodies in transfused patients. Transfusion. 2001;41(6):766-770.
[Crossref] [Google Scholar] [PubMed]
- Brand A. Alloimmune platelet refractoriness: Incidence declines, unsolved problems persist. Transfusion. 2001;41(6):724-726.
[Crossref] [Google Scholar] [PubMed]
- Vorholt SM, Hamker N, Sparka H, Enczmann J, Zeiler T, Reimer T, et al. High-throughput screening of blood donors for twelve human platelet antigen systems using next-generation sequencing reveals detection of rare polymorphisms and two novel protein-changing cariants. Transfus Med Hemother. 2020;47(1):33-44.
[Crossref] [Google Scholar] [PubMed]
- Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia: Perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus. 2015;13(3):380-390.
[Crossref] [Google Scholar] [PubMed]
- Kurz M, Greinix H, HOcker P, Kalhs P, Knobl P, Mayr WR, et al. Specificities of anti-platelet antibodies in multitransfused patients with haemato-oncological disorders. Br J Haematol. 1996;95(3):564-569.
[Crossref] [Google Scholar] [PubMed]
- Pavenski K, Freedman J, Semple JW. HLA alloimmunization against platelet transfusions: Pathophysiology, significance, prevention and management. Tissue Antigens. 2012;79(4):237-245.
[Crossref] [Google Scholar] [PubMed]
- Kekomaki S, Volin L, Koistinen P, Koivunen E, Koskimies S, Ruutu T, et al. Successful treatment of platelet transfusion refractoriness: The use of platelet transfusions matched for both Human Leucocyte Antigens (HLA) and Human Platelet Alloantigens (HPA) in alloimmunized patients with leukaemia. Europ J Haematol. 1998;60(2):112-118.
[Crossref] [Google Scholar] [PubMed]
- Bishop J, McGrath K, Wolf M, Matthews J, De Luise T, Holdsworth R, et al. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood. 1988;71(2):383-387.
[Crossref] [Google Scholar] [PubMed]
- Chenna D, Shastry S, Baliga P. Evaluation and monitoring of response to platelet transfusion therapy: Experience from a tertiary care center. Acta Clin Belg. 2021;76(4):300-303.
[Crossref] [Google Scholar] [PubMed]
- Kumawat V, Sharma R, Malhotra P, Marwaha N. Prevalence of risk factors for platelet transfusion refractoriness in multitransfused hemato-oncological patients at tertiary care centre in North India. Asian J Transfus Sci. 2015;9(1):61-64.
[Crossref] [Google Scholar] [PubMed]
- Comont T, Tavitian S, Bardiaux L, Fort M, Debiol B, Moree D, et al. Platelet transfusion refractoriness in patients with acute myeloid leukaemia treated by intensive chemotherapy. Leuk Res. 2017;61:62-67.
- Li Y, Ryan J, Xu F, Vostal JG. Macrophage depletion mitigates platelet aggregate formation in splenic marginal zone and alleviates LPS-associated thrombocytopenia in rats. Front Med. 2019;6:300.
[Crossref] [Google Scholar] [PubMed]
- Belizaire R, Makar RS. Non-alloimmune mechanisms of thrombocytopenia and refractoriness to platelet transfusion. Transfus Med Review. 2020;34(4):242-249.
[Crossref] [Google Scholar] [PubMed]
- Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17(6):585-589.
[Crossref] [Google Scholar] [PubMed]
- Ojha A, Nandi D, Batra H, Singhal R, Annarapu GK, Bhattacharyya S, et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep. 2017;7:41697.
[Crossref] [Google Scholar] [PubMed]
- Etulain J, Lapponi MJ, Patrucchi SJ, Romaniuk MA, Benzadon R, Klement GL, et al. Hyperthermia inhibits platelet haemostatic functions and selectively regulates the release of alpha-granule proteins. J Thromb Haemost. 2011;9(8):1562-1571.
[Crossref] [Google Scholar] [PubMed]
- El-Radhi AS. Pathogenesis of fever. Clin Manual Fever Children. 2019:53-68.
- Schmuckenschlager A, Pirabe A, Assinger A, Schrottmaier WC. Platelet count, temperature and pH value differentially affect haemostatic and immunomodulatory functions of platelets. Thromb Res. 2023;223:111-122.
[Crossref] [Google Scholar] [PubMed]
- Aster RH. Pooling of platelets in the spleen: Role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45(5):645-657.
[Crossref] [Google Scholar] [PubMed]
- Hill-Zobel RL, McCandless B, Kang SA, Chikkappa G, Tsan MF. Organ distribution and fate of human platelets: studies of asplenic and splenomegalic patients. Am J Hematol. 1986;23(3):231-238.
[Crossref] [Google Scholar] [PubMed]
- Hsu C, Patell R, Zwicker JI. The prevalence of thrombocytopenia in patients with acute cancer-associated thrombosis. Blood Adv. 2023;7(17):4721-4727.
[Crossref] [Google Scholar] [PubMed]
- Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(5):662-672.
[Crossref] [Google Scholar] [PubMed]
- Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176(3):365-394.
[Crossref] [Google Scholar] [PubMed]
- Murtasyidah A, Tambunan BA, Andarsini MR. Platelet refractoriness evaluation after platelet concentrate transfusion in pediatric leukemia. Res J Pharm Technol. 2021;14(8):4277-42780.
- Lieberman L, Liu Y, Barty R, Heddle NM. Platelet transfusion practice and platelet refractoriness for a cohort of pediatric oncology patients: A single-center study. Pediatric Blood and Cancer. 2020;67(12): 28734.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Sys Review. 2015;4(1):1.
[Crossref] [Google Scholar] [PubMed]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349.
[Crossref] [Google Scholar] [PubMed]
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: Software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9(1):80.
[Crossref] [Google Scholar] [PubMed]
- DeCoteau J, Haddad S, Blanchette V, Poon A. Refractoriness to platelet transfusions in children with acute leukemia. J Pediatr Hematol Oncol. 1995;17(4):306-310.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Cai H, Zheng L, Luo Y, Zhou J, Hui Y, et al. Clinical and immunological features of platelet transfusion refractoriness in young patients with de novo acute myeloid leukemia. Cancer Medicine. 2020;9(14):4941-4948.
[Crossref] [Google Scholar] [PubMed]
- Cheok KPL, Chhetri R, Wee LYA, Friel O, Pham A, Salvi A, et al. The burden of immune-mediated refractoriness to platelet transfusions in myelodysplastic syndromes. Transfusion. 2020;60(10):2192-2198.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Zhou H, Guo B, Guan Z. Clinical efficacy of platelet transfusion therapy in patients with leukemia and analysis of risk factors for ineffective transfusion. Oncol Lett. 2020;19(3):2554-2561.
[Crossref] [Google Scholar] [PubMed]
- Song X, Qi J, Fang K, Li X, Han Y. A meta-analysis of risk factors associated with platelet transfusion refractoriness. International Journal of Hematology. 2023;117(6):863-875.
[Crossref] [Google Scholar] [PubMed]
- Legler TJ, Fischer I, Dittmann J, Simson G, Lynen R, Humpe A, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997;74(4):185-189.
[Crossref] [Google Scholar] [PubMed]
- Saboo SS, Krajewski KM, ORegan KN, Giardino A, Brown JR, Ramaiya N, et al. Spleen in haematological malignancies: Spectrum of imaging findings. Br J Radiol. 2012;85(1009):81-92.
[Crossref] [Google Scholar] [PubMed]
- Allegra A, Tonacci A, Musolino C, Pioggia G, Gangemi S. Secondary immunodeficiency in hematological malignancies: Focus on multiple myeloma and chronic lymphocytic leukemia. Front Immunol. 2021;12:738915.
[Crossref] [Google Scholar] [PubMed]
Citation: Long X, Jackson DE (2024) The Significance of Immune and Non-immune Causes Associated with Platelet Refractoriness in Haemato-oncology Patients: A Systematic Review and Meta-Analysis. J Blood Disord Transfus. 15.579.
Copyright: © 2024 Long X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

