Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 10
The Sex-Ratio, Developmental Stages and Cycle of Gonad Maturation of Neolissochilus hexagonolepis in the Mid-Reaches of the River Tamor
Suren Subba1, Vinod Kumar Mahaseth2, Bharatraj Subba2 and Shyam Narayan Labh3*2Department of Zoology, Tribhuvan University, Biratnagar, Nepal
3Department of Aquaculture, University of Idaho, Moscow, USA
Received: 15-Nov-2019, Manuscript No. jard-24-2700; Editor assigned: 20-Nov-2019, Pre QC No. jard-24-2700 (PQ); Reviewed: 04-Dec-2019, QC No. jard-24-2700; Revised: 01-Jul-2024, Manuscript No. jard-24-2700 (R); Published: 29-Jul-2024, DOI: 10.35248/2155-9546.24.15.924
Abstract
A population of Neolissochilus hexagonolepis captured from the mid-reaches of the river Tamor, eastern Nepal were investigated between December, 2014 and November, 2016. Fish samples were collected in the second half of every month and were evaluated for the Gonado-Somatic Index (GSI), developmental stages and cycle of gonad maturation. The population structure showed asymmetric sexual pattern with females dominating the number of males for most part of the study period. Monthly mean GSI varied from 0.17 ± 0.05% to 3.27 ± 1.68% and from 0.13± 0.07% to 10.57 ± 4.56% for male and female fish, respectively. The gonads were observed to pass through six stages of maturation viz. immature, maturing virgin and recovering spent, ripening, mature, spawning and spent. The maturation cycle showed an extended breeding season of the fish from May to November with peak breeding activity in July. The information gathered during the present study could be incorporated in management plans for making sustainable conservational efforts for the near-threatened N. hexagonolepis population in the river Tamor.
Keywords
Neolissochilus hexagonolepis; River tamor; Gonado-somatic index; Developmental stages; Maturation cycle
Introduction
River Tamor is famous for its unmatched and refreshing beauty with snow-fed crystal clear water. This river serves as the home as well as the breeding ground for many species of fishes. Fishing practices have been taking place in this river since the time immemorial and the local fishermen engage in fishing activities for sustenance of life [1].
The assessment of Gonado-Somatic Index (GSI) finds a great application in fish culture and its management, particularly by predicting the periodicity and timing of the breeding time. Towers suggested that Gonado-Somatic Index (GSI) of a fish increases with maturation of the fish, being maximum during the peak period of maturity and declining abruptly after spawning. Several workers have reported on the GSI of hill stream fishes among which Mahapatra and Vinod, Arjamand et al., Verma, Wagle and Jan and Ahmed have reported on the GSI of N. hexagonolepis, Labeo dyocheilus, Tor putitora, S. richardsonii and S. plagiostomus respectively.
Noble and Jones opined that the knowledge about different stages of gonadal maturatiovn of fish provides important information necessary to prohibit fishing during the restoration of the fish stock.
Jyrwa and Bhuyan highlighted the importance of the detailed study of the gonadal cycle and the ultra-structural changes of gonads in fish before attempting its culture and conservation through artificial means [2].
N. hexagonolepis has the conservation status of near threatened according to IUCN. The urgent need for the present study was felt in view of the lack in the detailed scientific information on the reproductive biology of this species from its natural habitat.
Materials and Methods
The samples were collected for two years, from December 2014 till November 2016 from the mid-reaches of the river Tamor, eastern Nepal. The study area lies between the latitude and longitude coordinates of 26°56.700'N, 26°55.653'N and 87° 23.097'E, 87°17.653'E (Figure 1). Fish samples were captured in the second half of every month using hooks, cast net, gill net and locally constructed traps. Total Length (TL) was measured using a graduated ruler while Total body Weight (TW) and Gonad Weight (GW) were measured using digital balance with the precision of 0.01 gm.
Figure 1: Location map of study area.
Sex identification, sex ratio and Gonado-Somatic Index (GSI)
Sex identification was done through examination of gonads after dissection, which was further confirmed through histology. Monthly male to female sex-ratio was computed throughout the study period.
Gonado-Somatic Index (GSI) was calculated using the formula
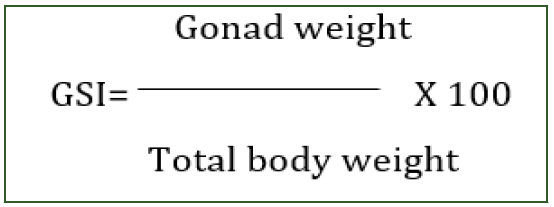
Stages and maturation cycle of gonads
The gonads were extracted in situ through longitudinal incision made along the ventral line of the body. They were then weighed and their morphological features noted. The gonads were washed in saline water and fixed in Bouin's fluid for 24 hours before transferring them into alcohol (70%) for preservation. The alcohol-preserved gonads were hydrated and dehydrated in alcohol series and embedded in paraffin wax [3]. They were then sectioned at 6 μ with a rotary microtome and the sections were double stained in hematoxylin and eosin before mounting in DPX. Finally, the sections were observed under a binocular microscope.
Gonad maturity stages were determined by examination of the histomorphological features of gonads according to the maturity scale modified after Brown-Peterson et al. The maturation cycle of gonads was investigated by calculating the frequency of a specific stage of gonad as percentage on monthly basis.
Statistical analysis
Descriptive statistics was used to outline the basic features of the data obtained during the study by giving simple summaries like the mean and standard deviation. Spearman rank correlation was performed to investigate the relationship between the weight and length with the maturity stages of either sex of the fish.
Results and Discussion
Altogether 198 fishes were sampled during the study period. Females dominated the number of males for most part of the study with the overall male to female sex ratio as 1:1.22. The asynchronous spawning migration and the partial segregation by sex during feeding caused the asymmetric sexual pattern of N. hexagonolepis in the river [4].
Gonado-Somatic Index (GSI)
The monthly mean GSI values varied from 0.17 ± 0.05% to 3.27 ± 1.68% and from 0.13 ± 0.07% to 10.57 ± 4.56% for male and female fish, respectively (Figure 2).
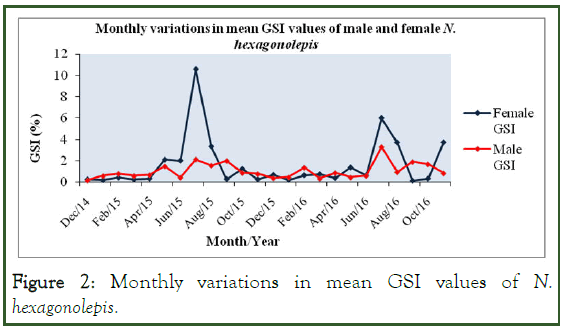
Figure 2: Monthly variations in mean GSI values of N. hexagonolepis.
The peak breeding season of N. hexagonolepis was found to occur in July as indicated by the higher GSI values for both the sexes in this month. Arruda, et al. suggested that the timing of reproduction and spawning can be identified from changes in the GSI and that the breeding season of fish corresponds to that during which the values of GSI for both male and female spike to the peak [5].
The single annual peak of GSI value for either sex gave a clear indication that the species is an annual breeder. Similar breeding behaviours were also reported in Labeo rohita, L. dyocheilus and Tor putitora.
We found that the mean GSI values of the fish declining abruptly after the peak breeding season and attributed this to the tremendous decrease in weight of the gonads after the spawning act. Similar trends in GSI values were also observed by Verma and Joshi, et al. in Labeo dyocheilus and Schizothorax richardsonii, respectively.
Developmental stages and maturation cycle of gonads
The testes and ovaries of N. hexagonolepis were observed at six different stages viz. stage I (immature stage), stage II (maturing virgin and recovering spent), stage III (ripening), stage IV (mature), stage V (spawning) and stage VI (spent) (Table 1).
| Gonad maturity stages | Gonad developmental status | Description of gonad | |
|---|---|---|---|
| Male | Female | ||
| I | Immature | Testes slender, thread-like, dirty white without vascular supply. Cysts of spermatogenic cells visible | Ovaries thin, slender, thread-like, dirty white in colour and translucent. Oocytes at stages I and II visible |
| II | Maturing virgin and recovering spent | Testes slender, whitish with vascular supply. Spermatogonia seemed to be dividing giving rise to spermatocytes | Ovaries still slender but slightly larger than at stage I. Oocytes at stages III and IV along with a few oocytes at stages I and II visible in ovigerous lamellae |
| III | Ripening | Increased in weight and volume occupying about 2/3 of the body cavity. All the stages of spermatogenesis viz. spermatocytes, spermatids and spermatozoa observed | Ovaries whitish-yellowish in colour with granular appearance. Ovigerous lamellae filled with large number of oocytes at stages IV and V along with a few oocytes at stage VI |
| IV | Mature | Testes large, turgid occupying almost the entire body cavity. Testicular wall thin and seminiferous lobules packed with spermatozoa. Few spermatids also visible | Ovaries large and deep yellowish in colour occupying almost the entire abdominal cavity. Large number of stage VII oocytes and some ripe eggs visible |
| V | Spawning | Testes pinkish white, turgid and occupied the entire body cavity. Milt was seen to be oozing out. Seminiferous lobules packed with spermatozoa with some empty lobules | Ovaries large and distended, occupying the entire abdominal cavity. Large number of jelly like yellowish translucent eggs present in the ovaries. Spawning was imminent |
| VI | Spent | Testes dull-white and appeared flaccid. Seminiferous lobules seen collapsing and empty | Ovaries flaccid, shrunken. Oocytes at stage VII along with oocytes at stages I and II visible in the ovigerous lamellae |
Table 1: Developmental stages of gonads of N. hexagonolepis in the mid-reaches of river Tamor.
Swar investigated the maturity stages of ovaries and testes of N. hexagonolepis based on their external features and established seven maturity stages for both the gonads. Jyrwa and Bhuyan also carried out the histological studies of gonads of the fish from Meghalaya, India and established their five developmental stages [6].
Intense spermatogenesis was observed during ripening stage of the testes where we examined all the stages of spermatogenesis viz. spermatocytes, spermatids and spermatozoa within the lobules (plate 1a).
Histologically, the seminiferous lobules of matured testes were seen to be packed with spermatozoa with some spermatids (plate 1b). Spent testes were seen to be collapsing and empty and contained only a small amount of unexpelled or residual sperms (plate 1c) (Figure 3).
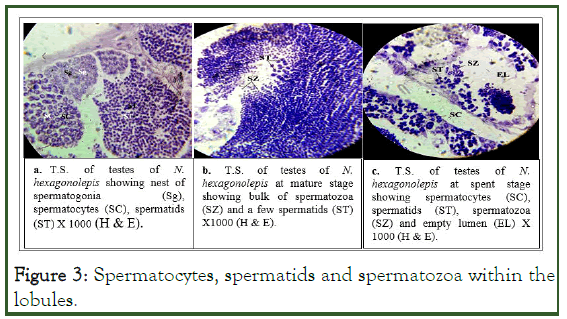
Figure 3: Spermatocytes, spermatids and spermatozoa within the lobules.
We observed oocytes at various stages of development in the ovigerous lamellae of the ovaries. Oocyte I, referred to as the chromatin nucleolus stage, contained a prominent nucleus with 3 to 4 nucleoli (plate 2a). The oocytes at II stage were recognized as the early and late perinucleolus stage on the basis of the distribution of the nucleoli within the nucleus [7]. Nucleoli increased in number as the oocytes matured and got distributed adjacent to the periphery of nuclear membrane (plate 2b and plate 2c). Oocyte III, conventionally termed as the early yolk vesicle stage, was characterized by the appearance of a large number of small, clear vacuoles in the cortical area of cytoplasm (plate 2d). Oocyte IV, referred to as the late yolk vesicle stage, showed further increase in size and number of the yolk vesicles which distributed randomly in the ooplasm (plate 2e) [8]. Yolk globules were seen to appear among the vesicles in the ooplasm of the oocyte at stage V. The oocyte at this stage is termed as the early yolk stage (plate 2f). Oocyte VI (late yolk stage) was characterized by the increase in number and size of yolk globules and the yolk vesicles became pushed towards the peripheral region of the oocyte (plate 2g). Oocyte VII showed a heavy deposition of yolk globules in the cytoplasm (plate 2h).
Sharma et al. also recognized the stages of oocytes as chromatin nucleolar stage, perinucleolar stage, early yolk vesicle stage, late yolk vesicle stage, early yolk stage, late yolk stage and ripe egg stage in garra gotyla gotyla.
The flaccid appearance of testes and ovaries of the fish during the post spawning season was attributed to the expulsion of spermatozoa and ripe eggs during the spawning season [9].
Significant positive correlations were observed between the various morphometric parameters and maturity stages of gonads for both the sexes of N. hexagonolepis (Table 2). These revealed that the matured fishes were heavier and larger in size and the gonads at peak maturity become heavier as they become fully packed with sperms or ripe eggs (Figure 4).
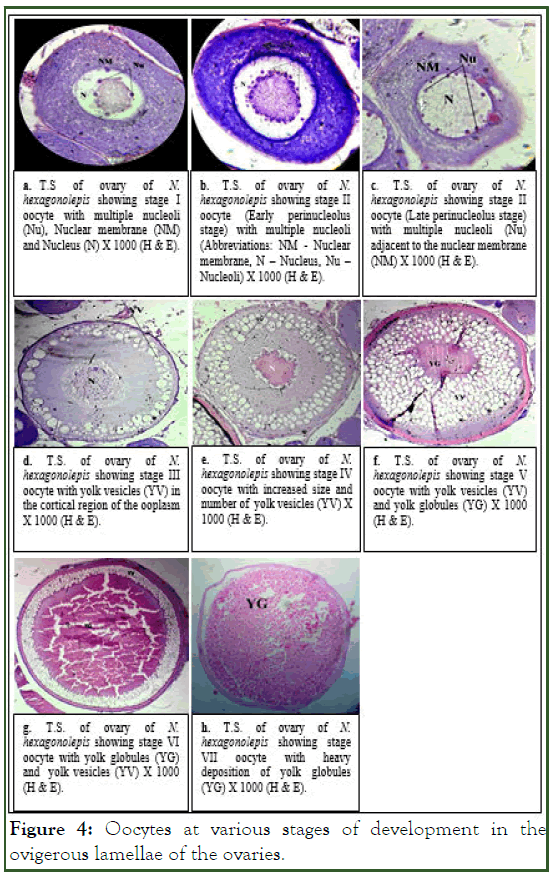
Figure 4: Oocytes at various stages of development in the ovigerous lamellae of the ovaries.
| Variables | Stage of gonad | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TW (gm) | TL (cm) | GW (gm) | TW (gm) | TL (cm) | GW (gm) | ||||
| Spearman's rho | Maturity stage of gonad | Correlation coefficient Sig. (2-tailed) | 1 | 0.708** | 0.685** | 0.901** | 0.708** | 0.685** | 0.901** |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
Note: ** Correlation is significant at the 0.01 level (2-tailed)
Table 2: Spearman rank correlations and significance values for morphometric parameters with stages of gonad of female N. hexagonolepis.
We found that the various stages of gonads of N. hexagonolepis showed monthly occurrence with varying frequencies. Stage II testes showed the highest occurrence among the months with their frequencies ranging from 11.11% in May to 71.43% in March.
Matured and spawning testes showed their occurrence from May onward. Similarly, spent testes occurred in August, September and October, constituting 50% of the total catch in the month of October (Figure 5).
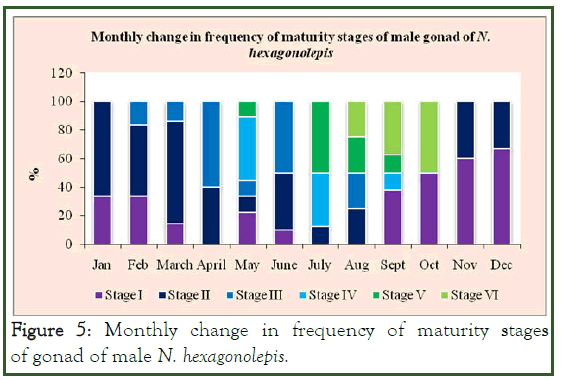
Figure 5: Monthly change in frequency of maturity stages of gonad of male N. hexagonolepis.
Stage II ovaries occurred throughout the year with their frequency ranging from 7.69% in July to 57.14% September [10]. Next in frequency were the ovaries at stage I constituting 20% in February to 69.23% of the total monthly catch in January. Ovaries at the mature and spawning stages were encountered till November. Spent ovaries (stage VI) occurred in November and December. We did not examine the spent ovaries from January onward (Figure 6).
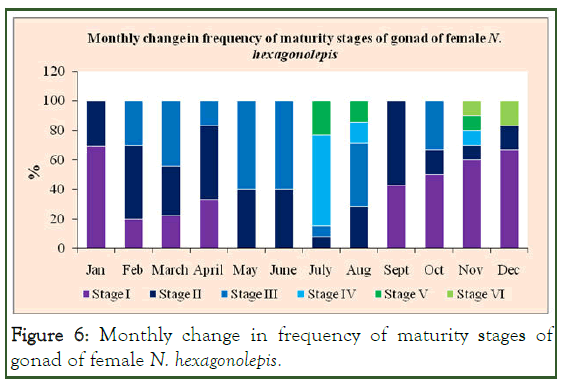
Figure 6: Monthly change in frequency of maturity stages of gonad of female N. hexagonolepis.
The capture of matured and spawning male fishes since May indicated the commencement of breeding season of the fish from this month [11]. Also, the capture of matured and spawning female fishes till November with the total absence of spent fishes from January onward gave a clear indication of the extended breeding season of N. hexagonolepis lasting from May till November. The extended breeding period of N. hexagonolepis has also been reported by Jhingran, Swar and Arunachalam. The extended breeding period of N. hexagonolepis could be considered as a part of the strategies developed by the species to withstand the environmental pressures.
Maximum number of matured and spawning fishes was captured during July. The highest GSI values for both the sexes were also assessed during the same month. Therefore, we concluded that the peak breeding season of N. hexagonolepis coincides the month of July [12].
Conclusion
The study indicated that N. hexagonolepis is an annual breeder with an extended breeding period from May till November showing peak breeding activity in July. The information gathered during the present study could be incorporated in management plans for making sustainable conservational efforts of the species in the wild. The finding of the study may also act as a baseline for understanding the reproductive biology as a pre-requisite for commercial domestication of the fish.
Conflict of Interest
Authors declared no conflict of interest.
References
- Arjamand S, Dar SA, Desai AY, Sayani AN, Yusufzai SI, Ashfaq M, et al. Reproductive biology of an endangered coldwater fish golden mahseer, Tor putitora (Ham.) from Anji Mahseer hatchery Reasi. IOSR J Pharm. 2013;3(10):13-16.
- Arruda LM, Azevedo JN, Neto AI. Abundance, age-structure and growth and reproduction of gobies (Pisces; Gobiidae) in the Ria de Aveiro Lagoon (Portugal). Estuar Coast Shelf Sci. 1993;37(5):509-523.
- Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK. A standardized terminology for describing reproductive development in fishes. Mar Coast Fish. 2011;3(1):52-70.
- Subba S, Mahaseth VK, Subba BR, Labh SN. Fecundity, histomorphology of the ovary and size at first maturity of Neolissochilus hexagonolepis (McClelland) in Tamor river, Nepal. J Nat Appl Sci. 2020;12(3):401-410.
- Jan M, Ahmed I. Assessment of fecundity, gonadosomatic index and hepatosomatic index of snow trout, Schizothorax plagiostomus in river Lidder, from Kashmir Himalaya, India. Int J Fish Aquat. 2016;4(2):370-375.
- Jyrwa LB, Bhuyan RN. Histological studies of gonads in Neolissochilus hexagonolepis (McClelland, 1839) (Teleostei: Cyprinidae) from Meghalaya, India. Iran J Ichthyol. 2017;4:140-149.
- Bagchi A, Jha P. Fish and fisheries in Indian heritage and development of pisciculture in India. Rev Fish Sci. 2011;19(2):85-118.
- Rahel FJ. Changing philosophies of fisheries management as illustrated by the history of fishing regulations in Wyoming. Fish. 2016;41(1):38-48.
- Wagle SK. Studies on gonadosomatic index, fecundity and hatchability of domesticated stock of asala Schizothorax richardsonii (Gray) from Nallu river of Lalitpur District. Our Nature. 2014;12(1):19-27.
- Subba S, K Mahaseth V, R Subba B, R Bhusal D. Monthly dynamics of reproductive indices of Neolissochilus hexagonolepis (McClelland, 1839) and their relationship with physico-chemical parameters along the mid-reaches of Tamor river, Nepal. Egypt J Aquatic Biol Fish. 2020;24(2):239-247.
- Sarma D, Mohan D, Posti R, Arya M, Ganie PA. The mighty mahseers of the genera Tor, Neolissochilus and Naziritor: A review on resource distribution, biology, ecotourism and conservation. Indian J Fish. 2022;69(4):146-169.
- Kechu M, Ezung S, Pankaj PP. Bionomics, length-weight relationship and condition factor of chocolate mahseer, Neolissochilus hexagonolepis from Dikhu river, Nagaland. J Environ Biol. 2023;44(5):706-712.
Citation: Subba S, Mahaseth VK, Subba B, Labh SN (2024) The Sex-Ratio, Developmental Stages and Cycle of Gonad Maturation of Neolissochilus hexagonolepis in the Mid-Reaches of the River Tamor. J Aquac Res Dev. 15:888.
Copyright: �?�© 2024 Subba S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

