Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 2
The Production of Biodiesel from Coconut Oil Using Base Catalyst Sodium Hydroxide and Methanol: Through Transesterification Method
H Y Bawah1 and Ibrahim Mustapha Muhammad2*2Department of Geology, Ahmadu Bello University, Zaria, Nigeria
Received: 08-Sep-2020, Manuscript No. ACE-24-6420; Editor assigned: 11-Sep-2020, Pre QC No. ACE-24-6420 (PQ); Reviewed: 25-Sep-2020, QC No. ACE-24-6420; Revised: 01-Aug-2024, Manuscript No. ACE-24-6420 (R); Published: 29-Aug-2024, DOI: 10.35248/2090-4568.24.14.333
Abstract
Biodiesel is synthesized from edible, non-edible and waste cooking oil or animal oil which can be regarded as an alternative diesel fuel. The various alternative fuel options tried in place of hydrocarbon oils are mainly biogas, producer gas, ethanol, methanol and vegetable oils. The aim of this research is to produce biodiesel from coconut oil using sodium hydroxide and methanol through transesterification method which is the most conventional method. In carrying out this research, different samples of coconut oil were subjected to a microwave power of different temperatures of 20°C, 30°C, 40°C, 50°C and 60°C for 1, 2, 3, 4 and 5 minutes respectively. The highest percentage yield was obtained at a short reaction time of 5 minutes as a result of increase in temperature. The graph of percentage yields against time which was plotted clearly showed that the longer the reaction time, the higher the yield of biodiesel. Properties such as density (852 kg/m3), viscosity (3.53 mm²/s at 40°C), flashpoint (119°C), pour point (-3°C), cetane (56) number, calorific value (38°C) and heating value (37.3°C) of the samples with the highest yield were obtained. This research shows that the highest percentage yield was obtained at a reaction time of 5 minutes using microwave oven at 60°C. This clearly shows that the higher the temperature the higher the percentage yield. From the experiments and results obtained, it can be concluded that biodiesel is more effective and less harmful than the fossil diesel, hence it is a good alternative for fossil diesel and can be produced in large scale.
Keywords
Biodiesel; Transesterification method; Sodium hydroxide; Coconut oil; Methanol
Introduction
Fuel and energy crisis and the society's concern for depleting world's nonrenewable energy resources led to the search for alternative fuels. Various global concerns such as the dwindling crude oil reserve, global warming and climatic change, air pollution and public health and more importantly the steady rise in the cost of fossil fuel, have altogether received great interest in the use of renewable fuels. One of the most promising alternatives fuel that has properties close to those of diesel is vegetable oils and one of which is coconut oil [1].
In 1912, Rudolf Diesel who invented and designed the diesel engine, predicted that the use of vegetable oils for engine fuels may seem insignificant today, but such oils may become in the course of time as. To overcome the problems associated with the direct use of vegetable oil, such as injector coking and the thickening of crankcase oil which results in piston ring sticking, various modifications of vegetable oils such as transesterification, microemulsion formation and the use of viscosity reducers have been tried. Among them, transesterification was considered the most suitable because the properties of the esters are similar to those of diesel. Through transesterification, these vegetable oils are converted to the alkyl esters of the fatty acids present in the vegetable oil. These esters are commonly referred to as biodiesel. The aim of this research is to produce biodiesel from coconut oil using sodium hydroxide and methanol through transesterification method which is the most conventional method [2]. This method is advantageous in respect of producing a less polar and corrosive biofuel with reduced cloud and pour points. Biodiesel is an alternative fuel that is renewable in the sense that its primary feedstock has a sustainable source.
Part of the advantages of using biodiesel is that it results in substantial reductions of unburned hydrocarbons, Carbon Monoxide (CO) and Particulate Matter (PM). For instance, the use of pure biodiesel can result in reductions of PM and CO by 50 percent and reductions of unburned hydrocarbons by 70 per cent. Also, biodiesel has no sulfur content and is thus compatible with the next generation of diesel engine pollution reduction devices, such as particulate filters, which require ultralow sulfur diesel fuel for effective operation [3]. When used in diesel engines there is a slight increase in nitrogen oxides (NOx; this increase is negligible when biodiesel is blended with petroleum diesel). Also, the increase does not occur when biodiesel is used in furnace heaters. Biodiesel is an easy alternative fuel to use because it can be used in most diesel equipment, in fact adding biodiesel increases the lubricity of the fuel, which can increase the life of the engine and its components. Because biodiesel can be produced domestically from renewable sources, such as canola or other oil seed crops or crop-residue, it is virtually CO2 neutral. Its use does not contribute to global warming [4].
In view of the current instability in oil prices, biodiesel stands as an attractive source of alternative energy. By adopting and increasing the use of biodiesel, European countries have reduced from her over-dependence on crude oil reserves. Besides, conventional fossil fuel has been reported as being finite. While it is worthy to note that biodiesel will not completely displace petroleum diesel, biodiesel has its place as an alternative fuel and can be a source of lubricity as an additive to diesel fuel. The emissions produced from biodiesel are cleaner compared to petroleum-based diesel fuel. Particulate emissions, soot and carbon monoxide are lower since biodiesel is an oxygenated fuel [5].
Materials and Methods
Material collection
Coconut oil, 2500 ml of pure coconut oil which is the raw material (vegetable oil) for biodiesel production was procured from a local market in Maiduguri, Borno state. Both methanol and sodium hydroxide were obtained from five-star chemical company, Maiduguri, Borno state. The methanol served as the reactant while sodium hydroxide was the catalyst (Table 1). Distilled water was used to wash the biodiesel after production to remove the impurities and soap produced during transesterification and was obtained from the department of chemical engineering laboratory, university of Maiduguri, Borno state (Figure 1) [6].

Figure 1: Methanol, sodium hydroxide, coconut oil.
| Equipment | Name of the company and place of manufacture | Type |
|---|---|---|
| Separation funnel | Bruker corporation, USA | Teflon straight |
| Heater (Microwave oven) | Shenzhen PCB, China | EMS |
| Conical flask (reactor) | Ablaze glass work, USA | Erlenmeyer flask |
| Stirrer | Microteknik, India | Anchor blade |
| Weigh balance | Dapeng, China | Multi-functional precision balance |
Table 1: List of equipment’s used.
Method
Transesterification process was adopted for the production of the biodiesel. 5.0 g of sodium hydroxide pellet was mixed with 100 ml of methanol inside a strong heat resistance glass beaker. Oil to alcohol ratio was 5:1. The mixture was stirred vigorously until sodium hydroxide pellets dissolved and formed a strong base known as sodium methoxide (NaOCH3). Then 500 ml of the treated coconut oil was poured into the reactor and heated gently at temperatures of 20°C, 30°C, 40°C, 50°C and 60°C for 1, 2, 3, 4 and 5 minutes respectively, then the methoxide was added to the oil and the mixture was stirred vigorously in order to obtain a homogeneous mixture. The final mixture obtained was poured into a separating funnel and made to settle for 24 hours. The mixture was separated into two layers with the biodiesel floating on top and glycerol at the bottom so the biodiesel was decanted [7]. The raw biodiesel was washed with water to remove some traces of soap and other contaminants and the water was allowed to settle down before removing it by draining.
The washed biodiesel was collected in a beaker and gently heated in an oven at 105°C to evaporate the excess water and methanol in the biodiesel. These steps were repeated for five different samples of coconut oil at different temperature and reaction time.
Biodiesel washing
The pure collected biodiesel after transesterification reaction was poured into a beaker. Hot water of 40°C was poured into the biodiesel slowly and the mixture was shaken and kept for 1 hour in a stable position. A layer of soap was formed at the bottom of the beaker. The biodiesel was then collected (Figure 2) [8].
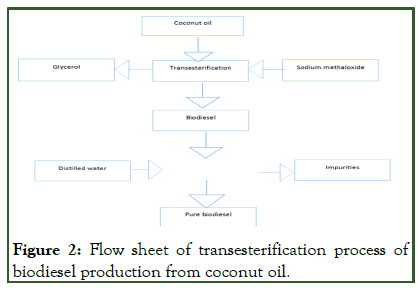
Figure 2: Flow sheet of transesterification process of biodiesel production from coconut oil.
Results
The percentage yield of biodiesel from coconut oil was obtained by subjecting it to a microwave power of 20°C, 30°C, 40°C, 50° C and 60°C for 1, 2, 3, 4 and 5 minutes respectively as shown in Tables 2-4 (Figure 3).
| S/N | Time (min) | Temperature (°C) | Percentage yield (%) |
|---|---|---|---|
| 1 | 1 | 20 | 83 |
| 2 | 2 | 30 | 86 |
| 3 | 3 | 40 | 90 |
| 4 | 4 | 50 | 91 |
| 5 | 5 | 60 | 93 |
Table 2: Yield of samples at various temperatures and reaction time.
| S/N | Property | Value |
|---|---|---|
| 1 | Viscosity (mm2/s at 40°C) | 3.53 |
| 2 | Density (kg/m3) | 852 |
| 3 | Flash point (°C) | 119 |
| 4 | Pour point (°C) | -3 |
| 5 | Calorific value (°C) | 38 |
| 6 | Heating value (°C) | 37.3 |
| 7 | Cetane number | 56 |
Table 3: Properties of biodiesel obtained from coconut oil.
| S/N | Time (mins) | Temperature (°C) | Theoretical weight of sample (g) | Actual weight of sample (g) | Percentage yield of samples (%) |
|---|---|---|---|---|---|
| 1 | 1 | 20 | 500 | 415 | 83 |
| 2 | 2 | 30 | 500 | 428 | 86 |
| 3 | 3 | 40 | 500 | 450 | 90 |
| 4 | 4 | 50 | 500 | 455 | 91 |
| 5 | 5 | 60 | 500 | 465 | 93 |
Table 4: Percent yield of the five samples.

Figure 3: Graph showing percentage yield against time.
DISCUSSION
Determination of percentage yield of biodiesel
The treated coconut oil was weighed before the transesterification process by weighing scale and the weight was referred to as theoretical yield. The biodiesel produced was weighed with the same weighing scale and this weight was referred to as actual yield [9].
Percent biodiesel yield=(Actual weight/Theoretical weight) × 100
The highest percentage yield of biodiesel from coconut was found to be 93%. It is very evident in that the highest yield of biodiesel was obtained at 60°C in 5 min reaction time. While in it is seen that the viscosity of the biodiesel is 3.53 mm2/s. So also the density is seen to be 852 kg/m³. However, the flash point and pour point are 119°C and -3°C respectively. The calorific and heating values are 38 MJ/kg and 37.3 MJ/kg. While the cetane number was found to be 56. These properties are much better than the fossil diesel [10].
From the graph of percentage yield against it is clearly seen that 60°C gives the highest yield within 5 minutes. Also, as the temperature increases, the yield increases. This is because at high temperatures NaOH reacts faster due to its fast reacting ability [11,12].
Conclusion
Transesterification is a commonly employed method for biodiesel production. The purpose of this method is to reduce the viscosity of oil or fat using acid or base catalyst in the presence of methanol or ethanol. It is very evident that coconut oil is a good feedstock for biodiesel production. It was observed that the highest percentage yield was obtained at a time of 5 minutes at 60°C. This shows that sodium hydroxide catalyst reacts faster as the temperature increases. The biodiesel produced can be used in convectional diesel engine, injectors and fuel pumps without modification. Finally, the emissions produced from biodiesel are cleaner compared to petroleumbased diesel fuel.
References
- Tiwari AK, Kumar A, Raheman H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenergy. 2007;31(8):569-575.
- Abdullah AZ, Razali N, Mootabadi H, Salamatinia B. Critical technical areas for future improvement in biodiesel technologies. Environ Res Lett. 2007;2(3):034001.
- Al-Widyan MI, Al-Shyoukh AO. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour Technol. 2002;85(3):253-256.
[Crossref] [Google Scholar] [PubMed]
- Alamu OJ, Waheed MA, Jekayinfa SO. Biodiesel production from Nigerian palm kernel oil: Effect of KOH concentration on yield. Energy Sustain Dev. 2007;11(3):77-82.
- Al‐Zuhair S. Production of biodiesel: Possibilities and challenges. Innova Sustain Econ. 2007;1(1):57-66.
- Azam MM, Waris A, Nahar NM. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy. 2005;29(4):293-302.
- Dunn RO, Shockley MW, Bagby MO. Improving the low‐temperature properties of alternative diesel fuels: Vegetable oil‐derived methyl esters. J Am Oil Chem Soc. 1996;73(12):1719-1728.
- Dunn RO. Antioxidants for improving storage stability of biodiesel. Innov Sustain Econ. 2008;2(4):304-318.
- Georgogianni KG, Katsoulidis AK, Pomonis PJ, Manos G, Kontominas MG. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process Technol. 2009;90(7-8):1016-1022.
- Kafuku G, Lam MK, Kansedo J, Lee KT, Mbarawa M. Croton megalocarpus oil: A feasible non-edible oil source for biodiesel production. Bioresour Technol. 2010;101(18):7011-7015.
[Crossref] [Google Scholar][PubMed]
- Goujard L, Villeneuve P, Barea B, Lecomte J, Pina M, Claude S, et al. A spectrophotometric transesterification-based assay for lipases in organic solvent. Analyt Biochem. 2009;385(1):161-167.
[Crossref] [Google Scholar] [PubMed]
- Hernandez-Martin E, Otero C. Different enzyme requirements for the synthesis of biodiesel: Novozym 435 and lipozyme TL IM. Bioresour Tech. 2008;99(2):277-286.
Citation: Bawah HY, Muhammad IM (2024) The Production of Biodiesel from Coconut Oil Using Base Catalyst Sodium Hydroxide and Methanol: Through Transesterification Method. Adv Chem Eng. 14:333.
Copyright: © 2024 Bawah HY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

