Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2024) Volume 12, Issue 4
The Peroxidation of Lipids, Cellular Senescence and Aging
T Blake Monroe, Paul D Robbins and David A Bernlohr*Received: 03-Dec-2024, Manuscript No. JASC-24-27682; Editor assigned: 05-Dec-2024, Pre QC No. JASC-24-27682 (PQ); Reviewed: 19-Dec-2024, QC No. JASC-24-27682; Revised: 26-Dec-2024, Manuscript No. JASC-24-27682 (R); Published: 02-Dec-2024, DOI: 10.35248/2329-8847.24.12.385
Abstract
The inducers of cellular senescence as a determinant in organismal aging are complex and likely driven by a combination of hormonal and metabolic factors. Lipids have recently been implicated as inducers of cellular senescence in vitro and in vivo across human and animal models and more directly, the electrophilic products of lipid peroxidation have been shown in a number of systems to initiate the senescence program. This review summarizes recent research at the interface of lipid biology and senescence. The review will emphasize the types of electrophilic lipids that induce senescence and how lipid scavengers are used to alleviate senescence burden and combat age- related disease.
Keywords
Lipid peroxides; Senescence; Aging; Oxidative stress
Abbreviations
4-HHE: 4-Hydroxyhexenal; 4-HNE: 4-Hydroxynonenal; 4-ONE: 4-Oxo-2-Nonenal; AGE: Advanced Glycation Endproduct; ALCAT1: Acyl-CoA:Lysocardiolipin Acyltransferase-1; ALDH2: Aldehyde Dehydrogenase 2; BLIS: Biogenic Lipid Induced Senescence; CML: Carboxymethyl Lysine; ELISA: Enzyme-Linked Immunosorbent Assay; FOXO3: Forkhead Box O3; GLO1: Glyoxalase I; GO: Glyoxal; GST: Glutathione-S-Transferases; MGO: Methylglyoxal; PPARγ: Peroxisome Proliferator-Activated Receptor Gamma; PUFA: Polyunsaturated Fatty Acid; TLR4: Toll-Like Receptor 4; TXNIP: Thioredoxin-Interacting Protein; SAMP8: Senescence-Accelerated Mouse-Prone 8; SASP: Senescence Associated Secretory Phenotype; SIRT1: Sirtuin 1
Introduction
Senescence in whole organisms is characterized by a progressive loss of physiological function and integrity. Over time, cells suffer insults such as mitochondrial damage, double stranded DNA breaks, telomeric attrition and disruptions to proteostasis. Accrual of cellular damage over time can bring about several cell fates (e.g. apoptosis, necrosis, etc.), one of which is senescence. Cellular senescence is a cellular fate characterized by the confluence of processes including withdrawal from the cell cycle, cell enlargement, overexpression of cyclin-dependent kinase inhibitors (e.g., p16ink4a and p21Cip1), loss of nuclear envelope protein Lamin B1 and secretion of an array of inflammatory factors referred to as the SASP. Cellular senescence is thought to play a prominent role in the etiology of a growing list of diseases associated with aging including cardiovascular disease, neurodegenerative diseases and cancer [1].
Cellular senescence is often triggered by one or more forms of cellular stress. Stressors can be endogenous factors such as telomeric attrition or exogenous factors such as genotoxic compounds or ionizing radiation [2]. Oxidative stress is such a physiological stressor long associated with both senescence and age-related pathologies [3]. In 1956, Denham Harmon outlined the “Free Radical Theory of Aging,” wherein he proposed that age-related degenerations can be “attributed basically to the deleterious side attacks of free radicals on cell constituents” [4]. Lipids that make up the phospholipid bilayer can react with free radicals to generate a diverse array of lipid electrophiles and such lipids have been broadly implicated as senescence inducers [5].
The connection between oxidative stress, cellular senescence and aging enjoys thorough coverage by literature reviews. This review uniquely examines biogenic electrophiles as senescence inducers, a less explored aspect distinct from oxidative stress, with implications for aging and therapeutic intervention. In this review, we will examine evidence relating to the association between lipid peroxidation and aging, electrophilic lipid peroxidation products as senescence inducers and scavenging of lipid peroxidation products as anti-aging therapies.
Literature Review
Lipid peroxidation potentiates cellular senescence and correlates inversely with longevity
Lipid peroxidation occurs when radical oxygen species, produced in the course of normal metabolism, react with lipid species those that comprise the phospholipid membrane. Although this can physically occur anywhere in the cell, the high content of oxygen radicals in the mitochondrion makes this organelle a central node in lipid peroxidation of biological membranes [5]. Membrane lipids with acyl chains containing multiple double bonds (i.e., polyunsaturated fatty acids) are particularly susceptible to peroxidation due to the resonance stabilization of the resulting lipid radical. A major class of such compounds include α,β-unsaturated alkenals such as 4-HNE, 4-HHE, acrolein, crotonaldehyde and malondialdehyde. Another lipid class is dicarbonyls comprised of species such as 4-ONE, glyoxal and methylglyoxal (Figure 1). Due to the stochastic nature of radical reactions that mediate lipid peroxidation, the sum total of lipid-derived electrophiles are a heterogenous mixture of all these compounds [6].
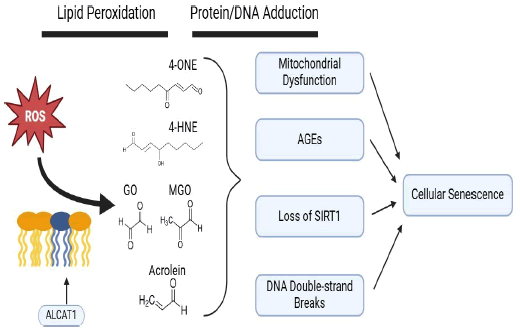
Figure 1: Graphical schematic of lipid electrophile biogenesis following peroxidation of the mitochondrial membrane and downstream effects leading to cellular senescence.
Lipid peroxidation is recognized as a driver of cellular senescence, specifically for the manner in which peroxidation can remodel biological membranes [5]. As polyunsaturated fatty acids in the membrane are oxidized, the polarity they acquire cause them realign from the internal hydrophobic regions where they originate to the external aqueous side of the lipid bilayer. These protuberances have been termed “lipid whiskers” and they are posited to mediate misfolding of the mitochondrial membrane in senescent cells as well as immune cell recognition of senescent and apoptotic cells [7].
Lipid peroxidation and 4-HNE have been studied in the context of therapy-induced senescence [8]. Many topoisomerase inhibitors used in anti-cancer chemotherapies, including camptothecin and its derivatives, have been shown to induce lipid peroxidation and subsequent accumulation of excess lipid- derived electrophiles, including 4-HNE and 4-ONE. Through alkylation of a critical cysteine residue in the active site of topoisomerase I, 4-HNE can crosslink topoisomerase I and DNA in a covalent complex leading to double-stranded DNA breaks [9].
Recently, it’s been proposed that enhanced lipid peroxidation in various age-related disease is a consequence of adverse cardiolipin remodeling specifically by ALCAT1, an acyltransferase that produces cardiolipin with highly polyunsaturated conjugated acyl chains susceptible to lipid peroxidation [10]. In contrast, tafazzin carries out a similar acytransferase reaction but instead incorporates typically 18:2 acyl chains into cardiolipin that are more peroxidation resistant. Genetic loss of tafazzin (Barth Syndrome) leads to cardiolipin peroxidation and high amounts of 4-HNE [11]. A body of work implicates ALCAT1’s role in an array of pathologies associated with aging (Figure 1). In contrast, loss of function of ALCAT1, either via pharmacological or genetic means, results in amelioration of a number of age-related diseases including obesity, type 2 diabetes, heart failure and diabetic cardiomyopathy as well as a concomitant lowering of HNE adduction [10].
The susceptibility of biological membranes to lipid peroxidation is acknowledged as an important determinant of longevity [12,13]. Famously long-lived species such as the naked mole rat and the ocean quahog have been observed to possess mitochondrial membranes that are especially resistant to lipid peroxidation due to lower n-3 and n-6 polyunsaturated fatty acid content [13,14]. In comparing strains of mice, others have reported that lower n-3 PUFA content in the phospholipid profiles of muscle and liver resulted in longer lifespans, an effect that was attributed to reduced lipid peroxidation [12]. In humans, centenarians have repeatedly been found to possess lipidomes that are less susceptible to peroxidation than their elderly counterparts, underscoring the significance of lipid peroxidation in longevity [15].
Lipid-derived electrophiles induce cellular senescence
Biogenic electrophiles have the ability to modify biomolecules with nucleophilic moieties such as nucleic acids and proteins (Figure 1). Lipid electrophiles will react with primary amines to form Schiff bases; this involves the addition of an aldehyde to an amine to make an imine. The predominant lipid elecrophile- protein products are Michael adducts wherein nucleophilic attack occurs on the beta carbon in the alpha/beta unsaturated chain. Further rearrangement can result in stable imidazole and hemi-acetal groups linking the peptide foundation to the akyl chain of the lipid electrophile [16]. Detection of reduced 4-HNE Michael adducts through the use of an antibody is frequently utilized to index biosynthesis of 4-HNE via Western blotting or ELISA [17]. Proteins that are alkylated by lipid electrophiles represent a major form of proteotoxicity and are targeted for degradation by the proteasomal apparatus [18]. Often, lipid alkylation will result in a loss in function for an adducted enzyme, as thiol and lysine groups in active sites tend to participate in chemistry critical for enzymatic catalysis. Accumulation of 4-HNE protein adducts have been observed in cellular senescence models in vitro as well as aging models in vivo in multiple tissue types [19,20].
Aside from amino acid side chains alkylation, 4-hydroxyalkenals can also form exocylic adducts with nucleic acids. 4-HNE can make Michael adducts directly with nucleosides to form a cyclized proprano-adduct. Alternatively, epoxidation of 4-HNE through prior autoxidation or interaction with other peroxides imparts the capacity to form etheno-adducts [21]. Since these etheno-adducts are removed through base excision repair and excreted from the cell, they are under investigation as potential biomarkers for DNA damage caused by 4-HNE and other biogenic aldehydes [22]. Multiplicity of reactive sites on some lipid electrophiles confers the ability to cross-link biomolecules (Figure 1). Aberrant protein aggregates, DNA crosslinks and protein-DNA complexes are all known features of several pathologies [23].
While oxygen radicals such as superoxide have biological half- lives measured in micro or milliseconds, these biogenic electrophiles can exist in their free state for minutes to hours. Therefore, these compounds can, theoretically, exert effects far more distant from their site of genesis than radical oxygen species. Excess 4-HNE adduction has been observed in multiple models of senescence, including replicative and irradiative senescence, posing the possibility that lipid-derived electrophile generation is a feature of multiple modes of senescence [19,24]. Direct exposure to lipid-derived electrophiles themselves cause cells to senesce in vitro, as documented in a body of work summarized as follows.
4-Hydroxynonenal and 4-Oxo-2-Nonenal: The chemistry of medium chain lipid-derived enals, particularly 4-HNE, has been well characterized and 4-HNE is known to exert genotoxicity and proteotoxicity through induction of carbonyl stress, a shared feature of multiple degenerative pathologies. 4-HNE and 4-ONE have been shown to induce cellular senescence in a number of cell culture models. A recently published study using IMR90 fibroblasts and murine adipose progenitor cells demonstrated the capacity of 4-HNE and 4-ONE to induce cellular senescence and provided detailed characterization of the accompanying senescence program, carbonyl stress, mitochondrial dysfunction and genotoxicity. The authors of this study termed this senescence program BLIS [25]. The observed incidence of BLIS spans multiple cells models and species, including human placental cells, human foreskin fibroblasts and bovine endothelial cells [26-28].
A comprehensive understanding of the molecular mechanism accounting for BLIS remains elusive due to the pleiotropic phenotype associated with BLIS and the stochastic nature of 4- HNE adduction.
Malondialdehyde: MDA is a dicarbonyl that tautomerizes to an enol. MDA is widely used as a biomarker of lipid peroxidation. Post-translational modification of proteins by MDA is thought to play a role the etiology of many age-related disease states [29]. However, to our knowledge, MDA has not been demonstrated to directly induce senescence in vitro and thus, molecular mechanisms tying MDA to cellular senescence remain an area for active investigation.
Glyoxal and methylgloxal: GO is a diketal byproduct of lipid peroxidation or the autoxidation of glucose. GO reacts with proteins to form AGEs, one of which is CML, a common biomarker used to index AGE biosynthesis. At concentrations ranging from hundreds of micromolar to millimolar, GO causes cellular senescence in human skin fibroblasts and bone-marrow derived, immortalized mesenchymal stem cells, as well as a concomitant increase in CML [30,31].
MGO, a related dicarbonyl produced in lipid peroxidation and glycolysis, induces senescence in murine adipose-derived stem cells. In cocultures with endothelial cells, stem cells made senescent with MGO were found to have hampered pro- angiogenic signaling capacity [32].
Acrolein: Acrolein is the simplest unsaturated alkene and is may be generated endogenously as a byproduct of lipid peroxidation or introduced exogenously as an environmental toxicant commonly found in air pollution and cigarette smoke. Acrolein has been shown to induce cellular senescence and accelerated telomere shortening in two different lines of human lung fibroblasts [33,34].
Scavenging biogenic electrophiles attenuates senescence
Abatement of carbonyl stress via direct sequestration or enhancement of biogenic electrophile disposal enzymes has been shown to effectively decrease senescence burden and ameliorate age-related disease.
TA293: Cytosolic hydroxyl radicals were found to generate oxidized phospholipids, which in turn, were determined to enact cellular senescence independent of TLR4 signaling and inflammatory response activation. In a mouse model of enhanced oxidative stress, administration of a small molecule hydroxyl radical scavenger (TA293) normalized levels of oxidized phospholipids and blunted expression of senescence markers SA- β-galactosidase, p21 and p16 in lung and kidney [35].
Aminoguanidine: Aminoguanidine is a scavenger of dicarbonyls such as GO and MGO. In two different strains of rat, aminoguanidine was demonstrated to attenuate age-related declines in renal and cardiovascular function, presumably through prevention of AGE accumulation. In primary human vascular endothelial cells, glyoxal-induced senescence as measured by p21 expression and formation of CML was abrogated with coadministration of aminoguanidine [36].
Glyoxalase 1: GLO1 is a member of the glyoxalase enzyme system that detoxifies glyoxal by conjugation to glutathione. Aged rats that overexpressed GLO1 exhibited lower closed proteins in renal tissue compared to their wild-type counterparts, coincident with reduced senescence markers (p53, p21 and p16) as well as improved markers of kidney function. Experiments done in vitro in the same study demonstrated that overexpression of GLO1 attenuates markers of senescence induced through exposure to the genotoxic topoisomerase poison, etoposide [37]. These results suggest that excessive glyoxal generation could be a common feature of multiple models of cellular senescence.
Ethanol: Exposure of human aortic endothelial cells to ethanol partially rescued serially passaged cells from replicative senescence through induction of ALDH2. As ALDH2 is a major detoxification enzyme for biogenic electrophiles, an increase in ALDH2 activity would be expected to relieve carbonyl stress. Indeed, it was found that ALDH2 induction through moderate ethanol exposure resulted in lower 4-HNE adducts. Of note, it was found that ethanol promoted nuclear translocation of SIRT1 [38].
Carnosine: L-carnosine is a dipeptide of histidine and beta alanine that’s abundant in millimolar concentrations in tissues with high bioenergetic demand such as skeletal muscle and brain. It is an aldehyde scavenger that makes an irreversible adduct with 4-HNE and other biogenic aldehydes, effectively acting to sequester reactive electrophilic moieties. Tissue quantity of L-carnosine is regulated by carnosine synthase and carnosinase, which biosynthesize and degrade carnosine, respectively.
L-carnosine has been shown to have anti-senescence effects in vitro [39]. Concurrent treatment of fibroblasts undergoing BLIS with a stoichiometric excess of L-carnosine with continuous 4- HNE exposure results in amelioration of the senescent phenotype as measured by SA-β-galactosidase activity and p21 expression [25]. Culturing fibroblasts with L-carnosine reduces 8-hydroxyguanine adducts upon starvation challenge, protects serially passaged cells from oxidative stress and telomeric shortening and rescues UV-exposed cells from DNA double stranded breaks and SIRT1 loss [40-43].
Studies in vivo evaluating degenerative disease have also seen benefits from carnosine. In mice fed a high fat and high sugar diet, L-carnosine administered in drinking water both improved metabolic parameters and reduced markers of senescence in adipose [25]. In a progeroid mouse model (SAMP8), treatment with L-carnosine rescued mitochondrial function and attenuated senescence-associated cognitive decline [44]. A 2023 meta-analysis of nine clinical studies testing the efficacy of L- carnosine against age-related disease concluded that L-carnosine demonstrated clear benefits in ameliorating type 2 diabetes and neurodegenerative disorders [45].
Discussion
Capacity to metabolize lipid-derived electrophiles seems to play a significant role in age-related disease and longevity. A major constituent of the electrophile disposal apparatus is GSTs, a family of enzymes that metabolize biogenic aldehydes by conjugating them to thiols in glutathione. In C elegans, ectopic expression of murine glutathione-s-transferase A4 or overexpression of glutathione-s-transferase 10 were associated with a longer lifespan while gene silencing of glutathione-s- transferase 10 was associated with a shorter one; an effect was recapitulated in mice [46]. In a study of the transcriptomes associated with 15 lifespan-lengthening interventions in mice, the gene most frequently upregulated (in 9 interventions) was GstA4 [47]. To our knowledge, biogenic electrophile disposal function has never been studied with regards to human longevity.
A significant obstacle to research in biogenic electrophiles as endogenous senescence inducers is the complexity of the molecular mechanisms underlying electrophile-induced senescence. Indeed, multitude cell signaling mechanisms are proposed to play a role in establishing the senescent phenotype.
Several interesting connections between senescence, disturbances in redox signaling, mitochondrial dysfunction and disruption of sirtuin signaling have been observed coincident with BLIS, brought on by exposure to medium chain lipid electrophiles such as 4-HNE and 4-ONE [25-28]. Mitochondrial dysfunction, itself an effector of cellular senescence, is a well- documented sequela of 4-HNE exposure [11]. One study found that part of BLIS is mediated by upregulation of pro-oxidant thioredoxin-interacting protein downstream of Peroxisome Proliferator Activated Receptor γ activation [28]. 4-HNE is known to possess the capacity to alkylate nuclear acetylase sirtuin 1, an inhibitor of p53 signaling and senescence. Interestingly, development of BLIS coincides with loss of SIRT1 and concurrent promotion of overall protein ubiquitination and acetylation [26]. Mitochondrial dysfunction and proteasomal degradation of acrolein-modified Werner’s syndrome protein, a helicase involved in telomere maintenance and DNA repair, mediate acrolein’s senogenic effects of acrolein [33].
The primary challenge of studying protein adducts of biogenic electrophiles are their diversity; characterization of post- translational modifications by lipid electrophiles are required for their quantification in vivo. Moving forward, researchers in the aging field can leverage increasingly complicated metabolomic, proteomic and transcriptomic technologies to fully elucidate complex molecular mechanisms basis biogenic electrophile- induced senescence.
Conclusion
Through generation of extremely reactive lipid electrophiles, lipid peroxidation can initiate a cascade of biomolecular and cell signaling modifications that ultimately lead to cellular senescence and potentiation of age-associated pathologies. Lipid electrophiles represent a diverse set of endogenously-generated senogenic compounds that accumulate with age. Scavenging lipid electrophiles represents a potential therapeutic method for alleviating age-related and degenerative disease. Further work should be done to glean a more complete understanding of molecular mechanisms underlying lipid electrophiles’ adverse influence on physiological aging.
Authors Contributions
TBM developed the outline and wrote the review. DAB edited the outline and manuscript. PDR edited the outline and the manuscript. All authors declare no conflict of interest exists in this review.
Acknowledgement
The authors would like to acknowledge the Robbins and Bernlohr laboratories for helpful suggestions. Supported by NIH 5R01AG069819 to DAB and NIH U54 AG079754 to PDR. TBM was supported by NIH 1F32AG086035.
References
- Childs BG, Durik M, Baker DJ, Van Deursen JM. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat Med. 2015;21:1424–1435.
[Crossref] [Google Scholar] [PubMed]
- Ben-Porath I, Weinberg RA. When cells get stressed: An integrative view of cellular senescence. J Clin Invest. 2004;113:8–13.
[Crossref] [Google Scholar] [PubMed]
- Pole A, Dimri M, Dimri GP. Oxidative stress, cellular senescence and ageing. AIMS Mol Sci. 2016;3.
- Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
[Crossref] [Google Scholar] [PubMed]
- Chen JJ, Yu BP. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic Biol Med. 1994;17:411–418.
[Crossref] [Google Scholar] [PubMed]
- Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021.
[Crossref] [Google Scholar] [PubMed]
- Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396.
[Crossref] [Google Scholar] [PubMed]
- Flor AC, Wolfgeher D, Wu D, Kron SJ. A signature of enhanced lipid metabolism, lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell Death Discov. 2017;3:1–12.
[Crossref] [Google Scholar] [PubMed]
- Flor A, Wolfgeher D, Li J, Hanakahi LA, Kron SJ. Lipid-derived electrophiles mediate the effects of chemotherapeutic topoisomerase I poisons. Cell Chem Biol. 2021;28:776–787.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Shi Y. In search of the holy grail: Toward a unified hypothesis on mitochondrial dysfunction in age-related diseases. Cells 2022;11:1906.
[Crossref] [Google Scholar] [PubMed]
- Xiao M, Zhong H, Xia L, Tao Y, Yin H. Pathophysiology of mitochondrial lipid oxidation: Role of 4- hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic Biol Med. 2017;111:316–327.
[Crossref] [Google Scholar] [PubMed]
- Hulbert A, Faulks SC, Harper JM, Miller RA, Buffenstein R. Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech Ageing Dev. 2006;127:653–657.
[Crossref] [Google Scholar] [PubMed]
- Munro D, Blier PU. The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell. 2012;11:845–855.
[Crossref] [Google Scholar] [PubMed]
- Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long- lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532.
[Crossref] [Google Scholar] [PubMed]
- Belenguer-Varea Á, Tarazona-Santabalbina FJ, Avellana-Zaragoza JA, Martínez-Reig M, Mas- Bargues C, Inglés M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020;149:51–63.
[Crossref] [Google Scholar] [PubMed]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128.
[Crossref] [Google Scholar] [PubMed]
- Xu Q, Hahn WS, Bernlohr DA. Detecting protein carbonylation in adipose tissue and in cultured adipocytes. Methods Enzymol. 2014. 249–261.
[Crossref] [Google Scholar] [PubMed]
- Grune T, Davies KJA. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204.
[Crossref] [Google Scholar] [PubMed]
- Petkovic I, Bresgen N, Gilardoni E, Regazzoni L, Uchida K, Aldini G, et al. In Vitro aging of human skin fibroblasts: Age-dependent changes in 4-hydroxynonenal metabolism. Antioxidants. 2020;9:150.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Morgan TE, Forman HJ. Age-related alteration in HNE elimination enzymes. Arch Biochem Biophys. 2021;699:108749.
[Crossref] [Google Scholar] [PubMed]
- Guéraud F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radic Biol Med. 2017;111:196–208.
[Crossref] [Google Scholar] [PubMed]
- Bin P, Shen M, Li H, Sun X, Niu Y, Meng T, et al. Increased levels of urinary biomarkers of lipid peroxidation products among workers occupationally exposed to diesel engine exhaust. Free Radic Res. 2016;50:820–830.
[Crossref] [Google Scholar] [PubMed]
- Schaur R, Siems W, Bresgen N, Eckl P. 4-Hydroxy-nonenal—a bioactive lipid peroxidation product. Biomolecules. 2015;5:2247–2337.
[Crossref] [Google Scholar] [PubMed]
- Suman S, Rodriguez OC, Winters TA, Fornace AJ, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging. 2013;5:607–622.
[Crossref] [Google Scholar] [PubMed]
- Monroe TB, Hertzel AV, Dickey DM, Hagen T, Santibanez SV, Berdaweel IA, et al. Lipid peroxidation products induce carbonyl stress, mitochondrial dysfunction, and cellular senescence in human and murine cells. Aging Cell. 2024;e14367.
[Crossref] [Google Scholar] [PubMed]
- Tasta O, Swiader A, Grazide MH, Rouahi M, Parant O, Vayssière C, et al. A role for 4-hydroxy-2- nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free Radic Biol Med. 2021;164:303–314.
[Crossref] [Google Scholar] [PubMed]
- Fafián-Labora JA, Rodríguez-Navarro JA, O’Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab. 2020;32:71–86.
[Crossref] [Google Scholar] [PubMed]
- Riahi Y, Kaiser N, Cohen G, Abd-Elrahman I, Blum G, Shapira OM, et al. Foam cell-derived 4- hydroxynonenal induces endothelial cell senescence in a TXNIP-dependent manner. J Cell Mol Med. 2015;19:1887–1899.
[Crossref] [Google Scholar] [PubMed]
- Halkoum R, Salnot V, Capallere C, Plaza C, L’honoré A, Pays K, et al. Glyoxal induces senescence in human keratinocytes through oxidative stress and activation of the protein kinase B/FOXO3a/p27KIP1 pathway. J Invest Dermatol. 2022;142:2068–2078.
[Crossref] [Google Scholar] [PubMed]
- Larsen SA, Kassem M, Rattan SI. Glucose metabolite glyoxal induces senescence in telomerase- immortalized human mesenchymal stem cells. Chem Cent J. 2012;6:1–13.
[Crossref] [Google Scholar] [PubMed]
- Leone A, Nicolò A, Prevenzano I, Zatterale F, Longo M, Desiderio A, et al. Methylglyoxal impairs the pro-angiogenic ability of mouse adipose-derived stem cells (madscs) via a senescence- associated mechanism. Cells. 2023;12:1741.
[Crossref] [Google Scholar] [PubMed]
- Jang JH, Bruse S, Huneidi S, Schrader RM, Monick MM, Lin Y, et al. Acrolein-exposed normal human lung fibroblasts in vitro: Cellular senescence, enhanced telomere erosion, and degradation of Werner’s syndrome protein. Environ Health Perspect. 2014;122:955–962.
[Crossref] [Google Scholar] [PubMed]
- Luo C, Li Y, Yang L, Feng Z, Li Y, Long J, et al. A cigarette component acrolein induces accelerated senescence in human diploid fibroblast IMR-90 cells. Biogerontology. 2013;14:503–511.
[Crossref] [Google Scholar] [PubMed]
- Sakai T, Imai J, Takagaki H, Ui M, Hatta S. Cytoplasmic OH scavenger TA293 attenuates cellular senescence and fibrosis by activating macrophages through oxidized phospholipids/TLR4. Life Sci. 2019;221:284–292. [Crossref]
[Google Scholar] [PubMed]
- Santos AN, Jacobs K, Simm A, Glaubitz N, Horstkorte R, Hofmann B. Dicarbonyls induce senescence of human vascular endothelial cells. Mech Ageing Dev. 2017;166:24–32.
[Crossref] [Google Scholar] [PubMed]
- Ikeda Y, Inagi R, Miyata T, Nagai R, Arai M, Miyashita M, et al. Glyoxalase I retards renal senescence. Am J Pathol. 2011;179:2810–2821.
[Crossref] [Google Scholar] [PubMed]
- Xue L, Yang F, Han Z, Cui S, Dai S, Xu F, et al. ALDH2 mediates the dose-response protection of chronic ethanol against endothelial senescence through SIRT1/p53 pathway. Biochem Biophys Res Commun. 2018;504:777–783.
[Crossref] [Google Scholar] [PubMed]
- Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry. 2000;65:866–868.
[Google Scholar] [PubMed]
- Kantha SS, Wada S ichi, Tanaka H, Takeuchi M, Watabe S, Ochi H. Carnosine sustains the retention of cell morphology in continuous fibroblast culture subjected to nutritional insult. Biochem Biophys Res Commun. 1996;223:278–282.
[Crossref] [Google Scholar] [PubMed]
- McFarland GA, Holliday R. Retardation of the senescence of cultured human diploid fibroblasts by carnosine. Exp Cell Res. 1994;212:167–175.
[Crossref] [Google Scholar] [PubMed]
- Shao L, Li Q huan, Tan Z. l-Carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts. Biochem Biophys Res Commun. 2004;324:931–936.
[Crossref] [Google Scholar] [PubMed]
- Swiader A, Camaré C, Guerby P, Salvayre R, Negre-Salvayre A. 4-hydroxynonenal contributes to fibroblast senescence in skin photoaging evoked by UV-a radiation. Antioxidants 2021;10:365.
[Crossref] [Google Scholar] [PubMed]
- Dai Z, Lu XY, Zhu WL, Liu XQ, Li BY, Song L, et al. Carnosine ameliorates age-related dementia via improving mitochondrial dysfunction in SAMP8 mice. Food Funct. 2020;11:2489–2497.
[Crossref] [Google Scholar] [PubMed]
- Sureshkumar K, Durairaj M, Srinivasan K, Goh KW, Undela K, Mahalingam VT, et al. Effect of L- carnosine in patients with age-related diseases: A systematic review and meta-analysis. Front Biosci (Landmark Ed). 2023;28:18.
[Crossref] [Google Scholar] [PubMed]
- Zimniak P. Relationship of electrophilic stress to aging. Free Radic Biol Med. 2011;51:1087–1095.
[Crossref] [Google Scholar] [PubMed]
- Tyshkovskiy A, Bozaykut P, Borodinova AA, Gerashchenko MV, Ables GP, Garratt M, et al. Identification and application of gene expression signatures associated with lifespan extension. Cell Metab. 2019;30:573-593.e8.
[Crossref] [Google Scholar] [PubMed]
- Sejersen H, Rattan SI. Glyoxal-induced premature senescence in human fibroblasts. Ann N Y Acad Sci. 2007;1100:518–523.
[Crossref] [Google Scholar] [PubMed]
Citation: Monroe TB, Robbins PD, Bernlohr DA (2024). The Peroxidation of Lipids, Cellular Senescence and Aging. J Aging Sci. 12:385.
Copyright: © 2024 Monroe TB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

