Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 11, Issue 4
The Mechanism of Heavy Metal Elements in various Biological Process and Its Deteriorate Effects on the Productivity of Different Crop Plants
Nazarul Hasan*, Sana Choudhary and Neha Naaz Nidhi SharmaReceived: 19-Feb-2021 Published: 02-Apr-2021, DOI: 10.35248/2252-5211.21.11.403
Abstract
Heavy metals are highly toxic elements with density five times more to the water molecule. In animals including humans, there are different processes such as ingestion, absorption, etc through which they enter into the body. When heavy metal accumulates at a higher rate than the discharge then they become harmful for the animal. Anthropogenic activities of humans are the major cause of contamination of the environment and of medium-size cities. Burning of the fossil fuels, mining, and use of the chemical substances in the crop, etc. are the parts of anthropogenic activities that contribute pollutants in the environment. Toxic metal elements like cadmium along with the nutrients also uptake by the plant and accumulates in over time resulting in infections. The production and quality of crops and atmospheric conditions can be influence by high concentration of heavy metals. To avoid the toxic effects of heavy metals, plants developed several mechanisms due to which severe toxic elements excluded, retained inside the root, and change into the physiological tolerant forms. The contamination by the heavy metal elements is the major concern due to raising the demand for food safety. In this review, we have discussed the severe effect of heavy metal on productivity and interference of metal elements with a particular biochemical process inside the various crop plants that make unable to plant for the tolerance of high level of heavy metals present in environmental soil.
Keywords
Heavy metal; Crop plant; Toxicity; Mechanism; Productivity
Introduction
Heavy metals are the group of naturally occurring chemical elements that consist of that consist essential (e.g., iron cooper nickel and zinc) and non-essential metal elements (cadmium, lead and mercury). Heavy metals have much high density than the water and may contribute the environmental pollution. The contamination in an ecosystem through heavy metals can be distinguished as major environmental concerns due to their persistence and their frequency. Heavy metals are not degraded easily in nature, therefore there is needs to develop more efficient and commercial techniques from which toxic elements could deployed rapidly in large number of physical settings such conducting polymer based on sensor and chemo sensor in particular. The toxicity and weight of the heavy metals are interrelated and some of the heavy metals may occur in metalloid form for example-arsenic, exposure in traces amount of arsenic to any organism induces the high toxicity in that organism. A big area of agricultural land has been polluted with heavy metals due to the use of fertilizers, pesticides, compost and municipal waste, and most of the waste (containing heavy metals) material of smelting industries and metlliferous mines release directly in to the agricultural land. The earth crust and rock are main sight where the heavy metals are present in form of oxide and sulphides ores. These metals elements can be separated from different ores such as sulphides of iron, lead mercury arsenic cobalt or cadmium. Soil is one the most important and basic part of an ecological system and the soil throughout the world has contaminated with the heavy metals Soil is the mixture of inorganic and organic material that found just below the earth’s surface and soil on earth is natural medium to support the plant growth and other developmental activities. Heavy metals are found naturally in soils and the soil is formed due to the activity of geological cycle which converts the material of underground part of the earth. Heavy metal pollution not only affects the production and quality of crops, but also influences the quality of the atmosphere and water bodies, and threatens the health and life of animals and human beings by way of the food chain. Most severe is that this kind of pollution is covert, long term and non-reversible. Heavy metals are also one of the major contaminating agents in our food supply. Vegetables are vital to the human diet and in particular provide the well-known trace elements and heavy metals. Minor or trace elements are essential for good health if they come from an organic or plant source. In contrast, if they come from an inorganic or metallic source, they become toxic. The processes of plant growth. depend on the cycle of nutrients including trace elements, from soil to plant. Vegetables, especially leafy vegetables, accumulate higher amounts of heavy metals because they absorb these metals in their leaves. The contamination of soil with heavy metals may cause of the threats and hazards to animals including human and an ecosystem may affect directly from these pollutants. Other problems like instability in the food chain (soils-plantshuman beings), contamination of drinking water, reduce the food quality and productivity (security and marketability) via toxicity in plants, reduction in agricultural land for the cultivation of crops resulting insecurity of food and land tenure problems. Plants accumulate more amounts of heavy metals which are generally grown on contaminated land with heavy metals and thus the contamination in food will starts. Due to the food chain contamination, the heavy metals may get entry into animals including human and affected animals with these metals suffering from acute or chronic disease such as dermatitis to the various types of cancers. The toxic effects of heavy metals have been shown in microflora present in soil and these effects result in alteration of diversity, size of the population and functional activity in communities of microorganisms present in soil [1,2] (Table 1).
| Pollutants | Impacts | ||
|---|---|---|---|
| Plants | Animals/Human Health | ||
| Cd | Brown margin to leaves, chlorosis, necrosis, cubed leaves, brown stunted roots, reddish vein and petioles, reduction in growth, purple coloration | Renal dysfunction, increased uric acid, Liver cirrhosis, lung cancer Vomiting, Itai- itai | |
| Ni | Chlorosis, necrosis, stunting, inhibition of root growth, decrease in leaf are | Nausea, vomiting, insomnia, irritability, tightness of the chest, non-productive, cough, dyspnoea cyanosis, tachycardia, palpitations, cardiac arrest | |
| As | Red brown necrotic spots on old leaves, yellow browning of roots, growth reduction | Gastrointestinal problem,cardio vascular proble Diarrhea, Colic pain, weak pulse, skin pigmentation renal dysfunction | |
| Pb | Dark green leaves, stunted foliage, increase amount of shoots Foliage increase amount of shoots |
Reduces synthesis of haemoglobin affect the central nervous system Renal dysfunction, loss of weight and loss of teeth, Vomiting and reduce immune | |
| Cr | Affect seed emergence, stunted plant growth and decrease dry matter production | Damages the kidneys, the liver and blood cells, Ulceration of the skin, premature dementia, cancer | |
| Se | Interveinal chlorosis, black spots, bleaching and yellowing of young leaves, pink spots on root | Nervousness, drowsiness, rapid and weak pulse Colic pain, respiratory failure, paralysis, Enlarge spleen, loss of appetite, loss of hair and nails, loss of fertility, heart problem, cirrhosis | |
| Hg | Severe stunting of seedlings and roots, chlorosis browning of leaf tips, reduction in growth | Affect the central nervous system Renal dysfunction, mental deterioration, Vomiting,insomnia and loss of smell sense | |
| Cu | Chlorosis yellow coloration, purple colouration of the lower side of the midrib, less branched roots inhibition of root growth | Wilson's disease - heaglobinuria, Jaundice Enlargement of Kidney, liver and spleen Hypertension, Neurological problem, paralysis and abdominal pain | |
It should be noted that how soil microorganisms function in an ecosystem to prolong exposed contamination of heavy metals. All the heavy metals do not have similar effects on the growth and development of the plants while they affect according to the developmental process of the plant. Heavy metals like Cd, Pb, As, and Hg have not any beneficiary role in growth and development, while these metals affect the plants adversely and adverse effects on plants have been observed at low concentration of heavy metals containing growth medium. It has been reported that the height of the rice plant growing on contaminated soil with very concentration (1mg Hg/Kg) reduces to a significant level. It was also noticed that shoot and root growth of wheat plant reduces which grown on the Cd contaminated soil at as low as 5mg/L in the soil. The activities like photosynthetic, mineral nutrition in plants and low activities of enzymes are affected due to the reduction in growth and developmental parameters of plants growing on heavy metal contaminated soil. Thus hazardous elements such as heavy metals may accumulate in different parts of crop plants growing on the contaminated agricultural soil with these elements. Like other living organisms, the crop plants are more sensitive to both low and high concentration of metal ions present in the soil as essential micronutrients, while other elements (such as Cd Hg and Pb) as ions form are more poisonous to the crop plant and effects to the various metabolic process at the same concentration. In case of animals contamination of food through the heavy metal elements deplete some of the essential mineral elements of the body which may be responsible for decreasing immunological defences, resulting in retardation of growth, disabilities associated with malnutrition, impaired psychological faculties and higher frequency of cancers in upper gastrointestinal region. Sever health conditions can be developed due to the intake of heavy metals (such as Cd, Ni, Pb and Cr) along with dietary products. Accumulated heavy metal elements of cultivated soil can be transferred to the animal including human by different processes of exposure that may result in the several adverse effects on the health of human. Heavy metals may pose the sever risk on the level of health through the contaminated water and determine by using the different indices such as Daily Intake of Metals (DIM), Transfer Factors (TF) and Health Quotient (HQ) or Health Risk Index (HRI). Animals and plants take the heavy metals from the wastewater and contaminated soil as well as from waste material deposited on the various parts of the plants that are exposed to air from the contaminated environment. It should be a critical environmental concern for people throughout the world that agricultural land has contaminated with heavy metals, most of which may have adverse ecological effects. In order to determine the toxic effects of heavy metals on plants, researches have been conducted throughout the world [3].
Sources of Heavy Metals
In the environment several anthropogenic chemical substances are released from the chemical industries or any other industries from the time of the beginning of industrialization. There are two sources from which these substances have released in environment one is natural sources and other is anthropogenic or human. Natural sources include erosion of the soil, volcanic activity, runoff from the urban areas and the aerosols particulate while anthropogenic or human sources include finishing of metals, mining of coal, textile industry, and atomic power station and electroplating process. Thus heavy metals release as pollutants along with the other chemical compound in soil from human activities. In nature environmental soil accumulates the heavy metals by the pedogenic process of weathering of the parent materials where the material present in traces amount (<1000 mg/kg) and are rarely toxic for life. The traces amounts of these heavy metals may have deteriorated effects on the quality of soil and water bodies in which heavy metals have found. The environmental soil becomes contaminated with the heavy metals because the rate of heavy metals generation via anthropogenic activities are more rapid random locations having a high potential of direct exposure receive these pollutants from the mines the concentration of heavy metals in discarded substances are more than other chemical compounds and the chance of bioavailability of heavy metals in the environmental system is more [4,5].
It is seemed that emission of heavy metals via anthropogenic activities has high magnitude then the natural sources. It is also considered that heavy metals release in soil from the anthropogenic processes are to be, mobile Heavy metals in the soil from anthropogenic sources tend to be more mobile and so bioavailability than the lithogenic or pedogenic ones. Metal containing solids can be originated at the contaminated site from different human activities in the form of mine tailing of metals, Metal-bearing solids at contaminated sites can originate from a wide variety of anthropogenic sources in the form of metal mine tailing, dumping off of substances containing metals elements in the protected land, disposal of high metal wastes in improperly protected landfills, the formation of petro-chemical compounds and various paints, fertilizer applications for land, use of pesticides and compost, combustion of coal residues, sewage sludge and deposition in the atmosphere are discussed here.
Fertilizers
The agricultural practices by a human were observed as major influences on the soil The heavy metals with other hazardous chemical elements may accumulate in large amounts in the soil through the frequent use of different fertilizers. Both macronutrients (N, P, K, Mg, S, and Ca) and micronutrients (Zn, Fe, Mo, Ni, Cu, Mn, and Co) are more essential for the different biochemical reactions in plants during their lifecycle, and these mineral elements play important roles in growth and development of crop plants. Sometimes soil of agricultural land is deficient for a particular mineral element then the soil is treated with that mineral element. Farmers add a large quantity of fertilizer (such as N, P, xand K) in agricultural land during the cultivation of their crops to support better growth and development of plants. Frequent use of this fertilizer in the crop may have an influence on the other living organisms residing in that agricultural land and quality of the soil may also be affected. The compounds used along with this fertilizer contain a small number of heavy metals (e.g., Pb and Cd) which impart impurities in the soil of that land and frequent supply of the fertilizer may significantly increase the content of this heavy metals in soil. Although, like other metal elements (Ni, Cu, Co, Mn. Mg Zn, etc.) heavy metals (such as Pb and Cd) have no significant role in the physiological activity of the plants. In some of the cases where phosphatic compounds are supplied to the agricultural land, adds heavy metals (Such as Cd, Hg, and Pb) [6].
Pesticides
To control the pest in agriculture and horticulture different types of pesticides containing a substantial amount of metals have used, extensively. In recent times it was approved in the UK that 10% chemical compounds used in prepare the insecticide and fungicides, should be contained with heavy metals (such as Hg, Zn, Pb, Mn, or Cd). Most important fungicides copper oxychloride and Bordeaux mixture (copper sulfate) are used to controlling the pest, contain traces amount of heavy metals. Several plant diseases caused by different pathogens control by particular lead-containing pesticides such as lead arsenate. To control other diseases in plants caused by pests (such as cattle ticks) and pests in banana, pesticides containing arsenic are used extensively in New Zealand and Australia. Sometimes heavy metals like Cu, As and Cr are used for the preservation of timbers and many derelict sites where the concentration of these elements increases in the soil of the site. If the site prepared again for agricultural purposes or any other purposes, it may be contaminated with the remains of chemical elements having the potential cause of serious problems. In contrast to fertilizer use of the pesticides has been localized and restricted to a specific site or crop plant [7].
Bio-Solids and Manures
The waste material (such as cattle, pig, and poultry manures) produce from animals in agricultural land is commonly applied to crop as solids or slurry . The heavy some fertilizers and manure metals such as Cd, As, Cu, Cr, Ni, Pb, Mo, Hg, Se, and so on obtained from the biosolids (e.g., composts, municipal sewage sludge, and livestock) accumulates in the soil. While most of the manures produce by animals are more valuable fertilizers, some of the heavy metals like Cu and Zn are added in diets used as growth promoters and As obtained from the health product of poultry have the potential to contaminate the soil. These heavy metals concentrate in soil by frequent use in cropland or other restricted land and metal elements increase considerably in the soil for a prolonged duration. The sewage sludge (biosolids) produce from wastewater treatment as primary organic products can be used for beneficial purposes and later they recycled (USEPA. 1994). The application of land for these organic products or biosolids has made a common practice in most of the countries of the world for allowing the reuse of these biosolids produced by the population of urban areas. Many references and regulatory definitions have been recognized for the use of sewage sludge and the term of biosolids is become more common for a replacement to sewage sludge because the sewage sludge reflect more accurately the many characteristics for beneficial purposes. According to the report of the United States it has estimated that more than 2.8 million dry tonnes of biosolids should be used or sewage sludge is disposed in land annually and biosolids occur in different parts of a country for the use of agriculture utilization. In most of the European countries more than 30% of sewage sludge is used as fertilizers in agriculture land. In Australia approximately 1.75 tonnes of dry sewage sludge or biosolids are produced every year through many metropolitan authorities, and in arable cropping situations most of the sewage sludge is used in agricultural land where biosolids can be incorporates into the soil.
Heavy metals such as Cd, Ni, Cr, Pb, Cu and Zn are most commonly found and limited concentration is administered by nature and activity of industrial intensity and different processes employed at the time of sewage sludge treatment. In some conditions, various heavy metals add to the soil during the biosolids applications and the heavy metals filtered to the different profile of soil and these heavy metals have the potential of contamination to the groundwater. In New Zealand through recent studies the treatment of soil with sewage sludge, it is reported that concentrations of heavy metals (such as Zn, Ni, and Cd) increase in drainage leachates [8,9].
Wastewater
Various applications of industrial wastewater and municipal wastes and other pollutants to agriculture land to the date 400 years ago and different practices in various regions of the world. It is estimated that throughout the world, millions of hectares of agricultural land are irrigated with polluted water. In many cities of Asian and African countries, In several Asian and African cities, different studies suggest that 50% of the vegetable provides to the different urban regions through irrigation of wastewater into agricultural land. Generally, many farmers are not aware of the benefits or hazards and maximizing the crop yield and profits. The concentrations of heavy metals in polluted water are relatively low and by continuous irrigation with the wastewater the content of hazardous heavy metals accumulates in the soil.
Metal mining and milling processes and Industrial wastes
In many countries of the world industries like the mining industry and the mill is the primary source of contamination of soil. Milling and mining of ores containing metal elements are responsible for wide rage distribution of metals as contaminants in soils. The metal elements directly discharge into natural environments from the tailing (large and heavy particles deposited at the bottom site of floating cell at the time of mining) and mining works and result in the elevated concentrations of the metal elements. Very much amount of Zn and Pb deposited on the soil as pollutants through the mining of and smelting of ores containing these metal elements, and the amount of metal elements is sufficient to pose the risk on human and ecological health. Various methods have been used at the contaminated site and the productivity of agriculture land not restored by these methods. Thus metal elements environmental risk to the human is related to the bioavailability. Direct ingestion (oral bioavailability) and ingestion by plant parts grown in (food chain) contaminated soil are the parts of assimilation pathways.
Other industries such as tanning, textile, petrochemical, or utilization of products containing petrol, pharmaceutical facilities, pesticides are generating the materials containing metal elements and this materials varies in compositions from each other. Even though some deposited material on land is useful for forestry or agricultural purposes while many of them containing heavy metals (such as Zn, Pb and Cr) having high potentiality of toxicity are applied to land seldom. Others materials are present in nutrition of plant in very small amount or they have no any properties in condition of soil [10-12].
Air-Borne Sources
Unlike other sources, heavy metals can contribute to soil and any other usable land from airborne sources such as pipe emission of air, gases, or stream of vapor and fumigation of affected areas with pest also contribute to metals due to the fumigations containing the heavy metals. Generally, these metals release into the environment as particulate matter in the stream of the gases. Some of the heavy metals like As, Pb, and, Cd have a property of volatility at the time of high-temperature processing and these heavy metals will convert into the metal oxides and become highly condense in the form of light particulates unless reduction of atmospheric gases is maintained. Incinerator or stack emission may distribute in a wide range area by the current of natural air until the rainfall separates them from the gas stream, while emissions by fugitive are spread to a very much small region because of the formation of this emission is confined to the ground. Generally, the amount of metal elements is much low in fugitive emission in comparison to emission from the stack or incinerators, and concentrations of metal elements emitted from both types of sources will depend on conditions of specific sites. All the emissions from the chimneys of a factory and solid particles of smoke eventually settle down on land or any other water body and another form of contamination is metal elements present in fossil fuel and the combustion of this fossil fuel since the industrial revolution result as contamination of the atmosphere. It was reported that a high concentration of heavy metals such as Zn, Cd, and Pb is present in the plants the agricultural land used in smelting work. The combustion of products containing petrol is another source of contamination and emission from these products contains a very large amount of Pb element; Pb content is substantial to contribute to the soil of urban regions and in those nearby major roads. Much content of metal elements such as Cd and Zn can also be added to the soil near to roads and the sources are being lubricant oils and tires [13].
Mechanism of heavy metals uptake by Plants
The heavy metal elements present in soil solution as soluble components absorb by the plant through root hairs or exudates release from the plant solubilize the heavy metals for absorption. Some of the metal elements are essential for the plant growth but in excess amount of these metals elements may be toxic for the plants and the plant which accumulates these essential metal elements are enables to acquire nonessential metal elements. Several studies of contamination of heavy metal during uptake and its mechanism have been performed by the different workers and result obtained from studies used in optimization of various factors to improve the processes of absorption of minerals especially metal elements Most of the plants act as both excluders and accumulators reported by Sinha. The plants those accumulates high concentrate of contaminants in the aerial tissues survive in high contaminated soil. Accumulator plants biodegrade or biotransform the harmful metal elements into inert form during the assimilation in tissues and excluder plants restrict the uptake of contaminants into the biomass. Plants have developed more efficient and highly specific mechanism to absorption of micronutrients from the environment, even micronutrients present at low ppm levels. Root hairs and root of plant, chelating agents [14] (Figure 1). releasing from the plant, pH changes induced by plant, the redox reactions play an important role in the solubilization and uptake mineral elements of very low concentration from the soil, even all these reactions help in mineral uptake from the nearly insoluble precipitate. Plants have also developed themselves for the translocation and accumulation of micronutrients and similar mechanism are also involved in translocation, absorption, and accumulation of toxic elements, the chemical properties of nonessential elements stimulates the essential elements. Plants have also evolved highly specific mechanisms to translocate and store micronutrients. Thus, the mechanisms of uptake of micronutrients are more important or are of great interest to phytoremediation. A range of special proteins aided to transportation mechanism of plants are embedded in the plasma membrane of the cell and this protein helps in translocation and uptake of ionic elements include(1) ATPases constitute the proton pump and proton pump use energy to the generation of electrochemical gradients, (2) Cotranspoters and anti-transporters (a protein that helps in the use of electrochemical gradients in active uptake of ions), and (3) carrier of the channel (a special protein that helps in the transport of ionic elements into the cell). The translocation of minerals elements taken by plants from the soil is highly regulated. The mineral elements do not accumulate in traces amount in a plant near beyond the metabolic needs and these amounts of most mineral elements are ranging from 10ppm to 15ppm that are sufficient for the needs of plants. But in some cases the plants takes toxic elements in high concentration (1000ppm), the plants are hyperaccumulator of toxic elements. When the toxic elements store in various parts of a plant in different forms arises as a serious problem that the plant how to avoid the toxicity of elements. Although, there is multiple mechanisms have been evolved due to which plants protect themselves from the toxicity of harmful elements and one of the mechanisms is accumulation of toxic elements in the vacuole of the cell. Heavy metals such as Co, Mn, Cd, Ni, Pb, and Zn accumulates in high concentration through the plants and up to 100 or 1000 time more than the plants which are incapable to accumulates the metals elements ( excluder or non-accumulator plants). Microorganisms like fungi and bacteria are live in the rhizosphere with close association with the plants may enhance the bioavailability of these metal elements due to their mobilization. Bacteria and fungi play an important role in the elimination of organic contaminants that is more significant than that in the case of inorganic compounds [15,16].
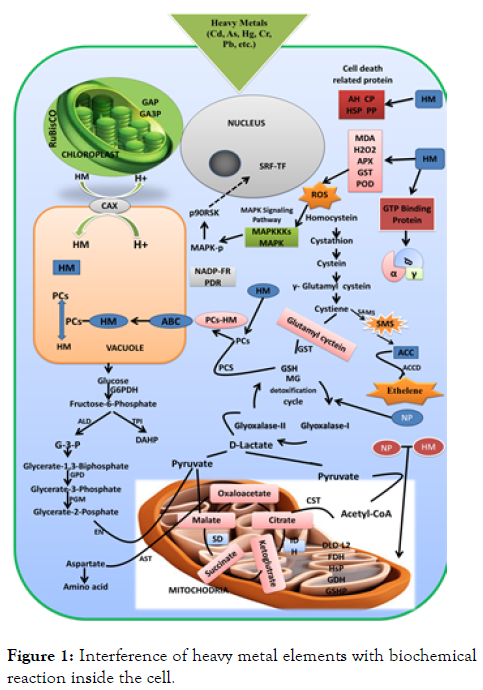
Figure 1: Interference of heavy metal elements with biochemical reaction inside the cell.
Biological Responses to Heavy Metals
Coagulation of protein, the formation of complexes with different coenzymes, and synthesis of ATP molecules may inhibit due to low concentration of arsenic in the medium. Arsenic can be a substitute for phosphorus in some of the biological reactions of cells as cadmium and mercury. Waste material release from the industries contains more amounts of chromium and chromium is considered as a serious pollutant for the environment. Chromium has very toxic effects on different growth as well as developmental process roots stem and leave and include alteration of the germination process, all the processes affected by this chromium element may result in a reduction in total dry weight of the plants. A low concentration of chromium element may result in chlorosis and necrosis of plants. The content of chlorophyll and carotenoid pigments decrease gradually in paddy crop with increasing concentration of chromium elements. Similarly, the reduction in chlorophyll contents has found in various crop plants that reported in pea, wheat, mungbean and cauliflower.
Sulfhydryl group (SH) in protein, hemoglobin, serum albumin and enzymes has a high strong affinity to mercury atoms. In animals, the central nervous system (CNS) is highly affected due to the damage of blood-brain barriers by the accumulation of mercury elements, and therefore, the transfer of essential metabolites such as amino acids is not properly regulated. In the case of the plant's cell membrane permeability, ADP or ATP, essential ionic molecules and active phosphorus groups damage in presence of mercury atoms and mercury may lead to the various oxidative stress in plants, among other effects.
The enzymes which are responsible for the absorption of different protein molecules through the kidney tubules get affected in presences of the Cd element. Thus, the functioning of different enzymes such as alcohol dehydrogenase (ADH) also affected due to the presence of the Cd element. Like other metal elements cadmium substituted to the zinc atom as the 2 metals are similar in solution.
It was reported that a significant change in the central monoaminigenic system of rats is due to the exposure of leadarsenic in combination.
The heavy metals interact with the antioxidant system of cells indirectly, change the properties of the electron transport chain, or metabolism of the essential biomolecule of the cell may disrupt.
Heavy metals induce the deleterious effects on their exposure to plants leading to the peroxidation of lipid molecule inside the cell due to which the plasma membrane may affect. Polyunsaturated fatty acids of membranes decompose in the presence of malondialdehyde (MDA), therefore it is suggested as indicators of oxidative stress.
In a study it has been observed that different tolerance levels on the exposure of Cd to two cultivars of flax, the content of chitinase enzymes of cell wall increase in both the cultivars, but the accumulation of protein were significantly high in both cultivars of flax.
Cd induces the refolding of protein in plants due to the activation of some enzymes involved in the refolding of protein, and the denaturation of protein in plants is a dramatic effect caused by Cd. Therefore, some of the enzymes belong to the chaperone family and chaperon like protein gets activated during response and adaptation to Cd pollution. The level of heat-shock protein (HSPs)which are involved in the refolding of denatured protein increased after the treatment of Cd, reported by Sergio.
Pb (lead) is the most abundant toxic element and ubiquitously distributed in the soil. In minute concentration lead exert an adverse effect on the morphology of plants, growth and developmental process, photosynthetic reactions of the plants. The traces of Pb inhibit the germination of seeds in Pinus helpers, Spartina alterniflora. Low concentration (60μM) of lead acetate deactivates the amylase and protease enzymes by about 50% in the endosperm of rice observed by Mukherji and Maitra. Growth at initial stages of seedling was also inhibited in different plant species such as barley and tomato, soya bean, rice, and legume plants. Reduction in growth and chlorosis of plants is due to administered of lead concentration (100-200 ppm) to the pots of sugar beet plants. The different morphological changes in plants such as irregular radial thickening of pea roots, lignifications of cortical parenchyma, and changes in the cell wall of endodermis are results of contamination of metal elements inside the plant. Lead elements interfere in various biological processes during the stem elongation and leaf expansion and inhibit this process in different plants such as barley, Allium sp., and Raphanus sativus. Lead concentration, its ionic composition, and pH of the medium affect the degree on which root elongation depends on this factor of lead element [17,18].
Genotoxic Effects of Heavy Metals
The mechanisms behind metal instigated genotoxicity are intricate in nature and still not surely knew. It has been resolved that heavy metal prompted genotoxicity/DNA harm happens by implication through the generation of ROS under oxidative pressure. Heavy metal toxicity induces chromosomal aberrations and also decreases the cell division rate.
Various investigations have recently distinguished heavy metal actuated nucleic acid debilitations in plants like Allium cepa. Genotoxic reactions vary among plant species to a similar metal and for the most part relies upon the number and all-out length of the diploid chromosomes and furthermore the number of metacentric chromosomes. The grouping of heavy metal, its oxidation state, and degree of introduction significantly influence the genotoxic reaction of any plant. Hydroxyl radical (OH) is the profoundly receptive species among ROS, harming all the segments of the DNA particle. ROS cooperation with DNA prompts base cancellation, base alteration, strand breaks, and harms to cross-connections and pyrimidine dimers. The introduction to ionization Cu prompted decreased mitotic record and chromosomal abnormalities ( viz. C-mitosis, heavy in chromosome tenacity, chromosome spans, and miniaturized scale core). Likewise, Cu poisonous quality caused debilitated microtubules to plan at various fixations and diminished substance of α-tubulin in contrast with controls. Reported Pb induced genotoxicity due to the amplification of new bands and the absence of normal amplicons in treated plants in random amplified polymorphic DNA (RAPD) analysis [19,20].
Conclusion
There are massive pressures on agriculture to satisfy the food demand for the world population and increase crop yields. In response to this demand, crop producers have adopted intensive agricultural practices that can contaminate the environment with heavy metals. Therefore, is extremely necessary to formulate appropriate agricultural public policies at a world level to enhance the extension services and educate farmers about better management risks without having a negative effect on food security and human health. The greater part of the observations to date centre around the investigation of HM impact on grownup plant development stage, however, it is of enormous hugeness to get data on the plant reactions to HM stress directly from the seed germination/seedling development stage, on the grounds that these are the most touchy phases of plant development cycle when confronting outside worry, as the plants that are utilized for phytoremediation need to confront pressure directly from beginning phases of development. Seed germination and early seedling development are likewise the most appropriate stages for bio observing. Another significant factor to remember is that the plants developed on debased soils need to confront different metal pressure instead of that of a solitary metal. It is clear that metals associate contrastingly when they are available in a mixture. For instance, particles of various metals will compete for binding sites of soil particles or plant metal transporters. In this manner, to comprehend the reaction system of the plant of interest under numerous metal stress is likewise a significant angle for the fruitful acknowledgment of phytoremediation in field conditions. The logical perceptions on a few of these plants have shown that glutathione is a significant player deciding their relative tolerance. Heavymetal stress induces the synthesis of reactive oxygen species (ROS) and may respond to generate oxidative pressure. It has been discovered that notwithstanding accumulated heavy metal elements, elevated levels of ROS antagonistically influenced the plants. Glutathione is engaged with detoxifying ROS through the ascorbate–glutathione cycle. While accumulated metal elements are detoxified by phytochelatins, which are synthesized from glutathione in plants during the exposure of heavy metals to crop plants. Phytochelatins structure complex with metal particles and sequestered them into the vacuole. This system of heavy metal tolerance in plants has emphatically proposed that glutathione ought not to be constraining. In this way, endeavours have been made to produce transgenic plants utilizing a few unique genes managing glutathione levels in plants. Especially, the job of glutathione, phytochelatin, cysteine union, and glyoxalase pathway genes has been accounted for in granting substantial metal pressure resistance. Additionally, several natural plant species have been identified showing the heavy metal accumulator behaviours. Initial indications in such plants documented the involvement of glutathione in the mechanism of heavy metal stress tolerance. However, this needs a further detailed account of experimental validation. These natural heavy metal accumulators could be a potential source for genetic manipulation of other important agricultural crop plants.
REFERENCES
- Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004;166(5), 1213-1218.
- Anderson AJ, Meyer DR, Mayer FK. Heavy metal toxicities: levels of nickel, cobalt and chromium in the soil and plants associated with visual symptoms and variation in growth of an oat crop. Aust J Agric Res. 1972;24(4):557-571.
- Andosch A, Affenzeller MJ, Lütz C, Lütz-Meindl U. A freshwater green alga under cad-mium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicel-lular model Micrasterias. J Plant Physiol. 2012;169(15):1489-1500.
- Arora A, Saxena S, Sharma DK. Tolerance and phytoaccumulation of chromium by three Azolla species. World J Microbiol Biotechnol. 2006;22(2):97-100.
- Ashraf R, Ali TA. Effect of heavy metals on soil microbial community and mung beans seed germination. Pakistan J Bot. 2007; 39 (2): 629-636.
- Aslam R, Bhat TM, Choudhary S, Ansari MYK. An overview on genotoxicity of heavy metal in a spice crop (Capsicum annuum L.) in respect to cyto-morphological behaviour. Caryologia. 2017;70(1): 42- 47.
- Baker AJM, Walker P. Physiological responses of plants to heavy metals and the quantification of tolerance and toxicity. Chemical Speciation & Bioavailability. 1989;1(1):7-17.
- Barbosa JS, Cabral TM, Ferreira DN, Agnez-Lima LF, De Medeiros SB. Genotoxicity assessment in aquatic environment impacted by the presence of heavy metals. Ecotoxicol Environ Saf. 2010; 73(3): 320- 325.
- Barceló J, Poschenrieder C. Plant water relations as affected by heavy metal stress: a review. J plant nutrition. 1990;13(1): 1-37.
- Bertrand M, Poirier I. Photosynthetic organisms and excess of metals. Photosynthetica. 2005; 43(3):345-353 .
- Bishnoi NR, Dua A, Gupta VK, Sawhney SK. Effect of chromium on seed germination, seedling growth and yield of peas. Agric ecosys environ. 1993;47(1):47-57.
- Keller C, McGrath SP, Dunham SJ. Trace metal leaching through a soil-grassland system after sewage sludge application. J Environ Quality. 2002; 31(5):1550-1560.
- Cervantes C, Campos-García J, Devars S, Gutierrez-Corona F, Loza- Tavera H, Torres-Guzmán JC et al. Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev. 2001;25(3):335-347.
- Chatterjee J, Chatterjee C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ pollution. 2000;109(1):69-74.
- Chugh LK, Sawhney SK. Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant physiol biochem. 1999;37(4):297-303.
- Cuypers A, Karen S, Jos R, Kelly O, Els K, Tony R et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J Plant Physiol. 2011;168(4):309- 316.
- Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plants: a review. Environ Pollut. 1997;98(1):29-36.
- Demiral T, Türkan I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot. 2005;53(3):247-257.
- Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hortic. 2010;123(4):521-530.
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signalling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005; 17(7):1866-1875.
Citation: Hasan N, Choudhary S, Sharma N (2021) The Mechanism of Heavy Metal Elements in various Biological Process and Its Deteriorate Effects on the Productivity of Different Crop Plants. Int J Waste Resour. 11:403.
Copyright: ©2021 Hasan N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.