Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 0, Issue 0
The Impact of Rotavirus Vaccine on the Prevalence Rotavirus Diarrhea on Omdurman Pediatric Teaching Hospital from November 2015 to August 2016
Qusay Eltayeb1* and Abdelwahid Ali Mohamed22Department of Clinical Microbiology, Sudan medical specialization board, Al Khurtum, Sudan
Received: 03-Jan-2022, Manuscript No. JVV-22-1192; Editor assigned: 05-Jan-2022, Pre QC No. JVV-22-1192; Reviewed: 19-Jan-2022, QC No. JVV-22-1192; Revised: 24-Jan-2022, Manuscript No. JVV-22-1192; Published: 31-Jan-2022, DOI: 10.35248/2157-7560.22.S17.001
Abstract
Rota virus diarrhea is a global health problem leading to high morbidity and mortality among children less than five years, the implementation of Rotavirus vaccine has made a change in this area. This study examined the impact of Rotavirus vaccine on prevalence of Diarrhea in Omdurman pediatric hospital, Khartoum, Sudan. A total of 368 children less than or equal to three years of age suffering from watery non bloody diarrhea were investigated by taking stool sample and examined by sandwich enzyme linked immunosorbent assay ((ELISA) to detect the Rotavirus antigen. Out of the 368 patient investigated, 28% tested positive for Rotavirus antigen, the highest rate of infection (49.5 %) was found among patient less than one year of age, patient with very sever disease were more in those whom were partially vaccinated (70.6%). Introduction of Rotavirus vaccine was found to decrease the prevalence of Rotavirus diarrhea from 33% to 28 %.
Keywords
Rotavirus vaccine; Rotavirus infection; Rotavirus diarrhea
Introduction
Rotavirus is a segmented double stranded RNA (dsRNA), non-enveloped capsid and icosahedral symmetry of Reoviridae. It causes acute gastroenteritis which leads to severe watery non bloody diarrhea and vomiting among infants and children worldwide [1]. In children less than five years old, there are about 450,000 deaths due to rotavirus gastroenteritis in the world yearly. It accounts for 37% of all deaths in children under five years worldwide [2]. the World Health Organization (WHO) and Federal Ministry of Health- Sudan (FMOHS) set up the virus gastroenteritis surveillance in June 2009 which showed that 33% of all stool samples (>9000 samples) collected from sentinel sites were positive for rotavirus [3] It is fortunate, that there is vaccine against rotavirus, which has been included in the program of vaccination carried out by the National Immunization Program (NIP) with a support from all members of the International Coordination Committee (ICC). The vaccine was submitted to Global Alliance for Vaccine Initiative (GAVI), and was approved in 2011[4]. On July 17, 2011 the first Sudanese child was vaccinated against rotavirus gastroenteritis [4]. So, Sudan is the first country in Africa where rotavirus vaccine was introduced with support from GAVI. Following the Sudan's introduction of the rotavirus vaccine, GAVI planned to introduce it in 40 GAVI eligible countries by 2015 [5]. The aim of this study is to assess impact of Rotavirus vaccine on prevalence of Rotavirus Diarrhoea among children under three year old.
Materials and Methods
This study is prospective hospital based study, was conducted at Omdurman Pediatric teaching hospital, during Nov 2015 up to Aug 2016, Survived children equal or less than three years of age who complained of three loose or watery motions in past 24 hours plus two or more episodes of unexplained vomiting.
Selection criteria
All Children less than three years old admitted and treated as a case of gastroenteritis as a primary illness and duration of symptoms is seven days or less were included , while children who acquired gastroenteritis during his hospitalization for treatment of another disease (hospital acquired gastroenteritis) or complained of bloody diarrhea we’re excluded.
Sampling technique and sample size
A random non-probability purposive sampling was followed.
The equation used for calculating the sample size was SS =ZXP (1-P)/P
SS= Sample Size, Z = 1. 96, d = 0. 05., p = prevalence = 0.33
SS =3.8416 × 0.33 × 0.67/0.0025=339.75=340 samples
A total of 368 children were recruited in the study.
Data collection
A questionnaire and formal consent were used to collect personal, demographic and clinical data. These include age, gender, weight, vaccination status, symptoms and classification of severity. Presence of vaccination card was important to consider the child as vaccinated, but we failed to obtain them from all candidates and considered the verbal information from parents.
Laboratory Examination
A single stool sample was collected during the attack of acute gastroenteritis in a dry, clean, wide mouth screw capped container and Placed in ice then sent directly to Omdurman pediatric Hospital laboratory.
Each sample investigated for the presence of group A rotavirus using VP6 monoclonal antibody in a solid phase sandwich enzyme immunoassay.
In room temperature (24 °C) a ratio of 1 ml from each sample was diluted in 0.1 ml of sample diluent in a clean dry tube, and time shift during pipetting was avoided. Then vortex for 30-60 seconds, the resulting dilution processed in a period between 15 minutes up to eight days at 2 °C-8 °C. Two negative control, two positive control and 0.1 ml of each diluted specimen were added to the separate microwells. After addition of all specimens and controls, (0.1 ml) of conjugate was added to each microwell and incubated at 20 °C-30 °C for 60 minutes. Each microwell was washed with diluted wash buffer of total five times. After the final wash, the plate was inverted and tapped on absorbent paper to remove the last traces of wash buffer. Then (0.1 ml) of substrate was added to each microwell and incubated at 20 °C-30 °C for 10 minutes and protected from light. The substrate reaction was stopped by adding (0.1 ml) of Stop Solution to each microwell. The colored product was read within 30 minutes after addition of stop solution because it is not stable. First macro reading was done by comparing the tested microwells to the negative and positive controls, then microwells were read photometrically as follow, the mean of negative control values should be less than 0.150 absorbable unit, the means of the positive control values must be greater than (0.500) absorbance units. The cut-off value was calculated by the addition of (0.200) absorbance units to the mean of the Negative Control Values. Any sample with absorbance value greater than the cut-off value was considered Positive. And any sample with absorbance value less than the cut-off value was considered Negative. An sample with absorbance value within (0.010) absorbance units of the cut-off value called equivocal and were retested or resampled.
Quality control of the Kits:
Each DAKO IDEIA rotavirus EIA kit contain a positive control and a negative control that should be performed with each assay to ensure that kit reagents are functioning correctly and that proper assay procedures have been followed.
Limitation of procedure
A negative test result does not exclude the possibility of rotavirus infection. Failure to detect rotavirus in stool specimen may result from many Factors such as collection of specimen after the peak period of viral shedding (3-5 days), improper sampling and/or handling of specimen, virus strain maybe not group (A) strain while test kit only detects group (A) rotavirus strain.
Data analysis
The collected data were checked, coded and entered into Statistical Package for Social Sciences (SPSS version 19). The results presented in tables and figures. The level of significance was considered significant with P value less than 0.05 using Chi square test.
Ethics statement
The study was approved from the ethics review committee of the Sudan Medical Specialization Board, council of clinical Microbiology. A Permission to conduct the research from the ministry of health Khartoum state, Administration of Omdurman pediatric teaching hospital. A written informed consent was obtained from the parents of all patients investigated.
Results
The result of present study involved (N = 368) children less than or equal 3 years of age with watery non-bloody diarrhea.
Descriptive statistics
Table (1) shows the percentage, absolute numbers and allocation of the sample 368 population under each one of the variables included in this study .
Table (2) shows the age distribution of patients was from 1 to 36 months (SD 7). Most of the patients study (196%/53.3%) were 1-12 month old, and (51/49.5%) of them were positive for rotavirus, and (144%/39.1%) were 13-24 month old, (46%/31.9%) were positive for rotavirus, children age 25-36 month old were least contributing to study (28%/7.6%) of them (6/21.4) were affected by Rota virus infection.
This association patient's age and rotavirus infection was found statistically not significant (P-0.35).
Table (3) shows that most of the participant were male (220%/59.8%) and (56%/25.5%) had Rotavirus infection, (148%/40.2%) of participants were female and (47/31.8%) were infected by Rota virus. Females were more liable to be infected than males but statistically not significant (P-0.11).
Table (4) shows that initial result of the total 368 stool samples, (99%/27%) were positive, (257%/70%) negative and (11%/3%) equivocal samples. These 11 equivocal samples were tested; the result was 4 positive and 7 negative samples. The end result of the 368 investigated clinical samples (103%/28%). positive, and (265%/72%) negative samples, All patients contributing in this study were vaccinated, (123%/33.4%) of them receive one dose of vaccine, (245%/69%) receive two doses of vaccine.
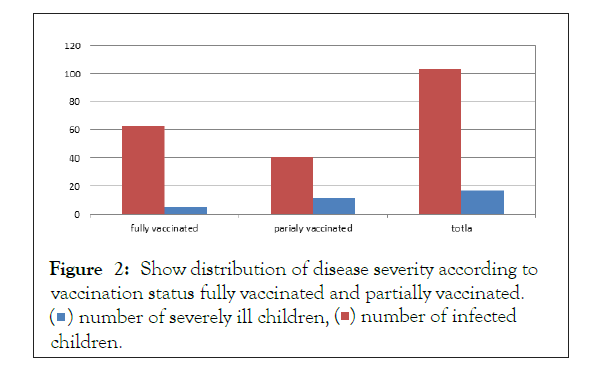
Table (5) shows relation between severity of the disease and number of rotavirus vaccine received (fully vaccinated or partially vaccinated) (63%/61%) of patient infected with Rotavirus were fully vaccinated (5%/7.9%) had very sever disease while (40%/39%) were partially vaccinated and (12%/30%) had very sever disease with (P value is 0.12) (Figures 1 and 2).
Table 1: Shows the percentage, absolute numbers of variable included in this study.
| Variable | Category | Absolute number | Percentage |
|---|---|---|---|
| Age | 1-12 months | 196 | 53.20% |
| 13-24 months | 144 | 39.10% | |
| 25-36 months | 28 | 7.70% | |
| Gender | Male | 220 | 59.80% |
| female | 148 | 40.20% | |
| Vaccination status | Partially vaccinate | 123 | 33.40% |
| Fully vaccinated | 245 | 66.60% | |
| Disease severity | Very Sever disease | 17 | 4.70% |
| Moderate sever disease | 82 | 22.30% | |
| Non infected | 269 | 73% |
Table 2: Distribution of rotavirus infection according to age.
| Age group | Total examined | Rotavirus positive | |
|---|---|---|---|
| Frequency | Percentage | ||
| 1-12 months | 196 | 51 | 26 |
| 13-24 months | 144 | 46 | 31.9 |
| 25-36 months | 28 | 6 | 21.4 |
| total | 368 | 103 | 28 |
Table 3: Distribution of Rotavirus infection according to gender.
| Gender | Number examined | Rotavirus positive | |
|---|---|---|---|
| Frequency | Percentage | ||
| Male | 220 | 56 | 25.5 |
| female | 148 | 47 | 31.8 |
| total | 368 | 103 | 28 |
Table 4: Shows the relation between Rotavirus infection and number of Rotavirus vaccine doses received.
| Number of vaccine doses | Number | Rotavirus positive | |
|---|---|---|---|
| Frequency | Percentage | ||
| One dose | 123 | 40 | 32.5 |
| Two doses | 245 | 63 | 25.7 |
| total | 368 | 103 | 28 |
Table 5: Show relation between severity of Rotavirus infection and number of Rotavirus vaccine received.
| Vaccination status | Number | Number of Very sever disease | |
|---|---|---|---|
| Frequency | Percentage | ||
| Fully vaccinated (two doses) | 63 | 5 | 7.9 |
| Partially vaccinated (one dose) | 40 | 12 | 30 |
| total | 103 | 17 | 16.5 |
Figure 2: Shows distribution of disease severity according to vaccination status fully vaccinated and partially vaccinated.  children.
children.
Discussion
In this study most infected children are less than one year of age, the rate of infection in this age group was (49.5%), this comparable to other study by Junaid (1), but it found to be less than rate documented by Magzoub (72.7%) (6), children less than one year were more prone to rotavirus infection because they are immunocompromised due to loss of passive immunization acquired from mother, and This is going with the assumption that the early peak of rotavirus may result from early exposure to contaminated sources (7).
In comparison of rotavirus infection rate between males and females, There was no significant difference observed (P value 0.11), this is similar to study in Cameron (8) but it is differ to that reported from Magzoub study (6) and other studies done in Italy (9) and Nigeria (1)
This study showed reduction in the prevalence of rotavirus diarrhea after the implementation of the Rotavirus vaccine. Prevalence dropped from 33% in 2009 [3] to 28% in this study 2016. This study prevalence (28%) was similar to that of S. Rahoud (27.3%) done in Gezira state, Sudan 2012 (10)· but less than that of Mustafa's study (36%) done in 2011 which is carried out before implantation of vaccine (11). and more than Magzoub’s study which showed prevalence of just 16% in 2013 (6).
This study prevalence rate was between 21.4% that reported form united Arab Emirate (12) and 32% reported from Brazil (13). But The figures in this study were higher than that reported from United States 18% (14) and from Valencia in Spain, 15% (15). These differences in studies may reflect different rate of infection with rotavirus in different countries as result of differences in hygiene and sanitation systems. However it may also be due to use of different Study designs and laboratory tools or had been carried out in different clinical situations.
There is no statistically significant differences in severity of Rotavirus infection between patients received one dose or two doses of the vaccine (P value-0.12), this differ from study in Canada which established that The higher the number of doses given to children, the higher the rate of documented reduced complications (16) and study in USA that reported considerable reduction in complications from acute gastroenteritis caused by rotavirus in patient received the full vaccine (17).
Conclusion
Vaccination of children with rotavirus vaccine reduces the prevalence, but not affects the severity of the disease, no significant association between rotavirus infection and age or gender of child.
This was a hospital based study and hence the result is unlikely to be a true reflection of the disease burden in the community, community base study should be carried out to detect the actual effect of vaccine in prevalence of Rotavirus infection.
References
- Junaid S, Umeh C, Olabode A, Banda J. Incidence of rotavirus infection in children with gastroenteritis attending Jos university teaching hospital, Nigeria. Virol J. 2011;8:233.
[Crossref], [Google Scholar], [PubMed]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and metaanalysis. Lancet Infect Dis. 2012;12(2):136-141.
[Crossref], [Google Scholar], [PubMed]
- Introduction of life-saving rotavirus vaccine in Sudan. Relief web. 2011.
- Incidence of rotavirus infection in children with gastroenteritis attending Jos university teaching hospital, Nigeria
- The Sudan introduces rotavirus vaccine-Gavi, the Vaccine Alliance.
- Magzoub MA bba, Bilal NE ldi, Bilal JAl, Osman OF. Rotavirus infection among Sudanese children younger than 5 years of age: A cross sectional hospital-based study. Pan Afr Med J. 2013;16:88.
[Crossref], [Google Scholar], [PubMed]
- Wobudeya E, Bachou H, Karamagi CK, Kalyango JN, Mutebi E, Wamani H. Breastfeeding and the risk of rotavirus diarrhea in hospitalized infants in Uganda: A matched case control study. BMC Pediatr. 2011;11:17.
[Crossref], [Google Scholar], [PubMed]
- Ndze VN, Akum AE, Kamga GH, Enjema LE, Esona MD, Banyai K, et al. Epidemiology of rotavirus diarrhea in children under 5 years in Northern Cameroon. Pan Afr Med J. 2012;11:73.
[Google Scholar], [PubMed]
- Marsella M, Raimondi L, Bergamini M, Sprocati M, Bigi E, De Sanctis V, et al. Epidemiology of rotavirus-associated hospital admissions in the province of Ferrara, Italy. Eur J Pediatr. 2009;168(12):1423-1427.
[Crossref], [Google Scholar], [PubMed]
- Rahoud S, Tajeldin IM. The Impact of Rotavirus Vaccination on The Diarrhea Admission and Mortality Rate in Children at WMTHC. Gezira State, Sudan. 2015.
Citation: Eltayeb Q, Mohamed AA (2022) The Impact of Rota virus Vaccine on the Prevalence Rota virus Diarrhoea on Omdurman Pediatric Teaching Hospital from November 2015 to August 2016. S17:001.
Copyright: © 2022 Eltayeb Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.