Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 10, Issue 5
The effect of Tamoxifen on Indomethacin induced hepato-nephrotoxicity in rats
Malak M Eljafari1*, Aisha M Dugani1, Soad A Treesh2 and Amal Musa22Department of Histology and Medical Genetic, University of Tripoli, Tripoli, Libya
Received: 20-Jul-2020 Published: 03-Aug-2020
Abstract
Background: Indomethacin is a widely prescribed nonsteroidal anti-inflammatory drug (NSAID) with a known hepato-renal toxicity. Tamoxifen is the treatment of choice for women with estrogen receptor-positive breast cancer.
Aim: This study was aimed to investigate the effects of tamoxifen (TAM) on renal toxicity and hepatic damage induced by indomethacin (IND) in rats.
Methods: Hepato-renal toxicity was induced in female Wistar rats by a single dose of IND (150 mg/kg) administered by gavage. TAM pretreatment involved Subcutaneous (SC) administration of the drug in a dose of 0.5 mg/kg/day for 5 consecutive days. Assessment of liver and kidney toxicities was based on determination of the activity of liver enzymes sGPT, sGOT, ALP, serum urea and serum creatinine as well as the ratio of liver and kidneys weight to total body weight and on histopathological evaluation.
Results: Oral administration of IND produced a significant elevation in liver and kidneys weight ratio, sGOT, sGPT, serum urea and serum creatinine levels compared to negative control group. The pretreatment with TAM 0.5mg/kg before induction of IND toxicity, resulted in significant reduction in serum urea, creatinine and liver enzymes activity and significantly protected the liver and the kidneys from injury induced by IND. Histopathological examination of kidneys and livers confirmed the protective effect of TMA. This protection by TAM may be related to its scavenging activity of free radicals, its antioxidant properties or to stimulation of ERs by TAM and its metabolites.
Conclusion: This study may suggest that the pretreatment with multiple low doses of TAM will significantly reduce INDinduced hepato-renal toxicity.
Keywords
Tamoxifen; Liver Enzymes; Indomethacin; Free Radical; Scavenging Activity
Introduction
Many drugs can attain high concentrations in the liver and kidneys making these organs highly vulnerable to toxicity [1]. The liver is the principal organ of drug metabolism while kidneys were considered as the primary eliminator of various waste products so it is not surprising that a broad spectrum of adverse drug effects on both organs have been documented [2,3]. Many drugs like statins, methotrexate, ketoconazol, acetaminophen and tamoxifen were known to induce hepatic damage [4,5]. While others could be linked to both hepatic and renal damage like acyclovir, ACE inhibitors, β-blockers and NSAIDs [6,7].
Indomethacin (IND) is one of the most potent non-selective NSAIDs [8]. It was reported that even at therapeutic dosages, IND can cause renal toxicity especially in susceptible patients (e.g., the elderly and patients with renal insufficiency, or decreased effective blood volume) [9]. And also can cause hepatotoxicity and alteration of liver function with elevations of serum aspartate and alanine aminotransferases (sGOT and sGPT), and necrosis of hepatic cells [10].
Tamoxifen (TAM), a non-steroidal selective estrogen receptor modulator (SERM) that has been used as a golden therapy especially for postmenopausal breast cancer [11,12]. In addition to its beneficial effect in the treatment of precocious puberty in McCune-Albright syndrome [13], in some cases of infertility [14], Gynecomastia [15], and sometimes reserved for management of resistant bipolar depression or mania, or when contra-indications to other psychotropic medications are present [16].
The use of TAM in treatment of breast cancer can be associated with many troublesome side effects like vaginal bleeding, endometrial cancer, cerebrovascular events, venous thromboembolic events [17], mood disturbances, bone pain, hypercalcemia, ocular toxicity, loss of libido and impotence in men, nephrotoxicity and hepatotoxicity [18,19].
Despite all those facts concerning TAM side effects, some studies highlighted its hepato-nephroprotective role [20-23] either in-vitro or in-vivo. However, upon literature search, no data were identified concerning the combined effect of tamoxifen/ indomethacin in particular the effect of TAM on the hepatic-renal toxicity induced by large dose of IND. Therefore, we conducted this study to evaluate the hepato-nephrotoxicity of the concomitant use of IND and TAM in female rats.
Materials and Methods
Experimental animals
Twenty-four female Albino Wistar rats (bred at the local Animal house, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, University of Tripoli, (Tripoli-Libya) (weighing 150 gm-200 gm). The rats were housed at a constant room temperature (20-25°C) under 12h light/12 dark cycle and with access to standard rat food pellet diet and given free access to water. Rats were deprived of food but allowed access to water for 24 hrs prior to the IND injection
Drugs and chemicals
Tamoxifen citrate (Sigma) dissolved in corn oil at concentration of 20 mg/1 ml [24] and ultra-sonicated for 30 minutes. IND capsules were from (Actavis), all the other chemicals used were of analytical grade and purchased from Merck (E. Merck, F.R. Germany) and (BDH Chemicals Ltd., Poole, England). The reagent kits used were from BIOLABO diagnostic Ltd (France).
Experimental Protocol
The animals were divided into four groups of six animals in each and were treated as follows:
Group І: negative control (NC): rats treated with vehicle (corn oil) only S.C for 5 consecutive days then on the 5th day injected with oral 5% NaHCO3.
Group IІ: Indomethacin treated group (IND): the rats were injected with 150 mg/kg of Indo dissolved in 5% NaHCO3 and given by gavage [25].
Group III: TAM 0.5 treated group (TAM): rats were injected with 0.5 mg /kg of TAM S.C for 5 consecutive days [5].
Group IV: Pre 0.5 group (PRE): rats were injected with 0.5 mg/kg of TAM S.C for 5 consecutive days then on the 5th day were orally received 150 mg/kg IND.
All rats were fasted for 24 hrs before the last dose and sacrificed 24 hrs later; blood samples were collected by cardiac puncture from all rats after being lightly anesthetized with diethyl ether; kidney and liver specimens were dissected and prepared for histopathological examination.
Serum biochemistry analysis
Blood samples was allowed to clot at room temperature and the serum was separated after centrifugation at 3000 rpm for 10 min. Serum specimens were stored frozen at (-20°C) until assayed for glutamate oxaloacetate transaminases (sGOT), glutamate pyruvate transaminases (sGPT), alkaline phosphatase (ALP), urea and creatinine (Vitalab Selectra E).
Relative liver and kidney weights and Histopathological examination
The liver and kidneys from each rat were carefully dissected, blotted free of blood and weighed to the nearest milligram. The relative organs weights were calculated as liver weight divided by total body weight while relative kidney weight calculated as kidney weight divided by total body weight.
Samples from each liver and kidney were placed in 10% formalin; paraffin-embedded sectioned at 5 μm and studied under light microscopy after staining with haematoxylin and eosin (HE). At least 2 slides were studied from each specimen in a blinded fashion for any histological changes [27].
Statistical analysis
The obtained results were expressed as mean ± SEM. Comparison between control and treatment groups were performed by oneway analysis of variance (ANOVA) followed by post-hoc LSD test. Differences were considered significant when the degree of confidence was 95% or better (P<0.05).
Results
Effect of drug treatments on Liver and renal biomarkers:
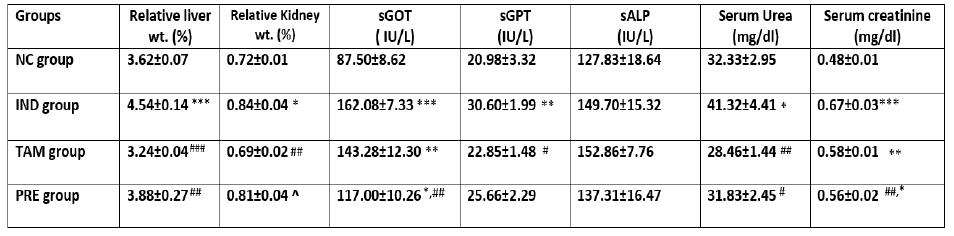
IND-treated group showed a significant increase in relative liver and kidney weights, sGOT (P<0.0001), sGPT (P<0.001) but not sALP, serum urea and serum creatinine were also significantly elevated compared to negative control. TAM-treated group showed significant increase in sGOT and serum creatinine compared to control group (P<0.001). In this group, the relative liver and kidney weights are significantly lower than IND group (P<0.001, P<0.01 respectively). sGOT and serum creatinine were significantly higher than control group (P<0.01). Pretreatment of rats with TAM before the induction of hepato-renal toxicity by IND resulted in significant reduction in relative liver weight, sGOT, serum urea and serum creatinine (P<0.01) as compared to IND group.
Histopathological Examination of Liver Sections:
The histopathological observations of the negative control liver showed normal liver section with normal structural and architectural intactness without any apparent damage or disruptions (Figure 1A). Rats treated with (150 mg/kg) IND showed dilated central vein (CV), congested blood vessels (CBV) in the portal area, portal tract inflammation with MNPCs infiltration, and no significant necrosis with distinct preservation of the hepatocytes (H) structural and architectural frame (Figure 1B). The hepatic damage in TAM 0.5 group showed slight portal tract inflammation with few MNPCs collection. There was distinct preservation of structural and architectural frame without significant changes from normal hepatic tissue as shown in (Figure 1C). The combination group (PRE group) showed improved hepatic picture of lobules with normal CV diameter, mild portal vein congestion and some macrophages infiltration (Figure 1D).
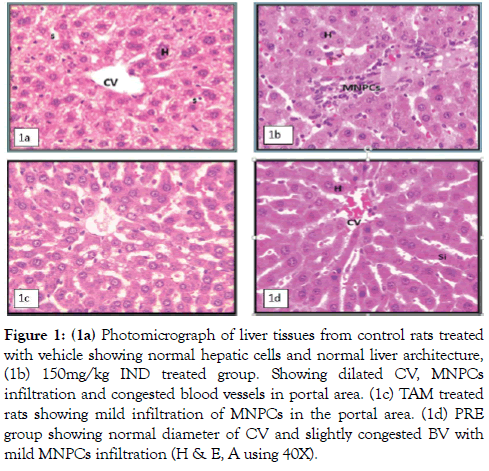
Figure 1: (1a) Photomicrograph of liver tissues from control rats treated with vehicle showing normal hepatic cells and normal liver architecture, (1b) 150mg/kg IND treated group. Showing dilated CV, MNPCs infiltration and congested blood vessels in portal area. (1c) TAM treated rats showing mild infiltration of MNPCs in the portal area. (1d) PRE group showing normal diameter of CV and slightly congested BV with mild MNPCs infiltration (H & E, A using 40X).
Histopathological Examination of the Kidney Sections
The kidneys of the control rats treated with vehicle, showed normal renal cells with normal architecture (Figure 2A). Light microscopic examination of the kidney sections from rats injected with 150 mg/kg of Indo (group II) showed cortical lesions included irregular areas of tubule, and conspicuous dilatation of renal space (RS), congested blood vessels (CB) with mild inflammatory changes, tubular necrosis as vacuolated cytoplasm (VAC) particularly in the proximal convoluted tubules (PCTs) (Figure 2B). TAM group showed mild inflammation with few infiltration of mononuclear phagocytic cells MNPCs, almost normal renal corpuscle, no congestion and the PCTs looks normal with small defect in brush border (Figure 2C). The PRE group showed that most of the renal tubules were having an architecture similar to those of the control group, normal RS, with preservation of a near to normal structure for many PCTs with regular brush border (Figure 2D).
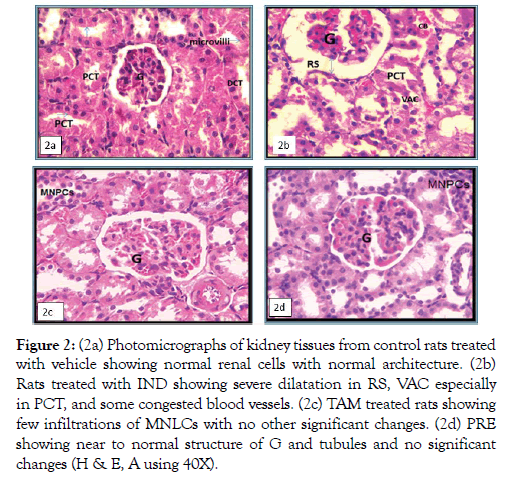
Figure 2: (2a) Photomicrographs of kidney tissues from control rats treated with vehicle showing normal renal cells with normal architecture. (2b) Rats treated with IND showing severe dilatation in RS, VAC especially in PCT, and some congested blood vessels. (2c) TAM treated rats showing few infiltrations of MNLCs with no other significant changes. (2d) PRE showing near to normal structure of G and tubules and no significant changes (H & E, A using 40X).
Discussion and Conclusion
Elevated activities of sGOT and sGPT are indications of hepatocellular injury and hepatic cell membrane damage [28,29]. And treatment with IND showed significant elevation in sGOT, sGPT and in liver index compared to negative control. This observed hepatotoxicity of indomethacin could be attributed to Indo active metabolites, activation of inflammatory cells, with increased synthesis of fibrinogen. Through, stimulatory action of interleukins TNF-α, IL-1 and IL-6, released by the inflammatory process and fibrinogen degradation products [30]. Oxidative stress and lipid peroxidation, mitochondrial dysfunction are among other reported causes [31,32]. However, pretreatment with TAM restored most of the above mentioned parameters significantly indicating the role of TAM protecting the liver. This protective effect confirmed by histopathological examination which reveals improved hepatic lobules with normal central vein diameter and near to normal hepatic architecture.
The hepatoprotective effect of TAM suggested to be related to lipid peroxidation prevention in rat liver microsomes and in phospholipid liposomes and suggested that the antioxidant property of TAM might contribute to its biological action and hepatoprotective effect [33].
From a different prospective the involvement of ERα in druginduced or chemical-induced liver injury and its exact functional mechanisms have yet been elucidated. But in a study done by Yoshikawa and his group found that TAM treatment before chemical hepato-intoxication produces a hepatoprotective effect against variety of chemicals including: acetaminophen, bromobenzene, diclofenac. Where the monocyte to macrophage differentiation-associated 2 (Mmd2) protein is commonly increased by TAM pretreated groups. Those results support the theory of ERα involvement in drug-induced or chemical-induced liver injury. Upregulation of Mmd2 protein in the liver was suggested as the mechanism of the hepatoprotective effect [20].
Table 1: The effects of different drug treatments on hepatic and renal biomarkers
Values are expressed as mean + S.E.M. (n=6). ***p<0.0001 **p<0.01, *p<0.05, respectively in comparison to NC group. ###p<0.0001 ##p<0.01, #p<0.05, respectively in comparison to IND group and ^p<0.05 in comparison to TAM group.
Another highlight of our study was the possible protective effects of TAM on IND induced nephrotoxicity. The PRE group produced a significant improvement in serum urea and creatinine levels compared to Indo intoxicated group. This protective behavior of TAM can be related to its free radical scavenging activity of TAM and its metabolite as indicated from in vivo and in vitro study by Moreira et al. [34]. The initial contention that Indo-induced adverse effects are solely due to the inhibition of COX enzyme has now been disputed. Several studies in the gastrointestinal tract and kidney have shown that Indo was also responsible for other effects in tissues. Such as induction of oxidative stress and mitochondrial dysfunction. Increase in the activity of myeloperoxidase (MPO), which is a marker of neutrophil infiltration, and decreased activity of glutathione reductase [35].
Even with the suggested increase in MPO enzyme after Indo dose, the results of our study agreed with previous in-vitro study [36]. Which found that MPO only weakly activate 4-OHTAM to its phenoxyl radical suggesting that 4-OHTAM is also a poor intracellular substrate for it and support the theory of antioxidant effect of 4-OHTAM.
Hydroxyl-TAM is a more powerful intramembranous inhibitor of lipid peroxidation compared to TAM. This effectiveness not correlating with alterations on membrane fluidity but may be due to the presence of a hydrogen-donating HO-group in the OHTAM molecule and its preferential location in the outer bilayer regions where it can donate the hydrogen atom to quench free radicals capable of initiating the membrane oxidative degeneration and also correlated to its higher partition in biomembranes [37].
Other study showed that TAM and its metabolites causes an acute vasorelaxation by inducing vasodilator prostanoids synthesis and this is another suggested mechanism for improved kidney damage caused by Indo intoxication. However, further study is necessary to elucidate the exact mechanism of the hepato-nephroprotection that TAM can provide.
The results of this study demonstrated the favorable hepatonephroprotective action of pretreatment with multiple low doses of TAM (0.5 mg/kg) before induction of IND toxicity will significantly reduce Indo nephrotoxicity and hepatotoxicity which was confirmed biochemically and histopathologically regardless the fact that TAM is hepato-nephrotoxic drug.
Competing Interests
The authors declared that they do not have any conflict of interest.
Funding
Not applicable
REFERENCES
- Levine RR, Walsh CT, Schwartz-Bloom RD, editors. Pharmacology: drug actions and reactions. CRC Press; 2000.
- Marasani A. Hepatoprotective activity of Bauhinia Variegata against isoniazid-and rifampicin-induced toxicity in experimental rats. Int J Pharm Pharm Sci. 2014;6:177-181.
- Perazella MA. Renal vulnerability to drug toxicity. Clin J of Ameri Soc of Nephrol. 2009;4:1275-1283.
- Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity: a review. J Appl Pharm Sci. 2012;2:233-243.
- Gafari MM, Najjar DG, Zriba KM, Dugani AM. Effects of tamoxifen on acetaminophen-induced hepatotoxicity in rats. Libyan J Med Res.2010;745-52.
- Elefsiniotis IS, Pantazis KD, Ilias A, Pallis L, Mariolis A, Glynou I, et al. Tamoxifen induced hepatotoxicity in breast cancer patients with pre-existing liver steatosis: the role of glucose intolerance. Eur J Gastroenterol Hepatol. 2004;16: 593-598.
- El-Beshbishy HA, Mohamadin AM, Nagy AA, Abdel-Naim AB. Amelioration of tamoxifen-induced liver injury in rats by grape seed extract, black seed extract and curcumin.Indian J Exp Biol. 2010;48:280-288.
- Wiseman H, Cannon M, Arnstein HR, Halliwell B. Tamoxifen inhibits lipid peroxidation in cardiac microsomes: comparison with liver microsomes and potential relevance to the cardiovascular benefits associated with cancer prevention and treatment by tamoxifen. Biochem Pharmacol. 1993;45:1851-1855.
- Kishore BK, Ecelbarger CM. Lithium: A versatile tool for understanding renal physiologyAm J Physiol Renal Physiol. 2013;304:1139-1149.
- Dodick DW, Jones JM, Capobianco DJ. Hypnic headache: another indomethacin‐responsive headache syndrome?. Headache: The Journal of Head and Face Pain. 2000; 40):830-835.
- Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat clin Pract Nephrol. 2006 Feb;2:80-91.
- Decensi A, Bonanni B, Guerrieri-Gonzaga A, Gandini S, Robertson C, Johansson H, et al. Biologic activity of tamoxifen at low doses in healthy women. JNCI: J of Natio Cancer Inst. 1998 7;90:1461-1467.
- Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN, et al. Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis. 2002 ;23:1897-1902.
- Pharma J. Endoxifen; 2016. Available from: http://jinapharma.com/?page_id=184.
- 15Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, et al. Evidence that transforming growth factor-β is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987; 48:417-428.
- Butta A, MacLennan K, Flanders KC, Sacks NP, Smith I, McKinna A, et al. Induction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Research. 1992;52:4261-4264.
- Pollak M, Costantino J, Polychronakos C, Blauer SA, Guyda H, Redmond C, et al. Effect of tamoxifen on serum insulinlike growth factor I levels in stage I breast cancer patients. JNCI: Journal of the National Cancer Institute. 1990 7;82:1693-1697.
- Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH, et al. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J pediat. 2003;143:60-66.
- Dhaliwal LK, Suri V, Gupta KR, Sahdev S. Tamoxifen: An alternative to clomiphene in women with polycystic ovary syndrome. J Hum Reprod Sci. 2011;4:76-79.
- Yoshikawa Y, Miyashita T, Higuchi S, Tsuneyama K, Endo S, Tsukui T, Toyoda Y, et al. Mechanisms of the hepatoprotective effects of tamoxifen against drug-induced and chemical-induced acute liver injuries. Toxicol and App Pharmacol. 2012; 264:42-50.
- Miyashita T, Toyoda Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T, et al. Hepatoprotective effect of tamoxifen on steatosis and non-alcoholic steatohepatitis in mouse models. J Toxicol Sci. 2012;37: 931-942.
- Kim D, Lee AS, Jung YJ, Yang KH, Lee S, Park SK, et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway. Nephrol Dial Transplant. 2014 ;29:2043-2053.
- Hernández-Esquivel L, Zazueta C, Buelna-Chontal M, Hernández-Reséndiz S, Pavón N, Chávez E, et al. Protective behavior of tamoxifen against Hg2+-induced toxicity on kidney mitochondria: In vitro and in vivo experiments. J Steroid Biochem Mol Biol. 2011;127: 345-350.
- Werawatganon D, Rakananurak N, Sallapant S, Prueksapanich P, Somanawat K, Klaikeaw N, et al. Aloe vera attenuated gastric injury on indomethacin-induced gastropathy in rats. World J Gastroenterol. 2014; 20:18330-18337.
- Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, et al. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41:108-114.
- Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Elsevier health sciences; 2008.
- Yue M, Ni Q, Yu CH, Ren KM, Chen WX, Li YM, et al. Transient elevation of hepatic enzymes in volunteers after intake of alcohol. Hepatobiliary Pancreat Dis Int. 2006; 5:52-55.
- Singh A, Bhat TK, Sharma OP. Clinical biochemistry of hepatotoxicity. J Clinic Toxicol 2011.
- Silva MA, Rao VS, Souza CM, Neves JC, Menezes DB, Santos FA, et al. Evaluation of thalidomide against indomethacin-induced small intestinal damage and systemic toxicity in rats. Biomedical Research. 2012; 23:125-133.
- Olusegun Taiwo V, Lawal Conteh O. The rodenticidal effect of indomethacin: pathogenesis and pathology. Veterinarski arhiv. Veterinarski Arhiv. 2008;78:167-178.
- Pennington SN, Smith CP. Indomethacin stimulation of lipid peroxidation and chemiluminescense in rat liver microsomes. Lipids. 1978; 13:636-643.
- Wiseman H, Laughton MJ, Arnstein HR, Cannon M, Halliwell B. The antioxidant action of tamoxifen and its metabolites Inhibition of lipid peroxidation. FEBS letters. 1990;263:192-194.
- Moreira PI, Custódio JB, Oliveira CR, Santos MS. Brain mitochondrial injury induced by oxidative stress-related events is prevented by tamoxifen. Neuropharmacology. 2005;48:435-447.
- Varghese J, Faith M, Jacob M. Zinc prevents indomethacin-induced renal damage in rats by ameliorating oxidative stress and mitochondrial dysfunction. Eur J Pharmaco. 2009;614:114-121.
- Day BW, Tyurin VA, Tyurina YY, Liu M, Facey JA, Carta G, et al. Peroxidase-catalyzed pro-versus antioxidant effects of 4-hydroxytamoxifen: enzyme specificity and biochemical sequelae. Chem Res Toxicol. 1999;12:28-37.
- Custódio JA, Dinis TC, Almeida LM, Madeira VM. Tamoxifen and hydroxytamoxifen as intramembraneous inhibitors of lipid peroxidation. Evidence for peroxyl radical scavenging activity.Biochem Pharmacol. 1994; 47:1989-1998.
- Montenegro MF, Ceron CS, Salgado MC, Desta Z, Flockhart DA, Tanus-Santos JE, et al. Tamoxifen and its metabolites cause acute vasorelaxation of aortic rings by inducing vasodilator prostanoid synthesis. J Cardiovasc Pharmacol. 2011;58:647-653.
Citation: Eljafari MM, Dugani AM, Treesh SA, Musa A (2020) The Effect of Tamoxifen on Indomethacin Induced Hepato-Nephrotoxicity in Rats. J Clin Exp Pharmacol. 10:269. doi: 10.35248/2161-1459.20.10.269
Copyright: © 2020 Eljafari MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Sources of funding : Not applicable

