Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 12, Issue 2
The Effect of Chemical Modifications of Chitosan on Intestinal Permeability and Oral Bioavailability of Carbamoylphosphonate JS403
Reut Bitton-Dotan1, Joerg Bohrisch2, Christian Schmidt2, Marina Tsuriel1, R. N. Prasad Tulichala1, Eli Breuer1, Reuven Reich1, Amnon Hoffman1* and Joachim Storsberg22Fraunhofer Institute Applied Polymer Research (IAP), Department of Biomaterials & Healthcare, Geiselbergstrasse 69, 14476 Potsdam, Germany
Received: 26-Feb-2020 Published: 07-Apr-2020, DOI: 10.35248/0975-0851.20.12.392
Abstract
JS403, Carbamoylphosphonate molecule, is a promising drug candidate with anti-metastatic activity. The polarity and water solubility of JS403 evolve from the phosphonate moiety that is ionized at physiologic pH. JS403 is regarded as a BCS class III molecule, and exhibit poor absolute oral bioavailability of less than 1%. The aim of this investigation is to identify proper chitosan based absorption enhancer compound(s) that upon oral co-administration with JS403 which would enhance its bioavailability. To determine the optimal properties of the chitosan derivative (CD), 11 relevant compounds were synthesized, each with different attribute, and their impact on the intestinal permeability of JS403 was examined in-vitro using the CaCO2 monolayer model. Then, the oral bioavailability of JS403 coadministered with the selected CD was examined in the freely moving rat model. The in-vitro study identified two leading trimethyl CD, KC13, (85% deacetylation, MW 20,000 g/mol and 66% trimethylation) and a novel derivative of hydroxypropyl chitosan KHC2 (trimethylation 71%) increased JS403 permeability in 2 and 10 folds, respectively. Similar permeability results were obtained when the same chitosan derivatives were coadministered with atenolol, another BSCIII drug. The paracellular absorption mechanism of these BCS III compounds was ascertained using palmitoyl carnitine as positive control. The pharmacokinetic investigation, following gavage coadministration with either KC13 or KHC2, showed a significant increase in JS403 oral AUC of 200%. The in-vitro permeation data correlated with the oral bioavailability outcomes. The relatively modest enhancement (~2 folds) of the chitosan derivatives in-vivo is anticipated as their effect on the enterocytes integrity and tight junction (TJ) need to be moderate. This is required because their noninvasive and reversible impact that is demanded in case of multiple treatment.
Keywords
Absorption enhancer; Intestinal permeability; Pharmaceutical approach; Oral bioavailability; BCS Class III; Chitosan derivatives; Paracellular; Palmitoyl carnitine
Introduction
JS403 is a small carbamoylphosphonates (CPO) molecule, with a structure of sulfonamide moiety and chain of 6 methylenes that link the anionic phosphonate moiety (Figure 1). JS403 in an important compound as it was shown to inhibit the activity on three cancer promoting zinc- enzyme: matrix metalloproteinase (MMP-II), carbonic anhydrase 9 and 12 (CA-IX, CA-XII) and autotaxin (ATX) [1]. Furthermore, in-vivo studies in murine melanoma models of B16F10 injection have shown promising results of 71 percent decrease in lung metastasis following JS403 oral administration [2].
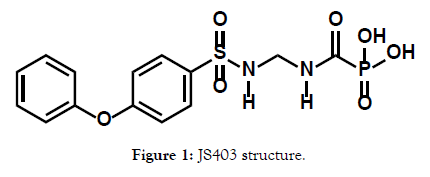
Figure 1: JS403 structure.
Those figures pose JS403 as a promising drug candidate for antimetastasis.
A key factor for JS403 as a drug candidate is the fact that it is ionized to anion in all physiological pH and for that reason cannot penetrate via cells membrane [1]. Therefore, JS403 hardly cross the intestinal wall, and once in the body its volume of distribution is restricted to the extracellular fluid. It is fortunate that the active sites of the relevant zinc-enzymes involved in metastases progression are distinctively exposed to the extracellular fluids. The restriction of the volume of distribution of JS403 is also important fact in lowering its potential intracellular associated toxicity [1].
JS403 is characterized by high water solubility and low membrane permeability therefore is considered as Biopharmaceutics Classification System (BSC) class III molecule [3]. The intestinal permeability of class III molecules is mainly limited to paracellular pathway. This pathway consists of diminutive surface area of the tight junctions (TJ) - less than 0.1% of the total intestinal epithelial layer surface [4]. Hence, the low oral availability of less than 1% of JS403 limit its development toward becoming clinically useful medication against metastasis in cancer pateints [2]. This is particularly important since JS403 is intended to be taken daily as a preventative anti-metastatic treatment. Thus, to ensure good patient compliance and comfort, the route of administration should be oral with reliable oral absorption.
A major strategy in overcoming the restricted bioavailability is the "pharmaceutical approach" of coadministration of the active pharmaceutical ingredient (API) with absorption enhancer(s) that modulate the tight junctions' opening. However, it is important to note that affecting the TJ opening should be in a noninvasive and reversible manner. Therefore, their expected impact is limited to relatively modest magnitude of effect.
To achieve this goal of elevating the JS403 oral bioavailability, a wide library of 11 trimethyl chitosan (TMC) derivatives were synthesized in this investigation. Chitosan was selected based on previous reports that implied their impact on enhancing the paracellular pathway (Figure 2). Chitosan is a biocompatible polymer with promising reversible tight junction modulating properties mainly by means of epithelial mucoadhesion [5,6]. However, a major drawback of the non-derived chitosan is its solubility that is limited to acidic environment [7]. Therefore, chitosan derivatives were generated, primarily TMC (Figure 3), which showed significant permeation enhancement and no apparent cytotoxic effect for mannitol an hydrophilic API that has been commonly used as a model drug to evaluate paracellular permeation kinetics [8].
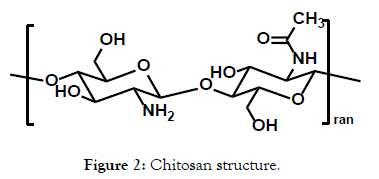
Figure 2: Chitosan structure.
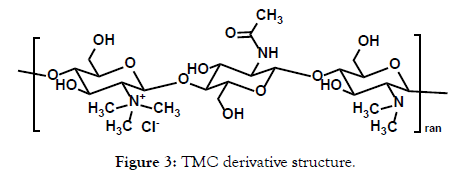
Figure 3: TMC derivative structure.
In order to determine the greatest impact on the TJ and on JS403 intestinal permeability, the effect of the different TMC derivatives has been investigated. All derivatives possess certain different parameter in different degrees and ranges in order to enable intelligent selection of the superior derivation. The parameters which have been studied are molecular weight in range of 104 to 106 (MW), deacetylation with 85% and 95% degree and trimethylation within range of 32%-71%. Following the knowledge obtained by identifying the optimal parameters from the tested chitosans, a novel library of N-methylated hydroxypropyl chitosan (KHC) derivatives (Figure 4) has been designed. This new derivative contributed the element of increased lipophilicity and its impact on JS403 intestinal permeability and oral bioavailability was established.
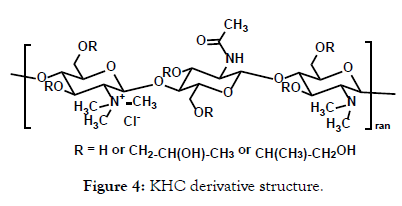
Figure 4: KHC derivative structure.
JS403 is negatively charged in physiological fluids thus may represent certain anionic BCS III APIs. In order to reach a broader conclusion for BCS III absorption enhancers, additional class III drug, atenolol (Figure 5) was used as model API and given together with the leading TMC derivatives in the CaCO2 permeability studies. Atenolol is a known low permeability marker and in contrast to JS403, it is a basic drug with pKa= 9.5 that has positive charge at physiological conditions [9]. These two molecules differ profoundly in their physico-chemical properties. Thus, the comparison between the impact of the co-administrated chitosan derivatives on the intestinal permeability of these two BCS III compounds, under similar experimental conditions, widen the understanding of this approach and enables to derive more sound conclusions. The direct influence of TMC derivatives on JS403 permeability enhancement were assessed first by in-vitro model at cell monolayer CaCO2 (mimic enterocytes layer). The outcomes of these studies paved the ground for in-vivo PK studies in the freely moving rat model that provides direct information on oral bioavailability of the tested API, which was the ultimate goal of this investigation. CaCO2 is regarded as good predictor for intestinal permeability process [10].
Figure 5: Atenolol structure.
Another known powerful enhancer for intestinal permeability is palmitoyl carnitine (PC). PC is a robust TJ modulator that when apply with BCS III medication in CaCO2 model increase the permeability. Therefore, PC were used as a positive control for comparing the other potentially absorption enhancers examined for hydrophilic molecules. However, its activity showed lytic effect with a decline in cell viability [11]. Since the use in enhancer required safety, PC cannot be used as enhancer. At this study, PC was used as a positive control for the permeability modification imposed by the examined TMC derivatives as a paracellular enhancer.
Ultimately, since the main goal of this study was to reveal a proper uptake enhancer for JS403 which will promote its development toward clinical utilization, the effect on JS403 oral bioavailability was assessed in the freely moving rat model taking into considerations the outcomes of the in-vitro permeability studies.
Materials and Methods
Chemicals
All materials, unless otherwise stated, were purchased from Sigma Aldrich (Rehovot, Israel).
Synthesis
JS403 was synthesized by Prof. Eli Breuer's laboratory, Institute for Drug Research, Hebrew University, Jerusalem, Israel. The N-trimethyl and hydroxypropyl N-trimethyl chitosan were synthesized by Dr. Joachim Storsberg’s laboratory, Fraunhofer- Institute für Angewandte Polymerforschung IAP, Potsdam, Germany.
N-trimethyl chitosan Synthesis details
The library of TMCs (Table 1) was synthesized adapting a known procedure Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride by Sieval AB et al. In 1998.
| Chitosan Name | Molecular Weight (g/mol) | Degree of Deacetylation |
|---|---|---|
| Low Molecular Weight | 70,000 | 80 |
| Medium Molecular Weight | 750,000 | 80 |
| High Molecular Weight | 2,000,000 | 80 |
Table 1: Chitosans examined in CaCO2 study.
Step 1 (KC21+ KC41): In a 2l 3-necked flask 25 g (150 mmol) of chitosan (50K), 60 g of NaI and 138 mL of aqueous NaOH (15%) were dissolved in 1000 mL of N-methylpyrrolidone. The mixture was heated to 60°C for 1 hour. With stirring, 326.4 g (2.3 mol) of iodomethane were added dropwise via dropping funnel. After 1 h, the light-yellow solution was precipitated in 6 l of ethanol and the flocculent precipitate was centrifuged off for 5 min at 4000 min-1. Then this was washed twice with diethylether (G1 frit) and dried in vacuum overnight. The I-/Cl- exchange was performed by dissolving the polymer in 200 mL NaCl solution (10%) overnight. The solution was filtrated again and then dialysed (5 days, 14 kD) and freeze-dried. The H-nmr analysis revealed a ratio quaternary/ tertiary amino group of 32/53 in case of KC21 and of 33/62 in case of KC41 [1].
Step 2 (KC22 + KC42): In the same manner as above 44 g of the solid (step 1, ahead of anion exchange) were mixed with 40 g NaI and 92 mL of aqueous NaOH (15%) in 660 mL of N-methylpyrrolidone and reacted with 132.2 g of iodomethane. Usual work-up with anion exchange yielded a polymer with ratio quaternary/tertiary amino group of 40/45 in the case of KC22 and of 64/31 in the case of KC42.
Step 3 (KC23 + KC43): In the same manner as above 18.4 g of the solid (step 2, ahead of the anion exchange) were dissolved in 330 mL of N-methylpyrrolidone together with 2.5 g of solid NaOH and reacted with 18.9 g of iodomethane. Usual work-up with anion exchange yielded a polymer with ratio quaternary/tertiary amino group of 57/23 in the case of KC23 and 79/16 in the case of KC43.
The 20K Chitosan (85 and 95% deacetylation degree) was reacted in the same way. Products of step 1 (KC31) with a ratio quaternary/ tertiary (q/t) amino group of 38/57, step 2 (KC12) with a ratio q/t amino group of 52/33 and of step 3 (KC13) with a ratio q/t amino group of 66/19 were taken for further investigation.
Hydroxypropyl N-trimethyl chitosan Synthesis details
Step 1: Hydroxypropylation of chitosan was performed adapting a known procedure of Influence of degree of molar etherification on critical liquid crystal behavior of hydroxypropyl chitosan. In a 250 mL 3-necked flask 7 g (42 mmol) of chitosan (20K) were mixed with 35 mL aqueous NaOH (13%) and stirred for 2 hours. After freezing overnight at -18 °C the mixture was thawed and 56 g (0.96 mol) of propylene oxide were added. The mixture was stirred and refluxed at 40°C for 30 min. The hydroxypropyl chitosan (substitution degree according to 1H-nmr was ~0.9) obtained after dialysis (5 days, 3.5 kD) was taken for the next steps.
Step 2 (KHC1): In a 250 mL 3-necked flask 4.4 g of this material were mixed with 10 g NaI and 22.6 mL aqueous NaOH (15%) in 160 mL N-methylpyrrolidone. The mixture was heated to 60°C. With stirring, 53.4 g of iodomethane were added dropwise via dropping funnel. After 1 hour the mixture was precipitated in 1.6 l of ethanol. The flocculent precipitate was filtrated (G3 frit), washed three times with diethylether (G1 frit). The I-/Cl- exchange was performed by dissolving the polymer in 150 mL NaCl solution (10%) overnight. The solution was dialysed (5 days, 3.5 kD) and freeze-dried. The 1H-nmr analysis revealed a ratio quaternary/ tertiary amino group of 42/43, assuming no N-hydroxypropylation.
Step 3 (KHC2): In the same manner 2.2 g of the precipitate of step 2 were mixed with 5 g NaI and 11 mL aqueous NaOH (15%) in 80 mL N-methylpyrrolidone and reacted with 16 g iodomethane. Usual work-up with anion exchange yielded a polymer with ratio quaternary/tertiary amino group of 71/14, assuming no hydroxypropylation.
in-vitro permeabillity studies: CaCO2 and cell model
CaCO2 cell monolayer assay
Growth and maintenance of cells: CaCO2 cells were obtained from ATCC (Manassas, VA, USA) and then grown in 75 cm2 flasks at 37°C in 5% CO2 atmosphere and a relative humidity of 95%. The culture growth medium consisted of Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heatinactivated fetal bovine serum (FBS), 1% nonessential amino acids, 2 mM L-glutamine, 2 mM sodium pyruvate and 2 mM penicillinstreptomycin solution.
Preparation of cells for transport studies: Cells in a passage range of 52-60 were seeded at a density of 25- 105 cells/cm2 on untreated culture inserts of polycarbonate membrane with 0.4 μm pores and a surface area of 1.1 cm2. The culture inserts containing the CaCO2 monolayer were placed in 24 trans-well plates 12 mm, Co-star. The culture medium was replaced every other day. Transport studies were performed 21-23 days after seeding, when the cells were fully differentiated and the TEER values were stable (300-500 Ω x cm2).
Transport experiment protocol for CaCO2: Transport study (apical to basolateral, A to B) was initiated by medium removal from both sides of the monolayer and replacement with 600 μL apical buffer (0.025M D-glucose monohydrate, 0.02 M MES biological Buffer, 1.25 mM calcium chloride and 0.5 mM magnesium chloride in Hanks Balanced Salt Solution, filtered and titrated to pH 6.5 with NaOH) and1500 μL basolateral buffer (0.025M D-glucose monohydrate, 0.02 M HEPES biological Buffer, 1.25mM calcium chloride, and 0.5mM magnesium chloride in Hanks Balanced Salt Solution, filtered and titrated to pH 7.4 with NaOH), both warmed to 37°C. The cells were incubated for 30 min at 37°C with shaking (100 cycles/min). Test solutions were dissolved in apical buffer and preheated to 37°C and added (600 μL) to the apical side of the monolayer. (In Chitosan private case, to ensure that the chitosans will dissolve freely in the buffer, the pH was adjusted to pH=5.5, according to a published protocol [6]. Model compound, JS403, apparent permeability was also assessed the same pH to ensure monolayer viability throughout the experiment. The rest of the protocol was carried out as explained in this section.) 50 μL samples were taken from the apical side immediately at the beginning of the experiment, resulting in 550 μL apical volume during the experiment. For the period of the experiment, cells were kept shaken at 37°C. At certain predetermined times (20, 40, 60, 80, 100, 120 and 150 min), 200 μL samples were taken from the basolateral side and replaced with the same volume of fresh basolateral buffer to maintain a constant volume.
Data analysis: The permeability enhancers (Papp) for JS403 and atenolol were calculated using the following equation:
Papp= (dQ/dt) X 1/A X C0
where ΔQ/Δt is the steady state rate of appearance of the drug on the receiver side (obtained from the linear plot of drug accumulated vs. time), C0 is the initial concentration of the drug on the donor side, and A is the surface area available for permeation [12].
In-vivo pharmacokinetic studies
Male Wistar rats (275 - 300 g) were purchased from Harlan laboratories (Rehovot, Israel). All rats receive free access to water and food up to three hours before the experiment. All surgical and experimental procedures were reviewed and approved by the Animal Experimentation Ethics Committee of the Hebrew University Hadassah Medical Center, Jerusalem. In order to enable convenient blood sampling, an indwelling cannula was implanted into the right jugular vein 24 hours before the pharmacokinetic experiment as described before [13]. During the cannula implantation rats were anesthetized by intra-peritoneal injection of 1 mL/kg of ketamine-xylazine solution (9:1, respectively). The cannula was tunneled beneath the skin and exteriorized at the dorsal part of the neck. After completion of the surgical procedure, the animals were kept overnight in individual cages. Four hours post oral administration free access to food and water was available. Animals were randomly assigned to the different experimental groups. JS403 formulation solution of (30 mg/mL) was freshly prepared prior to administration with 300 mg/kg of JS403 and100 mg/kg dose for chitosan derivatives. The solutions administered by using oral gavage to the rats (n=4). After dosing blood samples of 300 µL were taken at 0, 0.16, 0.5, 1, 3, 6, 10, 24h 36, and 48 h postdosing. Equal volumes of heparinized saline were administered after each blood sampling. Plasma samples were separated by centrifugation (4g, 10 min, and 4°C) and stored at -20°C pending analysis.
Analytical methods
150 µL of plasma were mixed with 15µL of internal standard TCH18, a CPO compound with a linker of seven methylenes. Then, 285µL of methanol were added and vortex-mixed for 1 min. After centrifugation at 40000 rpm for 10 min, the organic layer was transferred to fresh glass test tubes, filtered and evaporated to dryness (Vacuum Evaporation System, Labconco, Kansas City, MO) and reconstituted with 80 µL of mobile phase. JS403 amount was determined by using a high-performance liquid chromatography (HPLC) system (Waters 2695 Separation Module) with a massspectrometer (Waters Micro-mass ZQ, Waters Corporation, Milford, MA). The HPLC-MS conditions were as follows: Xterra MS C18, 3.5µm,100A, 100x2.1mm column (Waters), an isocratic mobile phase, methanol: water: 50mM ammonium formate buffer in water titrated with formic acid to pH- 3.8-3.9 (57:23:20 v/v/v), flow rate of 0.2 mL/min at 45°C.
Pharmacokinetic (PK) analysis
Noncompartmental pharmacokinetic analysis was performed using WinNonlin software (version 5.1, Pharsight, Mountain View, CA).
Statistical analysis
All values are expressed as mean ± standard deviation mean (SD). To determine statistically significant differences among the experimental groups, t-test or one-way ANOVA, followed by Tukey`s test, was used. A p value less than 0.05 were termed significant.
Results
Effect of various chitosan with different chain length on JS403 transport across CaCO2 monolayer
The effect of three different chitosans on the permeability coefficient (Papp) of JS403 when screened in CaCO2 cell model presented in Figure 6. The chitosans, listed in Table 1, varied in their average molecular weight, i.e. in their chain length, but shared the same degree of deacetylation of 80%. Shorter and longer chain lengths have shown significant enhancing permeability properties. Since shorter chain length chitosans displayed favorable viscosity, synthesis of future chitosans was done in the order of 104 g/mol.
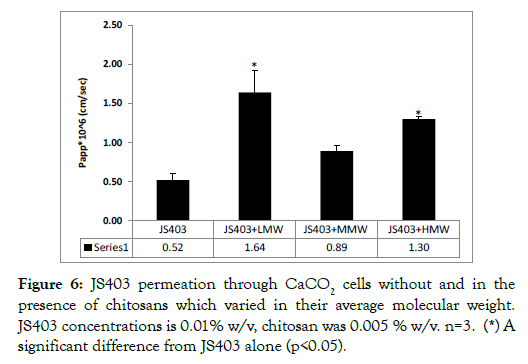
Figure 6: JS403 permeation through CaCO2 cells without and in the presence of chitosans which varied in their average molecular weight. JS403 concentrations is 0.01% w/v, chitosan was 0.005 % w/v. n=3. (*) A significant difference from JS403 alone (p<0.05).
N-trimethyl chitosan derivatives
Library of N-trimethyl chitosan derivatives synthesis and characterization: A library of 9 N-trimethyl chitosan (TMC) derivatives was synthesized varying in the chain length, degree of deacetylation and degree of trimethylation. For all TMCs, the counter-ion was Cl- and all were stable at room temperature and light. Table 2 depicts the specifications regarding each polymer.
| TMC derivative Name | Degree of Deacetylation | Molecular Weight (g/mol) | Content of trimethylation |
|---|---|---|---|
| KC12 | 85% | 20,000 | 52% |
| KC13 | 85% | 20,000 | 66% |
| KC21 | 85% | 50,000 | 32% |
| KC22 | 85% | 50,000 | 40% |
| KC23 | 85% | 50,000 | 57% |
| KC-3-1 | 95% | 20,000 | 38% |
| KC-4-1 | 95% | 50,000 | 33% |
| KC-4-2 | 95% | 50,000 | 64% |
| KC-4-3 | 95% | 50,000 | 79% |
Table 2: Library of N-trimethyl chitosans examined in CaCO2 study.
N-trimethyl chitosan derivatives in-vitro permeability study: Figures 7 and 8 show the permeability enhancing properties of each TMC group, categorized by their average molecular weight, degree of deacetylation and trimethylation. TMC with a high degree of deacetylation – 95%, showed no permeability enhancing properties in both chain lengths and all degrees of N-trimethylation (Figure 8).
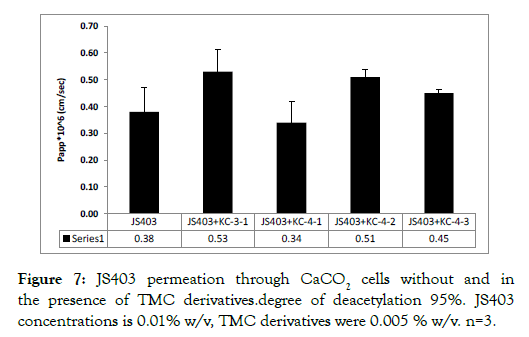
Figure 7: JS403 permeation through CaCO2 cells without and in the presence of TMC derivatives.degree of deacetylation 95%. JS403 concentrations is 0.01% w/v, TMC derivatives were 0.005 % w/v. n=3.
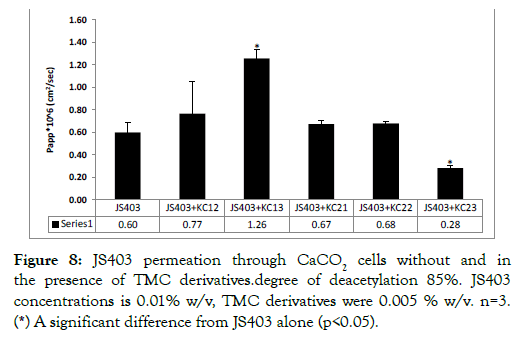
Figure 8: JS403 permeation through CaCO2 cells without and in the presence of TMC derivatives.degree of deacetylation 85%. JS403 concentrations is 0.01% w/v, TMC derivatives were 0.005 % w/v. n=3. (*) A significant difference from JS403 alone (p<0.05).
When TMC derivatives with a lower degree of deacetylation – 85% – were tested, apart from KC23, all exhibited enhancing properties of some sort. KC13, with 20,000 g/mol chain length and 66% degree of trimethylation showed the best enhancement properties.
N-methylated hydroxypropyl chitosan derivatives
Library of N-methylated hydroxypropyl chitosan synthesis and characterization: Library of N-methylated hydroxypropyl chitosan synthesis and characterization.
N-methylated hydroxypropyl chitosan derivatives in-vitro permeability study: Permeability enhancing effect of N-methylated hydroxypropyl chitosan derivatives (KHC) was evaluated in CaCO2 model as presented in Figure 9. KHC2, the derivative with the highest degree of N-trimethylation content (71%), showed 10.5- fold improvement in JS403 permeability, the best significant enhancement so far.
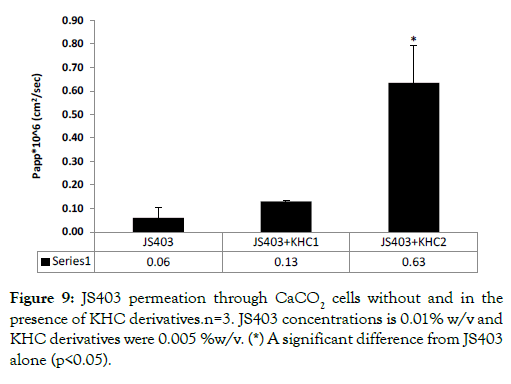
Figure 9: JS403 permeation through CaCO2 cells without and in the presence of KHC derivatives.n=3. JS403 concentrations is 0.01% w/v and KHC derivatives were 0.005 %w/v. (*) A significant difference from JS403 alone (p<0.05).
The effect of N-trimethyl chitosan derivatives on atenolol in-vitro permeability study: The effect of the chosen derivatives, KC13 and KHC2 on permeability coefficient of a different class III model drug, atenolol, was assessed in CaCO2 cell model. The findings showed significantly permeability enhancement of 3.5-fold and 10-fold caused by KC13 and KHC2 respectively. These results fit perfectly with JS403 finding.
The effect of PC on JS403 and atenolol in-vitro permeability study: In this study, PC served as a positive control for paracellular enhancer and model reliability. The permeability coefficient of JS403 and atenolol was examined without and in PC presence in CaCO2 model. As expected, the permeability of both drugs was significantly elevated with PC presence, as can be seen in Figure 10.
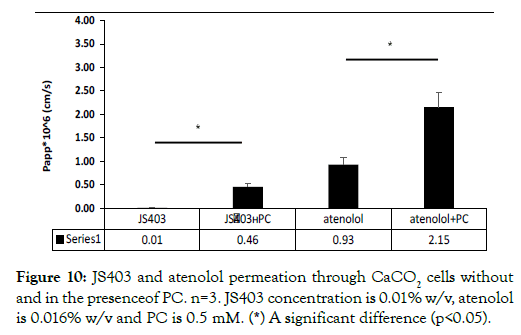
Figure 10: JS403 and atenolol permeation through CaCO2 cells without and in the presenceof PC. n=3. JS403 concentration is 0.01% w/v, atenolol is 0.016% w/v and PC is 0.5 mM. (*) A significant difference (p<0.05).
The effect of N-trimethyl chitosan derivatives on JS403 in-vivo absorption study: Plasma concentration time profiles for JS403 following oral administration of JS403 with chitosan derivatives: KC13, KHC2 to rats are depicted in Figure 11. In agreement with the in-vitro results, Oral administration of JS403-KC13 and JS403- KHC2 resulted in significantly increase of 2-fold in AUC values as compared to the oral administration of JS403 alone (Tables 3 and 4).
| Group name | AUCinf (h*µg/mL) | Cmax (µg/mL) | t1/2 (h) |
|---|---|---|---|
| JS403 | 10.2 ± 4.7 | 3 ± 0.3 | 5.53 ± 0.7 |
| JS403+KHC | 23.2 ± 7.9* | 7.33 ± 3.53* | 4.84 ± 1.7 |
| JS403+KC13 | 21.5 ± 4.5* | 5.59 ± 1.4 | 2.61 ± 1.04 |
Table 3: Library of N-trimethyl-hydroxypropyl chitosans examined in CaCO2 study.
| TMC derivative Name | Deacetylation degree | Molecular weight (g/mol) | Content of trimethylation |
|---|---|---|---|
| KHC1 | 85% | 20,000 | 42% |
| KHC2 | 85% | 20,000 | 71% |
Table 4: AUC,Cmax and t1/2 values (mean ± SD) obtained following single oral administration of JS403 (300 mg/kg) and co-administration of JS403 with enhancers: KHC, KC13 (100 mg/kg). n=4 for each group. (*) A significant difference (p<0.05) compare to group single oral administration of JS403.
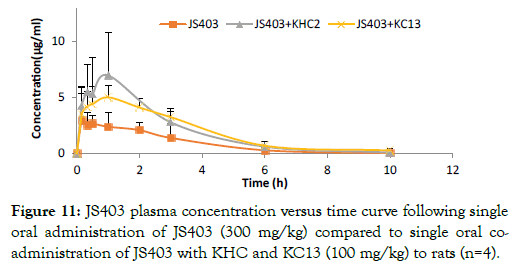
Figure 11: JS403 plasma concentration versus time curve following single oral administration of JS403 (300 mg/kg) compared to single oral coadministration of JS403 with KHC and KC13 (100 mg/kg) to rats (n=4).
Discussion
The CPO JS403 is a promising novel pharmaceutical entity that has shown efficacy in inhibiting cancer promoting enzymes in vitro and metastasis formation in vivo [2]. As a BCS class III compound it exhibits low oral bioavailability. The aim of the current investigation is to utilize the "pharmaceutical approach" to enhance the oral bioavailability of JS403 by co-administration with various chitosan derivatives. The motivation to pursue this systematic study was first to improve the oral bioavailability of the CPO making it an effective anti-metastatic oral treatment. In addition, to enrich the knowledge base regarding the impact of chitosan derivatives as gentle absorption enhancers for other APIs from the BCS III family. The chitosan derivatives tested in the current research were selected according to previous reports [14]. In addition, certain novel derivatives were synthesized based on intelligent design. The current information regarding the in-vivo utilization of TMC derivatives are sparse thus this additional systematic evaluation is required.
To perform this investigation, a library of 11 TMC derivatives in a different range of parameter was synthesized. In order to identify the optimal parameters to enhance JS403 intestinal permeability the TMCs were coadministered with the CPO and the impact on the permeability was determined by using the CaCO2 monolayer assay.
The first parameter investigated was chitosan chain length (MW). Three different chain lengths were studied but only two chain lengths had noteworthy impact on JS403 permeation. While short (7 × 104 g/mol) and long (2 × 106 g/mol) chain lengths increased JS403 permeation more prominently, the medium (7.5 × 105 g/mol) chain length elevated permeation but in a non-significant matter. Investigating this important parameter raised the issue of viscosity, where elevation of the molecular weight is associated with increased viscosity. Since high viscosity is not applicable for drug delivery, these findings indicated that it is preferable to synthesize short chain length chitosan in the order of 104 g/mol.
Another important parameter that was examined is the optimal degree of de-acetylation and trimethylation. For that, a 9 TMC derivatives library was synthesized with chain length in order of 104g/mol, in accordance with the previous results. All TMC with a high degree of deacetylation, 95%, had no significant enhancing effect on JS403 permeation. This implies that such high degree of deacetylation hinders the enhancing properties for JS403. This finding may result from exessive charge density that impairs the chitosan ability to interact with the epithelial membrane surface. Conversely, most of the TMC derivatives group with a lower degree of deacetylation- 85%, showed enhancing properties of varying power.
KC13, the derivative with 85% deacetylation, with the highest degree of trimethylation (66%) and shorter length chain (20,000 g/mol) exhibited the most significant permeability improvement of 2-fold. These results are in line with findings from previous studies, indicated that higher trimethylation degree, leads to more beneficial effect [8]. In contrast, KC23, derivative with a relatively high degree of trimethylation (57%), resulted in a significant reduction of JS403 permeability. This outcome may result from the relatively high molecular weight (50,000 g/mol) of KC23 yielding more viscose solution. It can be summarized that for this chitosan derivatives series, the most optimal degree parameters were related to 85% deacetylation, high degree of N-trimethylation and shorter chain lengths (20,000 g/mol).
However, the enhancement results are quite modest. The effect of increasing chitosan lipophilicity is one feature that has not been thoroughly studied yet. Benediktsdóttir et al. investigated the impact of lipophilicity of several derivatives of N-alkyl- chitosan on permeation enhancement in bronchial epithelium in-vitro [15]. They suggested that increasing the polymer lipophilicity could augment the permeation enhancement. Hence, in this study, a library of two novel chitosan derivatives- KHC was synthesized with all the optimal parameters, determined so far, and with a higher degree of lipophilicity by adding hydroxypropyl. Indeed, the novel derivative, KHC-2, have shown favorable effect over TMC derivatives on JS403 permeation enhancement. The greater ability of KHC2 to enhance JS403 permeability compare to KC13 may also stems from higher degree of trimethylation and the conformational modifications associated with this modification. This reasoning is along the same line that was stated previously by Dewald Snyman et al. that decrease in flexibility of TMC compare to chitosan can arise due to altered chitosan conformation [16].
The main purpose of this study was to investigate the effects of chitosan parameters on JS403 permeability. JS403 portrays part within BCS class III molecules. Therefore, it was important to examine if the anionic charge of JS403 in physiological fluids had electrostatic influence on the chitosan derivatives permeation enhancement capacity by comparing it to a positively charged BCS III compound such as atenolol [9,17]. The permeability of atenolol in the absence and presence of KC13 and KHC2 derivatives was found to be affected in the same way as JS403. These results corroborate the conclusion that the enhancement ability of the derivatives as observed in this study is not specific only to JS403 and probably can apply to a wider spectrum of class III molecules. This may also imply that the impact of the chitosan absorption enhancers is affecting the width of the TJ in a manner that is not dependent on the co-administered API.
In general, there may be a big gap between in-vitro permeability findings and the ultimate utilization of these findings in-vivo when dealing with the oral bioavailability. At the whole animal pharmacokinetic absorption study multi-threaded biological processes occurs that may affect the eventual outcome. On the other hand, the enterocyte monolayer that covers the intestinal surface is the major barrier for BCS III compounds. Thus, assessing this barrier behavior in the presence of the tested API and the coadministered enhancer provide realistic evaluation of what would be the impact of affecting the enterocyte monolayer permeability (as in the case of CaCO2 model), and the consequent overall bioavailability findings. This is because when dealing in the case of consecutive barriers cascade the rate determinant is the slowest process, which in the current case is the kinetics of crossing the enterocyte layer. Therefore, although generally it may be difficult to produce a passable in-vitro – in-vivo correlation (IVIVC), the rationale of conducting the in-vitro permeability study that is used to provide a predictive model for the in-vivo absorption outcome is quite reasonable, particularly, in the case of BCS III APIs [18].
According this rational the impact of the tested potential enhancers was studied also in-vivo. Indeed, following JS403 oral administration alone and combined with the leading derivatives KC13 and KHC2, the in-vivo findings provided a similar picture to the in-vitro predication. The results showed a statistically significant enhancement of 2-fold in JS403 AUC when given with KC13 and KHC2 derivatives, as correlate with the in-vitro results, but not in the same proportion. The differences in the proportion of KHC2 enhancement between the two models result most probably from the fact that the cell culture cannot fully simulate the additional processes that are associated the absorption process after oral administration into living body. Specifically, the in vitro monolayer model has a static mode that does not represent the processes associated with substances transport along the intestinal tract. It is important to note that the in-vivo studies in the current investigation where conducted in freely moving rats' and the API-enhancer combination was given by oral gavage. This administration modality mimics more closely the utilization of such combination in the clinical setting. In the past the in-vivo evaluations of chitosan impact on intestinal bioavailability where conducted following intra-duodenal administration [19,20]. The intra-duodenal administration has it own rationale but it for sure differs from what would ultimately happen following oral administration of the formulation. Needleless to mention that intra-duodenal necessitate abdominal surgery that by itself may affect the investigated pharmacokinetics.Another permeability enhancer which is known for its strong influence to enlarge TJ width is PC. Even though, PC is not clinically relevant due to safety concerns associated with the aggressive TJ opening. It was used in the current investigation in order to validate that the actual penetration pathway of the tested BSCIII APIs is related to the TJ width. Therefore, their co-administration with PC was used as a positive control for paracellular pathway enhancer. Indeed, the permeability of both atenolol and JS403 in the presence of PC has been significantly increased. Thus, confirming one of the investigations hypothesizes regarding the intestinal permeability mechanism of these BCS III APIs.
Conclusion
In conclusion, the challenge of increasing the paracellular permeability of JS403 as BCS III APIs is of importance due to their limited bioavailability restricted by the small diffusion space confined to the TJs. The potential enhancers have limited range of permeability enhancement as their impact has to be reversible and noninvasive which limits the fierceness of altering the TJ sites. As delineated in this work, chemical modifications of chitosan had quite modest impact on the permeability of JS403. Actually, in order to have immense impact on the degree of oral bioavailability the enhancer should be able to redirect the absorption mechanism from paracellular to transcellular where the absorptive surface is many folds larger. In general, the advantages of the pharmaceutical approach in enhancing bioavailability of BCS III compounds result from the fact that the API remains chemically untouched which makes it more accessible for advanced clinical utilization.
The investigation highlighted that KC13 and KHC2 chitosan derivatives can probably be used safely as absorption enhancer additives in formulation aimed to improve oral bioavailability of the BCS III APIs with physicochemical properties like JS403. Certain guidelines of methodologies to improve the chitosan derivatives absorption enhancing properties such as chain length, degree of acetylation, trimethylation and lipophilicity that were evaluated in the current research should be taken into consideration in future application and investigation of this approach.
Acknowledgement
The authors would like to acknowledge the financial support of this international “lighthouse” project funded by Fraunhofer Society, Germany and Sir Zelman Cowen university fund HUJI.
REFERENCES
- Reich R, Hoffman A, Ravi SR, Shai O, Frant J, Maresca A, et al. Carbamoylphosphonates inhibit autotaxin and metastasis formation in vivo. J Med Chem. 2014;30(5):767-772.
- Bitton R, Tsuriel M, Suresh RR, Breuer E, Reich R, Hoffman A. Investigation of Intestinal Absorption Enhancers : Individual vs. Blends with the Carbamoylphosphonate JS403. J Bioequiv Availab. 2018;10(2).
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification - solubilidade do CTZ. Pharm Res. 1995;12:413-420.
- Cano-Cebrián MJ, Zornoza T, Granero L, Polache A. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: a target for drug delivery. Curr Drug Deliv. 2005;2(1):9-22.
- van der Merwe SM, Verhoef JC, Verheijden JH, Kotzé AF, Junginger HE. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Biopharm. 2004;58(2):225-235.
- Schipper NG, Varum KM, Stenberg P, Ocklind G, Lennernäs H, Artursson P. Chitosans as absorption enhancers of poorly absorbable drugs 3: Influence of mucus on absorption enhancement. Eur J Pharm Sci. 1999;8(4):335-343.
- Jain A, Gulbake A, Shilpi S, Jain A, Hurkat P, Jai SK. A new horizon in modifications of chitosan: Syntheses and applications. Crit Rev Ther Drug Carrier Syst. 2013;30(2).
- Thanou MM, Kotzé AF, Scharringhausen T, Lueßen HL, de Boer AG, Verhoef JC, et al. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal CaCO2 cell monolayers. J Control Release. 2000;64(1-3):15-25.
- Lozoya-agullo I, González-álvarez I, González-álvarez M, Merino-sanjuán M, Bermejo M. Development of an ion-pair to improve the colon permeability of a low permeability drug : Atenolol. Eur J Pharm Sci. 2016;93:334-340.
- Artursson P, Palm K, Luthman K. CaCO2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;(1-3):27-43.
- Duizer E, van der Wulp C, Versantvoort CH, Groten JP. Absorption Enhancement, Structural Changes in Tight Junctions and Cytotoxicity Caused by Palmitoyl Carnitine in CaCO2 and IEC-18 Cells. J Pharmacol Exp Ther. 1998;287(1):395-402.
- Ovadia O, Greenberg S, Chatterjee J, Laufer B, Opperer F, Kessler H, et al. The Effect of Multiple N-Methylation on Intestinal Permeability of Cyclic Hexapeptides. Mol Pharmaceutics. 2011;8(2)479-487.
- Hoffman A, Levy G. Kinetics of drug action in disease states. XXIX. Effect of experimental nephrotic syndrome on the pharmacodynamics of heptabarbital: implications of severe hypoalbuminemia. J Pharmacol Exp Ther. 1989;249(1):117-122.
- Sahni JK, Chopra S, Ahmad FJ, Khar RK. Potential prospects of chitosan derivative trimethyl chitosan chloride (TMC) as a polymeric absorption enhancer : synthesis, characterization and applications. J Pharm Pharmacol. 2008;60(9):1111-1119.
- Benediktsdttir BE, Gudjónsson T, Baldursson Ó, Másson M. N-alkylation of highly quaternized chitosan derivatives affects the paracellular permeation enhancement in bronchial epithelia in-vitro. Eur J Pharm Biopharm. 2014;86(1):55-63.
- Snyman D, Hamman JH, Kotze AF. Evaluation of the mucoadhesive properties of N-trimethyl chitosan chloride. Drug Dev Ind Pharm. 2003;29(1):61-69.
- Frant J, Veerendhar A, Chernilovsky T, Nedvetzki S, Vaksman O, Hoffman A, et al. Orally active, antimetastatic, nontoxic diphenyl ether-derived carbamoylphosphonate matrix metalloproteinase inhibitors. Chem Med Chem. 2011;6(8):1471-1477.
- Lozoya-Agullo I, Gonzalez-Alvarez I, Zur M, Fine-Shamir N, Cohen Y, Markovic M, et al. Closed-loop doluisio (Colon, Small Intestine) and single-pass intestinal perfusion (Colon, Jejunum) in rat — biophysical model and predictions based on CaCO2 Pharm Res. 2017;35(1).
- Thanou M, Verhoef JC, Junginger HE. Chitosan and its derivatives as intestinal absorption enhancers. Adv Drug Deliv Rev. 2001;50:S91-S101.
- Luessen HL, De Leeuw BJ, Langemeÿer MW, De Boer AB, Verhoef JC, Junginger HE, et al. Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm Res. 1996;13(11):1668-1672.
Citation: Bitton-Dotan R, Bohrisch J, Schmidt C, Tsuriel M, Prasad Tulichala RN, Breuer E, et al. (2020) The Effect of Chemical Modifications of Chitosan on Intestinal Permeability and Oral Bioavailability of Carbamoylphosphonate JS403. J Bioequiv Availab 12:392. doi: 10.35248/0975-0851.20.12.392
Copyright: © Bitton-Dotan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

