Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 1
The Appropriate Use of O RhD Positive Units in RhD Negative Patients: A Systematic Review and Meta-Analysis
Leung W, Quiring S and Denise E Jackson*Received: 15-Jan-2024, Manuscript No. JBDT-24-24625; Editor assigned: 17-Jan-2024, Pre QC No. JBDT-24-24625 (PQ); Reviewed: 07-Feb-2024, QC No. JBDT-24-24625; Revised: 15-Feb-2024, Manuscript No. JBDT-24-24625 (R); Published: 21-Feb-2024, DOI: 10.4172/2155-9864.24.15.574
Abstract
The increasing use of O RhD-negative Red Blood Cells (RBCs) in transfusion practise requires a sustainable approach. We investigate the incidence of RhD alloimmunisation in RhD-negative patients to determine the efficacy and thereof the efficiency of using O RhD-positive as part of adapting protocols.
PubMed, Scopus, EMBASE and Google Scholar were used to search for relevant articles between August 2019 to August 2024 for articles with the use of RhD-positive RBCs in RhD-negative trauma patients.
Of 6 studies (458 cases) included in the meta-analyses, the incidence of RhD alloimmunisation of RhD-patients when given at least one RhD-positive RBC was 16.4% (CI 95%, 5.6%-31.3%). In the same studies, using eligible data, we found the incidence of non-RhD alloimmunisation in RhD-negative patients given RhD-positive RBCs (207 cases) to be 7.3% (CI 95%, 2.8%-13.6%) compared with non-RhD alloimmunisation in patients given RhD-negative RBCs (2119 cases) at 6.4% (CI 95%, 3.4%-10.3%).
The risk of RhD alloimmunisation given the protocols in place and the population frequency of RhD-positive individuals in Australia is minimal and not dissimilar to the alloimmunisation of non-RhD antibodies. Recommendations for the use of O RhD-positive RBCs in emergency transfusion remains a safe and viable alternative in the conservation of O RhD-negative RBCs.
Keywords
RhD positive transfusion; RhD alloimmunisation; Emergency transfusion
Introduction
The O RhD-negative blood group has been known as the ‘universal donor’ and elected as the choice red cell component in uncross matched emergency transfusions. A study of ABO blood groups in Australia using data from 2019 shows that the proportion of O RhD- negative Red Blood Cells (RBCs) issued have increased from 11.7% to 17.4% but first time O RhD-negative blood donors only make up 8.7% of donations. The same study shows that O RhD-negative people make up approximately 6.5% of the population while RhD-positive people make up 85.9% of the population [1].
Blood shortages have been experienced around the world particularly during the recent pandemic which has both caused an increase in demand of precious blood and also reduction in the provision of rare blood groups like O RhD-negative. Due to the demand of O RhD- negative RBCs for emergency transfusions, the frequent shortages of this precious blood group have prompted the need for a more permanent ongoing solution. In order to maintain a supply for this limited blood group so that they can be used for patients that need them most, in late 2022, the National Blood Authority (NBA) released a change in recommendations for the emergency use of group O red cells in Australia with considerations for the usage of O RhD-positive RBCs.
Emergency group O red cells in Australia
Currently the guidelines released by the NBA recommend the usage of emergency O RhD-negative blood for females with child bearing potential ( ≤ 50 years) and paediatric males ( ≤ 18 years). Females>50 years and adult males>18 are recommended O RhD-positive RBCs. These decisions apply until a valid group is obtained. If more than 4 units of uncross matched O RhD-negative RBCs are transfused and more are needed, the supply of RBCs is switched to O RhD- positive. The priority of red cell transfusions is to maintain life but the conservation of the precious supply of O RhD-negative units for specific use is also an important concept. Clinicians are responsible for evaluating the clinical need for the provision of RBCs and ultimately make the final decision based on these recommendations. There are provisions for combatting the alloimmunization of RhD using intravenous anti-D immunoglobulins when using RhD-positive RBCs [2].
Blood supply in Australia
Blood collection in Australia is on a voluntary basis, 1 in 30 Australians donate blood. The Australian Red Cross Lifeblood (ARCLB) oversees the collection of whole blood and plasma which is then processed into our blood components and batch products. The ARCLB is also responsible for the management of a continuous blood supply. Governance from the NBA in its National Blood Supply Contingency Plan (NBSCP) follows a tiered risk system that ensures that there is always an adequate supply of blood and blood products to all its services. The ARCLB makes provisions for finding and retaining donors of specific rare and in-demand blood types.
Emergency group O red cell usage in other countries
Since 2019, countries like Canada, UK and North America have made provisions for the usage of O RhD-positive RBCs in the clinical management of emergency transfusions, while others continue to only recommend O RhD-negative RBCs in their protocols [3-6]. These countries have a similar population frequency of RhD blood group to Australia.
Materials and Methods
The rationale for the recommendation to use RhD-positive RBCs is that the number of O RhD-positive RBCs used is proportionate to the O RhD-negative RBCs saved that can then be used in more clinically appropriately situations. Protocols are in place to circumvent the incidence of RhD alloimmunisation implicated in Haemolytic Disease of the Foetus and Newborn (HDFN) and Haemolytic Transfusion Reactions (HTR). It’s important to remember that all transfusions carry a risk, and that there is still an inherent risk of alloimmunization to other clinically significant antibodies, even when using O RhD- negative RBCs [7].
This systematic review and meta-analyses aim to assess the incidence of RhD alloimmunisation following the use of at least one RhD-positive RBCs in RhD-negative trauma patients. It also aims to assess and compare the incidence of non-RhD alloimmunisation following the transfusion with RhD-negative or RhD-positive RBCs. The hypothesis is that the incidence of alloimmunisation in the use of RhD-negative or RhD-positive RBCs following the recommendations is minimal.
Study design
This study follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocols. The quality of the literature was evaluated using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [8].
Search strategy
The databases PubMed, Scopus and EMBASE were employed to identify suitable articles between August 2019 to August 2023 [9]. The search terms used were “transfusion” and “RhD positive” and “Massive Transfusion” or “MTP”. Combinations and variations of “RhD positive” like “rhesus” and abbreviations like “pos” were also entered to ensure adequate coverage of terms used by authors. Further papers were identified by reviewing literature referenced by current articles and using Google Scholar within the search parameters.
Eligibility criteria
Articles were retrieved using the search strategy and duplicates were filtered out. Titles and abstracts were screened to ensure that included studies were based on transfusion of red cell component, assessed alloimmunisation rates and were not meta-analyses or case-reports. Reports assessed for eligibility excluded texts not available in English or translated, full-text articles not being available in the public domain or accessible viaRMIT’s library and articles not relevant to the protocol (such as, the use of Low Titre Whole Blood (LTWB) and where RhD- positive platelets were used).
Data extraction
Details of the included studies were compiled in Table 1, to illustrate the characteristics: author; study design; study period; country; total study size; RhD-negative patients who received RhD-positive red cells; mean number of units transfused and follow-up periods (Table 1). Raw data used in the statistical analyses was organised in Table 2, to show the incidence of alloimmunisation of RhD-negative patients given RhD-positive red cells or RhD-negative red cells for transfusion. The primary outcome was to assess the rate of RhD alloimmunisation with a secondary outcome on the incidence of non-RhD alloimmunisation.
| Study | Study design | Study period | Country | Total Study Size | RhD-neg patients who received RhD-pos RBCs | Mean number of units transfused | Follow-up period |
|---|---|---|---|---|---|---|---|
| Badami et al. 2022 [10] | Retrospective | 2011 - 2019 | New Zealand | 2585 | 227 | 10 (median) | - |
| Chowdury et al. 2023 [11] | Retrospective | Jun 2020 – Jun 2021 | Australia | 1013 | 66 | 2 (median) | 22 days (median) |
| Flommersfeld et al. 2018 [12] | Retrospective | Jan 2010 – Dec 2014 | Germany | 823 | 18 | 4 | - |
| Luyten et al. 2023 [13] | Retrospective | Jan 2019 – Oct 2022 | Belgium | 1199 | 141 | 4.1 | 56 days |
| Pandey et al. 2020 [14] | Prospective | Dec 2014 – Dec 2018 | India | 20658 | 57 | 4.42 | 3 months |
| Williams et al. 2019 [15] | Retrospective | Oct 2015 – Sep 2018 | United States | 1198 | 72 | 5.9 | 6 months min. |
Table 1: Characteristics of included studies.
| Badami et al. 2022 [10] | Chowdury et al. 2023 [11] | Flommersfeld et al. 2018 [12] | Luyten et al. 2023 [13] | Pandey et al. 2020 [14] | Williams et al. 2019 [15] | |
|---|---|---|---|---|---|---|
| Title and abstract | Y | Y | Y | Y | Y | Y |
| Objectives | Y | Y | Y | Y | Y | Y |
| Study design | Y | Y | Y | Y | Y | Y |
| Setting | Y | Y | Y | Y | Y | Y |
| Eligibility criteria | Y | Y | Y | Y | Y | Y |
| Study size | Y | Y | Y | Y | Y | Y |
| Outcome data | P* | P* | P* | Y | P* | P* |
| Discuss key results and limitations | Y | Y | Y | Y | Y | Y |
Note: Y: Yes (criteria fulfilled), P: Partial (criterial partially fulfilled) and N: No (criteria unfulfilled)
Table 2: Quality assessment of included studies using the STROBE checklist.
Statistical analysis
Meta-analyses were performed using Open Meta [Analyst] version 12.11.14 developed by Brown University. Data extracted. was subject to a binary random-effects model (Maximum Likelihood) using arcsine transformed proportions to calculate the incidence of alloimmunisation. Confidence intervals were set at 95%. I2 value measured heterogeneity and p-values measure statistical significance. A p-value<0.05 was considered statistically significant in this study.
Results
Study selection
Using the search strategy 8,914 articles was identified through PubMed, Scopus and EMBASE. The entries were extracted from the databases and input into Endnote (ver. 21.1) to filter and remove duplicates. 6,361 articles were then screened based on their title and abstracts. Of these, the full-text of 43 relevant articles was read to determine their eligibility. A total of 6 articles were accepted with 4 articles from the initial search and a further 2 articles identified by reference checks of included articles and manual search terms through Google Scholar shown in Figure 1 [10-15].
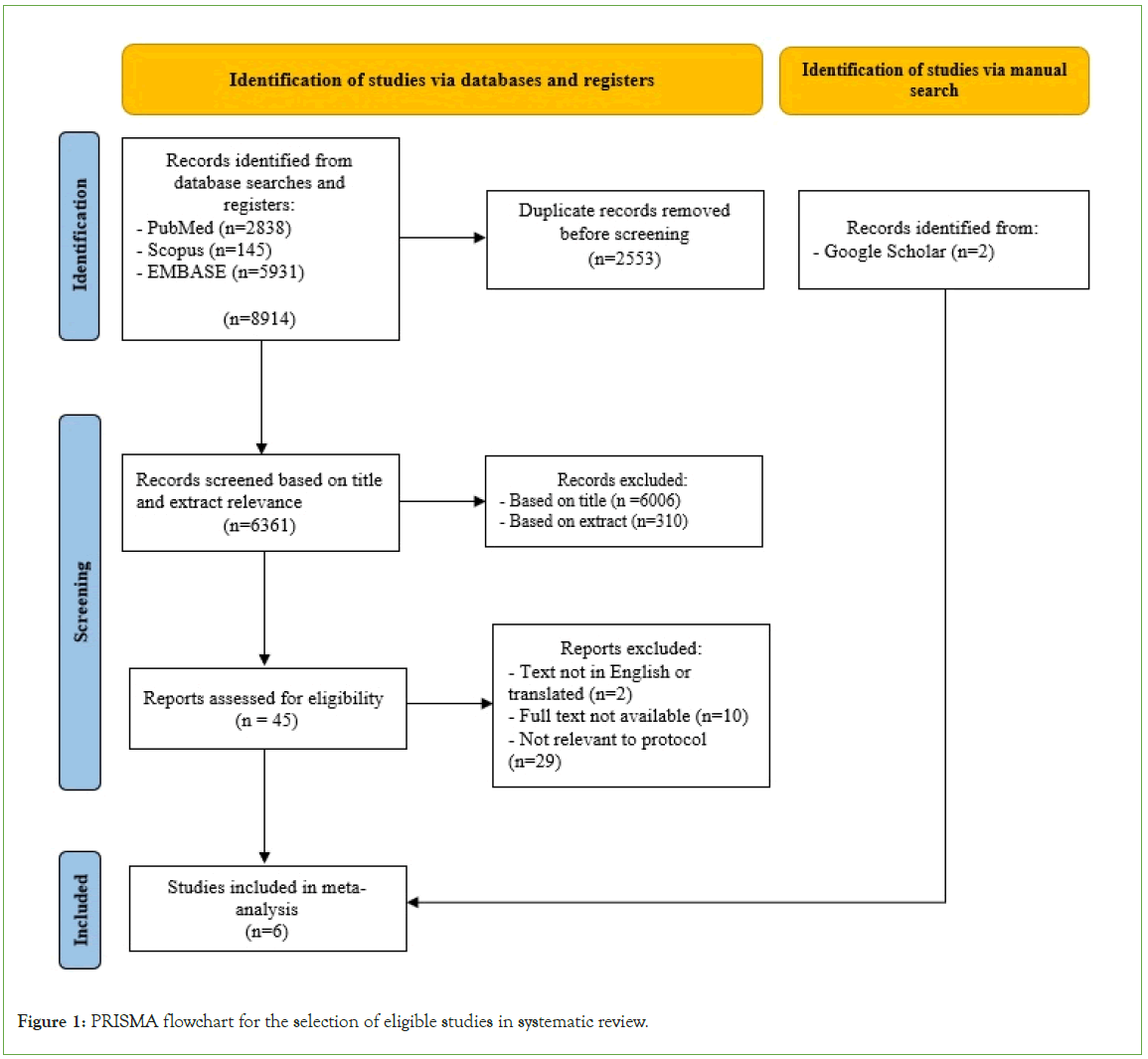
Figure 1: PRISMA flowchart for the selection of eligible studies in systematic review.
Study characteristics
The 6 included studies in this meta-analysis identified the incidence of alloimmunisation in either O uncross matched emergency issue red cells or RhD mismatched red cells in trauma patients. The studies were retrospective and prospective, conducted in six different countries covering a period from 2011 to 2022 [10-15]. Total study sizes were listed, however, eligible data extracted was further limited due to exclusion criteria to better represent sample sizes used in this study. The mean number of units transfused varied as one study assessed immunisation rates following massive transfusion only and the other studies included use of either/or these red cells in their whole hospital population [10,11-15]. Follow-up periods were highlighted if reported (Table 1).
Study quality assessment
The included studies were independently assessed for quality using the STROBE checklist summarised in Table 2. The studies fulfilled most of the criteria included in the STROBE checklist; this demonstrates significantly high quality of the included studies. Outcome data for some studies was reported as partially fulfilled as non-RhD alloimmunisation for our protocol was not part of those studies aim and therefore was not available to assess our secondary outcomes [10-12,14,15]. Follow-up periods for two studies were not specifically reported but the limitations and associated bias for this parameter was discussed in all studies [10-15].
Incidence of RhD alloimmunisation
RhD-negative patients given RhD-positive RBCs: All 6 studies were included in the meta-analysis for the incidence of RhD alloimmunisation of RhD-negative patients given RhD-positive red cells, this is demonstrated as a forest plot in Figure 2a [10-15]. The overall incidence of RhD alloimmunisation was found to be 16.4% (CI 95%, 5.6%-31.3%) with a p-value of<0.001 which makes this finding statistically significant. An I2 value of 91.68% (p<0.001) indicates that the studies included are considerably heterogeneous (Table 3).
| Study | RhD-negative patients transfused with RhD-positive red cells | Patients transfused with RhD-negative red cells | |
|---|---|---|---|
| Alloimmunisation of RhD antibody | Alloimmunisation of non-RhD antibody(s)* | Alloimmunisation of non-RhD antibody(s) | |
| Badami et al. 2022 [10] | 0 | - | 95/1320 |
| Chowdury et al. 2023 [11] | 7 | - | 12/419 |
| Flommersfeld et al. 2018 [12] | 9 | 3** | - |
| Luyten et al. 2023 [13] | 12 | 8** | 39/380 |
| Pandey et al. 2020 [14] | 7 | 1 | - |
| Williams et al. 2019 [15] | 10 | 4** | - |
Note: *Reported alloimmunisation of non-RhD antibodies include: Anti-C, E, Jka and K. **Alloimmunisation of non-RhD antibodies observed on patients with development of RhD antibody.
Table 3: Analysis of immunological response to RhD-negative patients transfused with RhD-positive red blood cells.
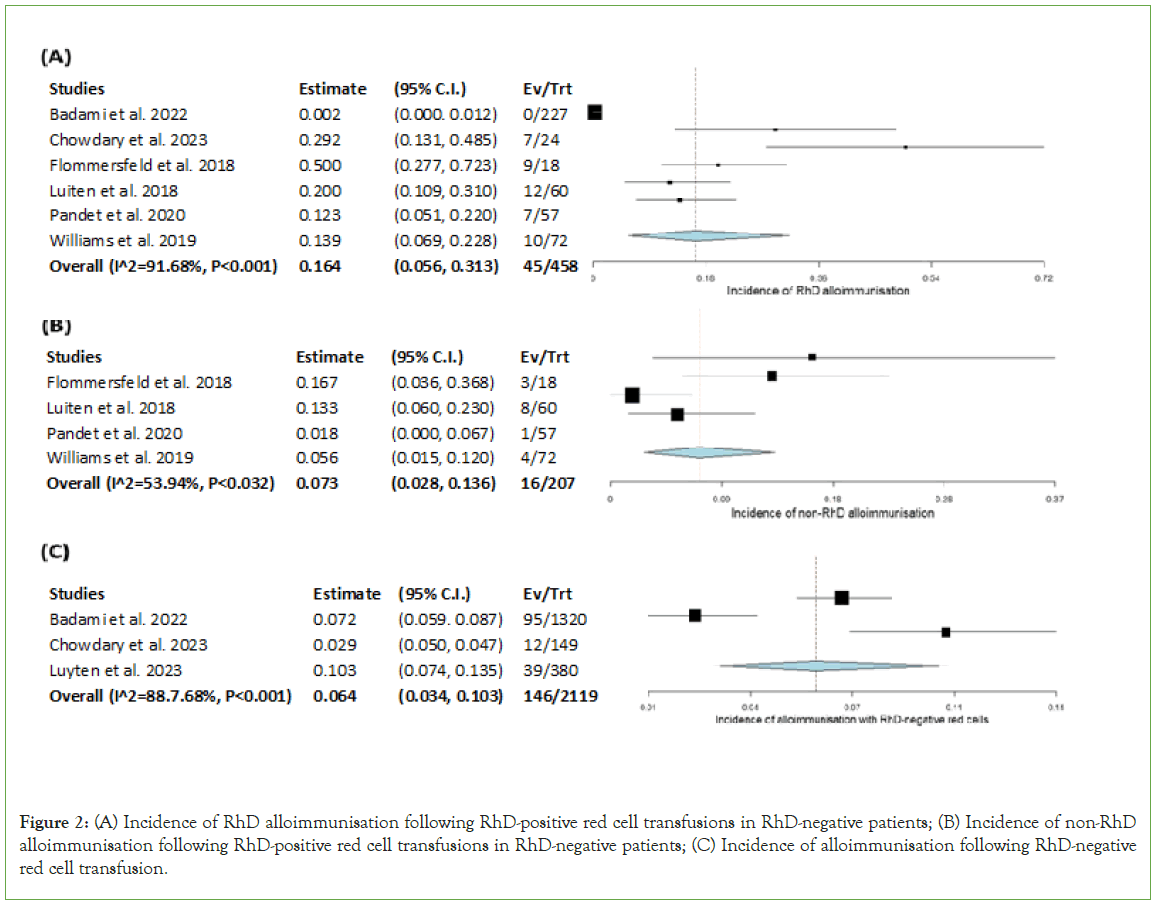
Figure 2: (A) Incidence of RhD alloimmunisation following RhD-positive red cell transfusions in RhD-negative patients; (B) Incidence of non-RhD alloimmunisation following RhD-positive red cell transfusions in RhD-negative patients; (C) Incidence of alloimmunisation following RhD-negative red cell transfusion.
Incidence of non-RhD alloimmunisation
RhD-negative patients given RhD-positive RBCs: 4 studies were included in the meta-analysis for the incidence of non-RhD alloimmunisation of RhD-negative patients given RhD-positive red cells, this is demonstrated as a forest plot in Figure 2b [12-15]. Badami et al. and Chowdury et al. did not release independent statistics for this parameter. The specificity of immunised antibodies includes one-of or a combination of anti-C, E, Jka and K. The overall incidence of non-RhD alloimmunisation of this cohort was found to be 7.3% (CI 95%, 2.8%-13.6%) with a p-value of<0.001 which makes this finding statistically significant. An I2 value of 53.94% (p<0.032) indicates that the studies included are moderately heterogenous.
Incidence of non-RhD alloimmunisation
Following transfusion of RhD-negative RBCs: 3 studies were included in the meta-analysis for the incidence of non-RhD alloimmunisation following the transfusion of RhD-negative red cells, this is demonstrated as a forest plot in Figure 2c [10,11,13]. Flommersfeld et al., Pandey et al. and Williams et al. did not release independent statistics for this parameter. The specificity of immunised antibodies was not particular to this cohort and therefore has not been reported. The overall incidence of non-RhD alloimmunisation for patients given RhD-negative red cells was 6.4% (CI 95%, 3.4%-10.3%) with a p-value of<0.001 which makes this finding statistically significant. An I2 value of 88.7% (p<0.001) indicates that the studies included are considerably heterogenous (Figure 2).
Discussion
RhD alloimmunisation
The clinical implications of the incidence of RhD alloimmunisation in HDFN and HTR are mostly circumvented through the use of protocols established to ensure that RhD-mismatched RBCs are not provided to women of child bearing age and paediatric males. In transfusion practise, this may not always be possible due to limited inventory of O RhD-negative RBCs or group specific RhD-matched RBCs. This is especially the case in trauma or massive transfusions where large volumes of RBCs are required.
Our study shows the incidence of RhD alloimmunisation in RhD-negative patients that receive at least one RhD-positive RBCs to be 16.4% (CI 95%, 5.6%-31.3%) which is concordant with a previously reported incidence of 14%-40% in trauma patients. Variability in RhD alloimmunisation have been reported when assessing different cohorts with a healthy population having a proportionally higher incidence of alloimmunisation (55%-74%) compared to immune- compromised (0%-8) and oncology patients (8%-16%), this is thought to be due to the immune system’s inability to react to foreign bodies as readily as healthy individuals [16].
Studies by Yazer and Seheult suggest that the number of transfusions do not necessarily influence the incidence of RhD alloimmunisation, where the risk of RhD alloimmunisation is mostly attributed to the first RBC given in a multiple RBC transfusion. This is supported by a study on the risk of alloimmunisation in massive transfusions versus regularly transfused individuals that showed negligible difference [17,18]. However, it has been reported that multiple transfusions of an RhD-positive RBC in a short amount of time have shown to increase the risk of RhD alloimmunisation [19].
We must consider that transfusion practises are adjuvant to surgical intervention in the prevention of anaemia in emergencies and that RBCs and other components used are chosen carefully [20]. Laboratory guidelines for the provision of emergency uncross matched RBCs include the collection of the patient’s blood so that group and antibody matching can commence timeframes for testing and having matched allocations of blood components available in 30 minutes. Group specific issuing will decrease our risk of transfusion related incidences and also help to better manage our blood inventory. We never expect large amounts of uncross matched red cells to be issued but it is important to be ready and available.
Non-RhD alloimmunisation
While there are efforts to reduce transfusion reactions to the highly immunogenic ABO and Rh blood groups, it’s important to remember all transfusions carry a risk of alloimmunisation to other non-RhD/ ABO blood groups which may be clinically significant and have equal implications to HDFN and HTRs.
We found the incidence of non-RhD alloimmunisation when RhD-negative patients are given at least one RhD-positive RBCs to be 7.3% (CI 95%, 2.8%-13.6%) with antibody specificity to anti-C, E, Jka and K. The incidence of non-RhD alloimmunisation of all other patients given RhD-positive RBCs was 6.4% (CI 95%, 3.4%-10.3%), however, the antibody specificity of this target cohort was unable to be extrapolated. A study by Sood of multiply transfused patients was shown to have an incidence of alloimmunisation of 4.24% with reference to alloimmunisation rates of previous studies between 5%, 10% and even 20%. A study on the specificity of antibodies on 1710 immunised individuals showed that the most common antibodies developed included anti-E (34%) and anti-K (24.9%) followed by anti-Fya, c and Jka (9%, 8.5% and 7.9% respectively) [21]. Incidence of non-RhD alloimmunisation in RhD-negative patients given RhD-positive RBCs and all other individuals given RhD-negative RBCs are comparable and similar to previously reported findings along with the antibody specificities [22]. Our finding suggests that the incidence of alloimmunisation is the same independent of the RhD blood group and is further proof that when the RhD blood group is accounted for that the risks of transfusion remain the same.
Limitations
A study on RBC alloantibodies shows that antibody specificity had a large role in antibody formation and subsequent detection, while they found 16.8% who developed antibodies did so within 14 days after transfusion, it was shown that testing at intervals (1 month, 3 months, 6 months, and up to 5 years) still detected the development of new antibodies, this study however excluded RhD as it was said by the author to be common practice to match the RhD blood group in transfusions. Despite that fact, the findings can be used to highlight that low incidence of alloimmunisation may be due to alloimmunisation being missed [22]. Patient follow-up in our studies range from an average of 22 days to a 6-month minimum, with exclusion criteria and data loss resulting from failure to follow-up with patients. This limitation is also a caveat of retrospective study which forms a large portion of this analysis.
The eligible sample size of our study cohort is small at 1.6% of the total study sizes included. This is unfortunately a reflection of the population frequency of RhD-negative individuals and from further exclusions to define our sample population (exclusion of LTWB and RhD-positive platelet). Recommendations for the select use of RhD- positive RBCs may help us to collect more data for future analysis.
Conclusion
In conclusion, data collected from recent studies suggest the judicious usage of O RhD-positive blood group as emergency O uncross matched RBCs poses no additional risks compared to O RhD-negative only emergency issue RBCs. Further studies should be done to assess the outcomes of O RhD-positive RBCs given the new protocols and to continue monitoring the efficacy and improvement in blood inventory conservation.
Conflict of Interest
Authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Hirani R, Weinert N, Irving DO. The distribution of ABO RhD blood groups in Australia, based on blood donor and blood sample pathology data. Med J Aust. 2022;216(6): 291-295.
[Crossref] [Google Scholar] [PubMed]
- National Blood Authority Australia. National statement for the emergency use of group O red blood cells. 2022.
- Trudeau JD, Dawe P, Shih AW. Clinical guide to transfusion: Chapter 11 Massive hemorrhage and emergency transfusion. Canadian Blood Ser. 2021;54(1):19-24.
- National Blood Transfusion Committee. Appropriate Specification for Emergency Red Cells. NHS Blood and Transplant. 2020.
- Murphy M, BenAvram D. Association Bulletin#19-02: Recommendations on the Use of Group O Red blood cells. AABB Advanc Transfus Cel Therap Worldwide. 2019.
- Sanderson BJ, Field JD. Transfusion medicine handbook 3rd edition-Chapter 3: Guide to good transfusion practice. New Zealand Blood Ser. 2023;49(3).
- Dean L. Blood groups and red cell antigens. Bethesda. 2015.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009; 339:1-8.
[Crossref] [Google Scholar] [PubMed]
- Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014; 12(12):1495-1499.
- Badami KG, Neal C, Sparrow RL. Red blood cell alloantibodies in the context of critical bleeding and massive transfusion. Blood Transfus. 2023; 21:390-399.
[Crossref] [Google Scholar] [PubMed]
- Chowdury R, Williams BA, Williams S, Casey J. Quality improvement review of O positive blood in emergency transfusion. Transfusion. 2023; 63:1841-1848.
[Crossref] [Google Scholar] [PubMed]
- Flommersfeld S, Mand C, Kuhne CA, Bein G, Ruchholtz S, Sachs UJ. Unmatched Type O RhD+ red bood cells in multiple injured patients. Transfus Med Hemother. 2018; 45:158-161.
[Crossref] [Google Scholar] [PubMed]
- Luyten U, Peeraer S, Pirlet C, Khaouch Y, Streel C, Deneys V. O-negative blood shortage management in a university hospital: Impact of transfusing RhD-positive red blood cells to RhD-negative patients. Transfus Clin Biol. 2022;30(4):402-409.
[Crossref] [Google Scholar] [PubMed]
- Pandey P, Setya D, Singh MK. Anti-D alloimmunization after RhD positive red cell transfusion to selected RhD negative patients. Indian J Hematol Blood Transfus. 2022; 38(3):577-584.
[Crossref] [Google Scholar] [PubMed]
- Williams LA, Sikora J, Aldrees R, Pham HP, Marques MB. Anti-Rh alloimmunization after trauma resuscitation. Transfus Apher Sci. 2019;58(6):102652.
[Crossref] [Google Scholar] [PubMed]
- Ji YL, Luo GP, Fu YS. Incidence of anti-alloimmunization in D-negative individuals receiving D-positive red blood cell transfusion: A systematic review and meta-analysis. Vox Sang. 2022; 117:633-640.
[Crossref] [Google Scholar] [PubMed]
- Yazer MH, Triulzi DJ, Sperry JL, Seheult JN. Rate of RhD-alloimmunization after the transfusion of multiple RhD-positive primary red blood cell-containing products. Transfusion. 2021;61:150-158.
[Crossref] [Google Scholar] [PubMed]
- Seheult JN, Callum J, Delaney M. Rate of D-alloimmunization in trauma does not depend on the number of RhD-positive units transfused: The BEST collaborative study. Transfusion. 2022;62:185-192.
[Crossref] [Google Scholar] [PubMed]
- Zalpuri S, Middelburg RA, Schonewille H. Intensive red blood cell transfusions and risk of alloimmunzation. Transfusion. 2014; 54:278-284.
[Crossref] [Google Scholar] [PubMed]
- Yazer MH, Spinella PC, Sperry J, Triulzi DJ, Leeper C. Timing of RhD-positive red blood cell administration is associated with D-alloimmunization in injured patients. Transfusion. 2023; 63:54-59.
[Crossref] [Google Scholar] [PubMed]
- Sood R, Makroo RN, Riana V, Rosamma NL. Detection of alloimmunization to ensure safer transfusion practice. Asian J Transfus Sci. 2013; 7(2): 135-139.
[Crossref] [Google Scholar] [PubMed]
- Schonewille H, van de Watering LMG, Loomans SEL, Brand A. Red blood cell alloantibodies after transfusion: Factors influencing incidence and specificity. Transfusion. 2006; 46:250-256.
[Crossref] [Google Scholar] [PubMed]
Citation: Leung W, Quiring S, Jackson DE (2024) The Appropriate Use of O RhD Positive Units in RhD Negative Patients: A Systematic Review and Meta-Analysis. J Blood Disord Transfus. 15.574.
Copyright: © 2024 Leung W et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

