Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 16, Issue 10
Tentative Analysis of Biomarkers Associated with Filariasis in Moungo Division, Littoral, Cameroon
Jean Baptiste Hzounda Fokou1*, Syntiche Teudem Biyong1, Francine Kouemo2, Fru Awah Akumwah1, Ambassa Reine1, Véronique Simone Fannang3, Juliette Koube1 and Jules Clement Assob32Department of Health Sciences, University of Buea, Buea, Cameroon
3Department of Biological Sciences, University of Douala, Douala, Cameroon
Received: 17-Sep-2024, Manuscript No. BLM-24-26941; Editor assigned: 19-Sep-2024, Pre QC No. BLM-24-26941 (PQ); Reviewed: 03-Oct-2024, QC No. BLM-24-26941; Revised: 10-Oct-2024, Manuscript No. BLM-24-26941 (R); Published: 17-Oct-2024, DOI: 10.35248/0974-8369.24.16.733
Abstract
Filariasis is a significant cause of morbidity and a public health concern in most tropical countries. These diseases are usually contracted in childhood and most often diagnosed in adulthood. This study aimed to identify biochemical markers associated with filariasis and the endobacteria present in microfilariae. This was a cross-sectional analytical study within a hospital setting. Persons aged from six years and above were included in the study. They were then clustered into asymptomatic and symptomatic. Data was collected from February, 2023 to June, 2023. Blood, urine and skin samples were collected and analysed by microscopy to identify microfilariae. The plasmatic and urinary markers were evaluated by spectrophotometry and protein fraction was monitored by electrophoresis. The data was analysed using software-SPSS 25 with the significant threshold set at 0.005.
A total of 55 persons were included in the study, representing 74.32% of the total population questioned. The sex ratio (F/M) was 2.66. The infection rate was 18.2% Onchocerca volvulus, 54.54% Mansonella perstans, 22.72% Loa loa and 4.54% Wuchereria bancrofti. Protein levels were 35% higher in the asymptomatic with no parasite, 80% in the asymptomatic with detected parasite and 91.67% in the symptomatic. The decrease in albumin fraction and increase in gamma fractions seem to be correlated with the presence of parasites in the blood. These results suggest that protidemia and serum protein are significant indicators in the diagnosis of filariasis.
Keywords
Microfilariae; Biomarkers; Moungo; Cameroon
Introduction
Filariasis is defined by The World Health Organisation (WHO) as a parasitic disease caused by the presence of filarial nematodes and their embryos in the body, microfilariae, which for a long time was classified as a “neglected” tropical disease [1,2]. Filariasis is a group of diseases that vary according to the parasite species, the clinical manifestations and the nature of the immune response induced [3-6]. Their socioeconomic impact makes them a major cause of morbidity in most tropical countries [7-10].
It is estimated that over 200 million people worldwide are affected by filariasis out of approximately one billion people at risk [11]. Current global estimates suggest that by 2019, 863 million people in 47 countries will be at risk of lymphatic filariasis, with 30% of these people living in the African region [9,12]. The number of persons infected with onchocerciasis is estimated at around 18 million, with over 99% living in intertropical Africa [12]. In Central Africa, 15 to 20 million people are infected with schistosomiasis and more than 50 million people are infected with dracunculiasis Medina filaria [13]. Several outbreaks have been described in Cameroon, notably in the town of Nkongsamba since 1937 [5]. According to the distribution of endemicity of neglected tropical diseases in Cameroon in 2017, 152 districts are endemic for lymphatic filariasis, 113 districts are endemic for onchocerciasis and the prevalence of co-endemicity for schistosomiasis ranges from 0.2% to 6.2% [14].
Over the past few years, several therapeutic strategies have been developed, yet mass treatment is not recommended in regions where there is co-endemicity with loiasis, a type of filariasis that is just as challenging as onchocerciasis and volvulus [1,15,16]. Low-specific antigenic tests and loa-scop have been developed for the diagnosis of filariasis, yet remain inaccessible in most endemic regions. The selection of populations infected with onchocerciasis or loiasis, respectively, means that they can be treated with ivermectin (Mectizan®), without the risk of developing a serious adverse reaction requiring palliative care. However, those who are not eligible for this mass treatment are given an individual long-lasting treatment [17-19]. Moreover, the risk of resistant microfilariae is a major concern and the ineffectiveness of available antiparasitic drugs on adult worms represents a significant impairment to breaking the chain of transmission [19].
Filariasis is typically contracted during early childhood, with the majority of cases presenting with no symptoms. However, acute and chronic symptoms may subsequently emerge in adulthood [9]. Therefore, it is of the utmost importance to identify specific markers for filariasis, which could help in the development of an early diagnosis method, the creation of a novel treatment, or even a vaccine. To achieve this, it is first necessary to conduct a preliminary mapping of all the metabolites that are directly or indirectly involved in the pathogenicity of the different forms of filariasis. The objective of this study is to identify biochemical markers associated with filariasis and the metabolome of the endobacteria present in the microfilariae identified.
Materials and Methods
Type of study
A cross-sectional and analytical study was conducted.
Study site: Specimens were collected in public and private hospitals in the Moungo Division, with particular focus on the Nkongsamba Regional Hospital (NRH), the Ekol-Mbeng Integrated Health Centre (CSI-Ekol-mbeng), the Manengole Catholic Medical Health Centre (CSMCM) and the Bon Samaritain Private Health Centre in Njombe.
The samples were analysed at the Laboratory of Pharmacology, Faculty of Medicine and Pharmaceutical Sciences at the University of Douala, at Douala General Hospital and in the bacteriology laboratory at the Centre Pasteur du Cameroun in Yaoundé.
The study population
The study population consisted of patients of any sex aged at least six years.
Inclusion criteria: Any person of any sex, aged at least 6 years, who consented and who visited the hospitals concerned during the study period was included in the study.
Non-inclusion criteria: The following individuals were not selected for this study: Hospital patients and children under the age of six.
Exclusion criteria: The study excluded three categories of participants: Pregnant women; volunteers who had withdrawn their informed consent; and any individual with HIV, AIDS, or cancer, or another serious disease requiring specific monitoring, due to the potential for bias in our results caused by other diseases present.
Sampling
The sampling was non-exhaustive and consecutive and the sample size was determined by the following Cochran formula.
N=(T2 × p(1-p)/m2)
N: The size of the sample
T: The standard deviation for the normal distribution, with a 95% confidence interval of 1.96
p: The prevalence of filariasis
m: The margin of error at 10%
Standard value for the margin of error: 0.1
The prevalence of filariasis in Cameroon is estimated to be 17% [20].
Consequently, N can be calculated as follows:
N = (1.96² × 0.17(1-0.17)/0.1²=54.20)
Consequently, the minimum sample size was set at 54 participants.
Technical procedures
Patient recruitment: Some patients were contacted by telephone, while others were approached and recruited in the consultation and sampling units of the laboratory departments of the hospitals involved in the study. Prior to their participation, all patients were given a detailed verbal presentation of the aim of the study. For those who agreed to take part, an informed consent form was provided for their signature, along with a data collection form and they were assisted when needed in filing the form.
Data collection: Data were collected using an individual survey form (adults and minors). Data were collected from interviews with patients and/or parents or guardians who had read, understood and consented to participate in the study after the purpose of the study had been explained.
The following variables were collected: Socio-demographic data (age, sex, occupation, place and duration of residence in the region); Epidemiological and clinical data; Biological data
Pre-analytical steps
Blood sampling: Venipuncture was done at the elbow or back of the hand during the daytime (12:00-16:00) in a plain tube for the determination of plasma markers (procalcitonin, total proteins, electrophoresis). An EDTA tube was used for the detection of Loa loa and Mansonella perstans microfilariae (thick film, leuco-concentration) and a simple prick at the fingertip was employed to collect the first drop of blood (fresh state) when possible. The nightly sampling period (22:00-01:00) entailed a simple venipuncture procedure at the elbow or back of the hand, conducted in an Ethylenediaminetetraacetic Acid (EDTA) tube, with the objective of testing for Wuchereria brancrofti.
The sampling procedure employed was as follows
Once the required equipment has been assembled and the patient’s previously identified tubes and slides have been located, a tourniquet is applied approximately 7cm to 10 cm above the venipuncture site. The patient is then asked to point the bandage so that the vein is turgid, after which the vein is palpated and the puncture site is cleaned using a cotton wool pad soaked in povidone iodine 10%, which is then disposed of in the bin. Subsequently, the needle and pump body should be assembled and the needle inserted into the vein, with the bevel facing upwards. The collection tube is then inserted into the vacutainer and filled to the mark. Subsequently, the tube was removed from the holder and a dry swab was placed over the puncture site. Subsequently, the needle was also removed and a dry pad was applied to the lesion area in order to facilitate haemostasis. Once this had been achieved, the pad was removed and a dry dressing was applied.
The needle was then disconnected and discarded into the provided safety box. The sample was later secured and coded, the participant’s little finger was massaged and the fingertip was disinfected. A brief puncture was made and the first drop was collected on a slide that had been previously identified and coded. Next, a dry cotton pad was placed over the puncture site to prevent further bleeding. A slide cover was placed over the freshly collected drop of blood and observed under an optical microscope as soon as possible.
Skin sampling: Skin sampling was conducted at the Ekol-Mbeng Centre for the Study of Infectious Diseases (CSI), also known as the “Centre des Grands Endemiques”, which specialises in filarial infections and at the Nkongsamba Regional Hospital.
Following disinfection of the sampling area, a sterile needle was employed to pinch a flap of skin. This was then incised with a scalpel blade and placed on a slide with a drop of physiological water between the slide and the slide covers. The flap was observed twice under a light microscope, with an interval of 60 minutes between observations.
Urine collection: Following the labelling and coding of the urine jars, the participants were provided with instructions on how to collect the urine. The urine was collected by the participants themselves in the toilets of the hospitals.
Transport and storage: Following sampling, the samples collected were transported to the parasitology and biochemistry laboratories. Samples from dry tubes were centrifuged at 3500 rpm for five (05) minutes and the sera obtained were aliquoted and stored at -20°C until the day of analysis. Serums in EDTA tubes were stored at room temperature for three weeks before analysis. Finally, the urine samples were analysed without delay in the biochemistry laboratories of the various hospitals where the samples were taken.
Analytical stage
The microscopic samples were analysed as follows
Microscopy: Identification, quantification and isolation of microfilariae.
Identification and quantification of microfilariae-Giemsa staining of the thick film (GE) and blood smear.
Principle: The Giemsa solution is a mixture of eosin and methylene blue (azure). Methylene blue colours the parasite cytoplasm in blue. The ideal pH for highlighting the granulations associated with the parasites and identifying the different species is 7.2. Several methods are used to stain EG/blood smears. For this study, we opted for the slow method, which involves preparing a 3% Giemsa dye solution for staining times of 45-60 minutes.
Procedure: After spreading the drop of blood on a slide and drawing the smear, allow to air dry, then fix the smear by dipping it for two seconds in a jar containing methanol, then allow to air dry. Place the slides on the staining rack making sure that they do not touch each other and then carefully pour the staining solution over each slide until the spreads are covered. Allow to stain for 45 minutes, then carefully remove the remaining stain and gently rinse the slides in a jar of water buffered at pH 7.2. Then allow the slides to air dry and discard the remaining Giemsa solution. The prepared slides were read at objective 10 and with a drop of immersion oil at objective 100.
Observations: Presence of a head, tail, sheath (optional), one or two internal bodies and somatic nuclei (purple).
Isolation of microfilariae by haemolysis
Principle: This consists of destroying the red blood cells contained in a whole blood sample to better identify the microfilariae.
Reagents: 2% saponin solution (pure saponin (S 7900 Sigma® 0.2 g) 0.9% NaCl solution.
In a Falcons-type centrifuge tube of at least 20 ml, place 5 ml of blood taken on anticoagulant (or 1 volume). Add 10 ml of NaCl 0.9% (or two volumes) and turn to mix well. Then add the saponin solution dropwise (250 μL). Close and invert the tube several times to ensure complete haemolysis, particularly at the end cone of the tube, as the liquid should be completely translucent. Centrifuge at 500 g (approximately 1,500 rpm) for 10 minutes and discard the supernatant, without inverting the tube completely, by wiping the sides with cotton wool or sponge paper mounted on forceps so that the haemolysis liquid does not dilute the pellet when it is turned upside down. Add one drop of NaCl 0.9%. Homogenise the pellet well, then take 20 μL for quantification by the calibrated GE and keep the rest for bacteriological and spectrometric analysis [21].
Evaluation of plasma and urinary biochemical markers
Determination of Procalcitonin (PCT) was carried out by following procedure
Principle: Finecare PCT rapid quantitative tests are based on fluorescence immunoassay technology for the quantitative determination of PCT concentrations in whole blood, serum or plasma. Add the sample to the intended sample dilution solution and mix; then add the mixed sample to the sample hole of the corresponding test cartridge, where it will combine with the fluorescently labelled PCT antibody in the labelling buffer to form reaction complexes. As the compounds migrate onto the nitrocellulose matrix by capillary action, they are captured by the PCT antibodies that have been immobilised on the detection line of the nitrocellulose membrane. The more PCT is present in the sample, the more the complexes accumulate on the detection line. The intensity of the PCT antibody detection signal is proportional to the concentration of PCT in the specimen. The procedure strictly follows the manufacturer’s instructions.
Evaluation of the urine strip with the URS-11 kit
The literature review informs us that in certain patients with a high parasite load, microfilariae may be found in the urine and their presence in the urinary tract could not be the cause of certain urinary tract infections in the patient [3]. We evaluated three (03) of the eleven parameters available in the URS-11 kit. The procedure was as describe by the manufacturer.
Determination of total protein using the Mindray BA-80 semi-automatic spectrophotometer
Principle: The Mindray BA-88A semi-automatic analyser is a spectrophotometer used for the quantitative determination of many biochemical tests. Its principle is based on spectrophotometry by measuring the absorbance of a solution at a given wavelength according to Beer Lambert’s law, which stipulates that the absorbance of a solution is proportional to the concentration of the element it contains.
Serum protein electrophoresis on the MINICAP® instrument Serum protein electrophoresis is a technique used to separate proteins into fractions of different mobility, obtaining their relative percentages.
Principle: The MINICAP system uses the principle of capillary electrophoresis in free solution, allowing the separation of charged molecules according to their electrophoretic mobility in a given pH buffer and depending on the pH of the electrolyte, a greater or lesser electroosmotic flow. The Miniature Capillary Electrophoresis system comprises 2 capillaries in parallel, allowing 2 simultaneous analyses. With this system, the sample (diluted in the analysis buffer) is injected into the capillaries by suction at the anode. Separation is then achieved by applying a potential difference of several thousand volts across each capillary and the different fractions migrate according to their electrophoretic mobilities.
The direct detection of proteins is carried out by absorption spectrophotometry spectrophotometry at 200 nm on the cathode side. The capillaries are then washed with a washing solution, followed by the analysis buffer. Interpretation of protein electrophoresis is made by relating each fraction to the level of total serum protein (g/l). With the buffer used at basic pH (9.9), the order of migration of proteins with their serum constituents is as follows gamma globulins (7.5 g/L to 16 g/L); beta-2 globulins (containing transferrin, fibrinogen transferrin, fibrinogen and the C3 fraction of complement); beta-1 globulins; alpha-2 globulins (6 g/L to 10 g/L) consisting in particular of alpha-2-macroglobulin, haptoglobin; alpha-1 globulins (1.5 g/L to 4 g/L) including alpha-1 -antitrypsin, alpha-1 -anti chymotrypsin and orosomucoid; and albumin (33 g/L to 50 g/L).
Statistical analysis
Microsoft Office Excel 2016 was used to enter our data; SPSS 25 for Windows (Statistical Package for Social Sciences) was used to analyse the data; Quantitative variables were represented as histograms and categorical variables as pie charts;
The Chi-square test was used to compare discontinuous quantitative variables (frequencies, proportions); and the Student t-test for continuous quantitative variables (mean and standard deviation); the difference was considered statistically significant for a value of p<0.05.
Results
Sociodemographic characteristics and clinical history of the population
Sociodemographic characteristics of the population: Our population was predominated by female (73%), they represented around 1/3 of our study population (sex ratio F/H of 2.66). The mean age of the population was 35 ± 2 years with extremes of 6 and 71 years. Distribution of the population according to collection sites, location, lifestyle, knowledge of the disease and sampling periods.
29.09% of our population was sampled at the HRN, 20% at the Ekole Mbeng CSI, 23.63% at the CSCM and 27.27% at the private health centres of Njombé-Penja. The majority came from Nkongsamba, Manengolè, Njombé and Mboébo, with a higher distribution in Nkongsamba. Most (54%) had been born and raised in these localities and 24% had been living there for more than 10 years. 51% of our population were working in the rural area and 31% were still scholars. Nearly 80% of the population had knowledge of filariasis and 25% did not sleep under an impregnated mosquito net as recommended. 52.72% agreed to have their samples taken during the two sampling periods and the rest of the participants agreed to just one sampling period (Table 1).
| Variables | Characters | Number (n=55) | Percentage (%) |
|---|---|---|---|
| Sampling Sites | HRN | 16 | 29,09 |
| CSI d’Ekol-Mbeng | 11 | 20 | |
| CSMCM | 13 | 23.63 | |
| CSP. N-P | 15 | 27.27 | |
| Localisations | Nkongsamba | 14 | 25.45 |
| Manengole | 11 | 20 | |
| Njombe | 11 | 20 | |
| Pendja | 2 | 3.64 | |
| Mbanga | 2 | 3.64 | |
| Mboebo | 9 | 16.36 | |
| Douala-Bonaberie | 2 | 3.64 | |
| Loum | 1 | 1.81 | |
| Mandjo | 2 | 3.64 | |
| Bafoussam | 1 | 1.81 | |
| Note:HRN: Nkongsamba Regional Hospital; CSI: Centre de Sante Integre; Centre de Sante Medicalise Catholique de Manengole; CSP.N-P: Njombe-Pendja Private Health Centres. | |||
Table 1: Various sampling sites and localisations.
Clinical history of the population
Distribution of the population according to the administration of mass treatment: 9.09% of the population had never taken the mass treatment offered free of charge by the government, due to their fear of developing serious and disfiguring side effects. As the Mectizan® distribution campaign is carried out once a year; we noted during this study that people who had taken the treatment 1 year ago were statistically more numerous than others (Figure 1).
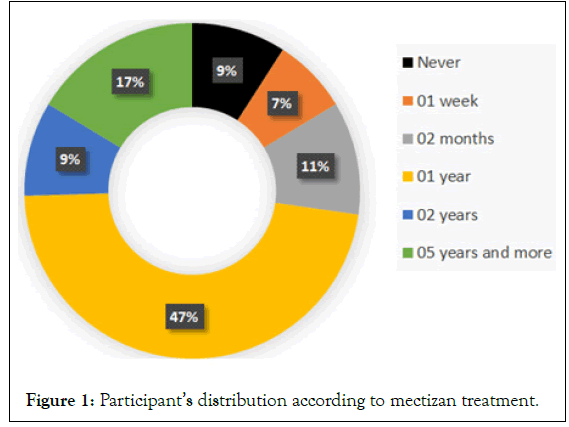
Figure 1: Participant’s distribution according to mectizan treatment.
Distribution of the population according to the presence or absence of symptoms: Figure 2 shows that of the 55 participants selected for our study, 49% had no symptoms at the time of sampling, while the other 51% had some symptoms of filariasis, in particular elephantiasis of the lower limbs, subcutaneous nodules, itching on the hands and buttocks, etc.
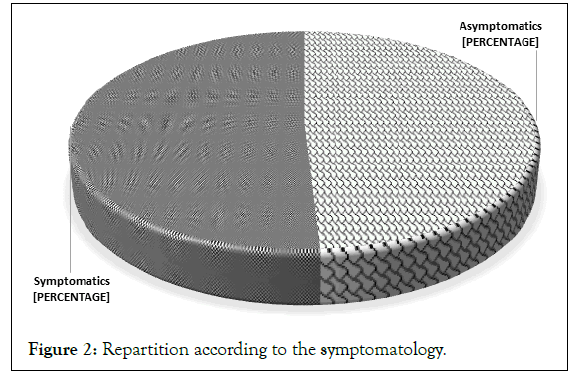
Figure 2: Repartition according to the symptomatology.
Identification of microfilariae: Breakdown of participants into groups of Symptomatic (S) and Asymptomatic (AS)
Out of 27 asymptomatic participants, 18.18% were positive cases, i.e. infected but asymptomatic patients and out of 28 symptomatic participants, 21.81% were positive cases, i.e. infected and symptomatic patients (Figure 3).
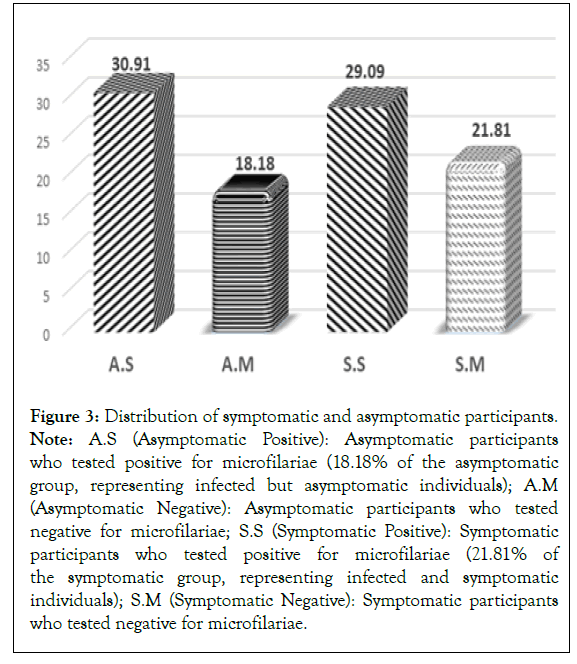
Figure 3: Distribution of symptomatic and asymptomatic participants. Note: A.S (Asymptomatic Positive): Asymptomatic participants who tested positive for microfilariae (18.18% of the asymptomatic group, representing infected but asymptomatic individuals); A.M (Asymptomatic Negative): Asymptomatic participants who tested negative for microfilariae; S.S (Symptomatic Positive): Symptomatic participants who tested positive for microfilariae (21.81% of the symptomatic group, representing infected and symptomatic individuals); S.M (Symptomatic Negative): Symptomatic participants who tested negative for microfilariae.
Identification of microfilariae and distribution according to type of sample and infection: A total of 22 samples tested positive for microfilariae, present in mono infection and/or co-infection with another type of microfilaria. We recorded 01 co-infection out of 10 in asymptomatic patients and 5 co-infections out of 12 in symptomatic patients (Tables 2 and 3).
| Occupations | Farmer | 17 | 30.9 |
| Students | 28 | 50.91 | |
| Others | 10 | 18,18 | |
| Life style | Alcohol | 42 | 76.36 |
| Smoker | 6 | 10.9 | |
| Knowledge | Yes | 44 | 80 |
| No | 11 | 20 | |
| Impregnated mosquito nets | Yes | 41 | 74.54 |
| No | 14 | 25.45 | |
| Sampling period | Diurne | 19 | 34.55 |
| Nocturne | 7 | 12.72 | |
| Diurne+Nocturne | 29 | 52.72 | |
| Note : HRN: Nkongsamba Regional Hospital; CSI: Centre De Sante Integre; Centre De Sante Medicalise Catholique De Manengole; CSP.N-P: Njombe-Pendja Private Health Centres. | |||
Table 2: Patient distribution and lifestyle.
| Parameter | Variables | Number (n=22) | Percentage (%) |
|---|---|---|---|
| Positive sample | BCE | 4 | 7,27 |
| Blood | 18 | 32,72 | |
| Urine | 0 | 0 | |
| Type of infection | Co-infections | 6 | 27,27 |
| Mono infections | 16 | 72,72 | |
| Mono infections | S.M | 7 | 58,33 |
| A.M | 9 | 90 | |
| Co-infections | S.M | 5 | 41,67 |
| A.M | 1 | 10 | |
| Note: A.M: Asymptomatic patients, S.M: Symptomatic patients, BCE: Bloodless skin biopsy. | |||
Table 3: Distribution of various occupational groups and lifestyle habits.
Microfilariae identified and parasitic loads: Onchocerca volvulus was identified in 4 patients (18.2%), Wuchereria brancrofti in a single patient (4.54%), Loa loa in 5 patients (22.72%) and Mansonella perstans in 12 patients (54.54%). Parasitic loads were a minimum of 1 mf/μL of blood and a maximum of 4 mf/20μL of blood (Table 4).
| Microfilaires | Cases | Parasitic loads (Mf/Ml) |
|---|---|---|
| Onchocerca volvulus | 4 | (1-2) |
| Wuchereria brancrofti | 1 | 1 |
| Loa loa | 5 | (1-2) |
| Mansonella perstans | 12 | (2-4) |
Table 4: Microfilariae identified and parasite loads.
Results of biochemical parameters of participants
To avoid bias in the results of biochemical parameters, we subsequently excluded all symptomatic healthy patients, as the fact of presenting symptoms without any microfilaria found could be due to pathology (Figure 4).
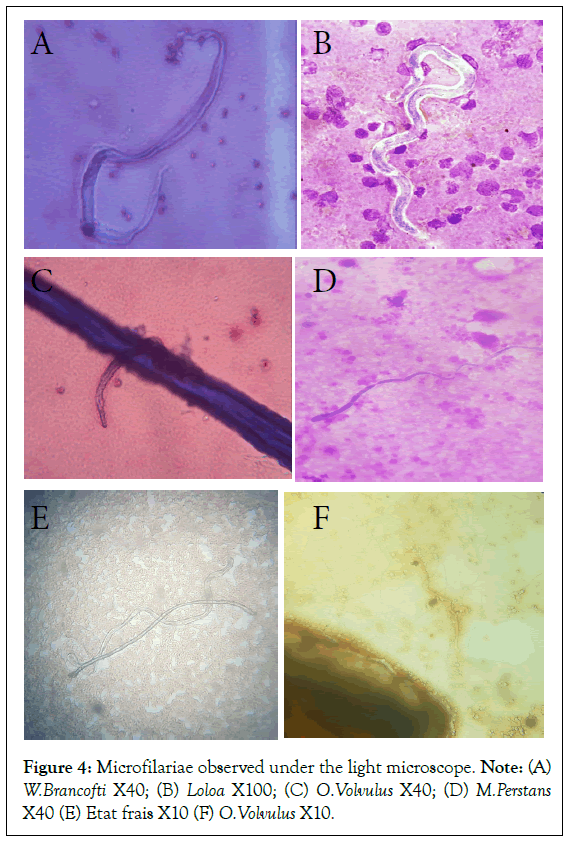
Figure 4: Microfilariae observed under the light microscope. Note: (A) W.Brancofti X40; (B) Loloa X100; (C) O.Volvulus X40; (D) M.Perstans X40 (E) Etat frais X10 (F) O.Volvulus X10.
Urine dipstick and procalcitonin results: The results of the urinary biochemistry are presented (Table 5). From the table, it came out that, 15.38% had proteinuria while 2.56% of patients had leukocytes in their urine. There were no positive nitrites and no procalcitonin was detected in the urine of the participants.
| Parameters | Variables | Number (N=39) | Percentage (%) |
|---|---|---|---|
| Uinary proteins | Positive | 6 | 15,38 |
| Negative | 33 | 84,62 | |
| Nitrites | Positive | 0 | 0 |
| Negative | 39 | 100 | |
| Leucocytes | Positive | 1 | 2,56 |
| Negative | 38 | 97,44 | |
| Procalcitonin | Positive | 0 | 0 |
| Negative | 39 | 100 |
Table 5: Urine dipstick and procalcitonin results.
Total serum protein: 65% of asymptomatic patients had normal serum proteins and 35% abnormal serum proteins, with a minimum of 48.05 g/L and a maximum of 91.70 g/L. In asymptomatic infected patients, we obtained 80% elevated protidemia compared. 8.33% of symptomatic patients had normal protidemia compared to 91.67% with elevated protidemia. They had a minimum of 73.90 g/L, a maximum of 98.20 g/L and a mean of 88.7408 ± 2.04 g/L; the standard deviation was 7.06 g/L and the median was 86.80 g/L. The characteristics of these different groups are summarised in the figure below.
Results of serum protein electrophoresis: All the albumin fractions showed a relative decrease in all our patients, with a minimum of 44.90% (for asymptomatic infected) and a maximum of 54.60% (for asymptomatic uninfected). All the symptomatic and infected patients had abnormal α-1 globulin fractions. 50% had a decrease in the α-2 globulin fraction. β-1 and β-2 globulin fractions were normal for all the patients. All gamma globulin fractions were higher in all patients, with a mean of 26.25% ± 2.49% and extremes of 21.7 and 37%.
Discussion
This study aimed to determine the biochemical markers associated with filariasis in the hospital at the Moungo Division. The study population was highly dominated by females. The sex ratio (F/M) was 2.66. In most articles in the literature, the population is dominated by males [22-24]. 35% of the population have microfilaria and 65% were not infected. These data are higher than that of Wanji et al., on top of these changes; these authors did not find Loa loa., in their studies sites in Cameroon [25]. Moreover, these results are higher than that of Nana-Djeunga et al., who drew the map Loa loa throughout Cameroon and found that the prevalence of detection of microfilaria was between 0% and 1% in the same study area [26]. Out of Cameroon, these data are lower compared to that of Lamontellerie et al., found in Burkina Faso, 85.4% of the same filariasis [27]. More specifically, 18% of our study population was infected but still asymptomatic. This goes in the same line with the report from Nana-Djeunga et al., [26]. These authors found that even though the filarial load was low in many regions of Cameroon, the global circulating filarial antigen was at around 20% of his study population. 21.81% were symptomatic and had microfilaria in their blood. From this positive population, Onchocerca (18.2%), Mansonella perstans (54%), Loa loa (22.72%) and Loa loa (4.54%) were detected. This does not correspond to the report from Wandji et al., [25] who found that μwas the most present in localities of the East Region of Cameroon followed by Mansonella perstans. In Burkina Faso, Lamontellerie et al., found Onchocerca (32,4%), Loa loa (13,2%) and Mansonella perstans (33,8%). Moreover, Kuété et al., found that O.volvulus (26.38%), Loa loa (5,53%) and Mansonella perstans (4,7%) were found in Ntui in Cameroon [27]. These differences observed in different regions and periods in Cameroon might be due to the massive drug administration done by the Cameroon state against lymphatic filariasis [28-32].
Taking into consideration the time after the massive drug administration campaign, 33.33% were asymptomatic and the same size were symptomatic two months after the campaign [33-36]. 15.38% were still symptomatic and infected one year after the campaign. After two years after the campaign, 40% of the patients were symptomatic and infected. These findings are different from those of Camara et al., at Sikasso region, Mali [28].
These authors found those 9 months after the massive drug administration campaign, 2.33% of the population was still symptomatic and infected. This difference might be associated with the re-introduction of the disease from the neighbouring area where the disease is present and mainly by the crisis in North West and South West regions of Cameroon that has led to a massive displacement of the population [26].
No microfilaria was detected in the urine of the patients. This is in accordance with the literature [30,31]. These research groups found out that the search for microfilaria in urine is important for diagnosis but the detection remains difficult and is mostly associated with another clinical feature. In addition, the results obtained from the urinary stick do not correlate significantly with the presence of microfilaria in the blood. Therefore, the presence of leukocytes in urine was probably associated with urinary infection by pathogens other than lymphatic filaria.
Procalcitonin is a valuable tool to assess the inflammatory response of the organism due to the infection by microorganisms. It is more specific with bacterial infections and slightly interesting for the diagnosis of parasitic diseases [32-33]. The level of procalcitonin does not depict the presence of any infection and concerning the abundant literature on that particular test, the results confirm that our participants do not suffer from bacteraemia or fungemia [33-37].
The lymphatic system is involved in several functions, including the regulation of blood volume through the transport and return of plasma proteins that have escaped from the bloodstream into the blood and this function could be altered by the presence of microfilariae in the lymphatic system [37].
Therefore, monitoring proteinemia and proteinuria can be of help in the early diagnosis of filariasis [38]. In this study, 91.67% of symptomatic infected and non-infected patients have abnormal proteinemia. This falls in the same line with the observation by Nana-Djeunga et al., who found that the biochemical parameter goes down slowly after clearance of the filaria in the body [26]. We also observed that the proteinemia was 80% higher in asymptomatic infected patients in contrast; the asymptomatic non-infected patients had their protidemia 35% higher than the normal range. We did not find in the literature authors that analysed the protidemia of the uninfected population from that particular rural area.
On the other hand, the albumin fraction was globally low in all study populations; it was lower in infected asymptomatic and symptomatic patients. Regarding the other protein fraction, we found that gamma globulin level was high in all infected patients. This result goes in the same line with that of Moreau et al., in France who found that Wuchereria Brancrofti, Loa loa and Mansonella perstans infection are correlated with an elevation of immunoglobulin G [39].
Conclusion
This study reports the presence of four parasitic species-Onchocerca volvulus, Loa loa, Wuchereria bancrofti and Mansonella perstans in the Moungo division of the Littoral region, indicating a significant public health concern. The research reveals that the detection of microfilariae in the blood shows a poor correlation with overall protein levels (proteinemia), suggesting that traditional protein markers may not effectively reflect parasitic infection. In contrast, the presence of microfilariae is positively correlated with albumin and gamma globulin levels, indicating that these proteins may play a role in the immune response to these parasites. This finding enhances our understanding of the relationship between parasitic infections and specific blood protein levels, potentially informing diagnostic and treatment strategies.
Acknowledgements
The authors are grateful to all participants for their cooperation. The authors are grateful to all the heads of health care centres in the Moungo division for their support during data collection. Also, the authors would like to thank the Director of the gyneco-obstetric Hospital of Douala for the support during data analysis.
References
- French Association of Teachers of Parasitology and Mycology. Human filariasis. The French Virtual Medical University. 2014:1-22.
- World Health Organization (WHO). Manual for National Programs: Global Program to Eliminate Lymphatic Filariasis. 2018:10-22.
- HAS. Updating the Nomenclature of Medical Biology Procedures for the Diagnosis and Monitoring of Filariasis. High Health Authority. 2018;1-85.
- Moyou RS, Ouambe MA, Fon E BJ. Survey on lymphatic filariasis in seven villages of the district. Med Trop. 2003;63:583-586.
- Pasteur Center Laboratory. Lymphatic filariasis in Cameroon: State of knowledge. Bull link doc. 1987;32(4).
- Valérie D. Onchocerciasis: Definition, Causes and Prevention. Health/disease report.
- Adrian H. Elimination of onchocerciasis and lymphatic filariasis. 2015;12:6-14.
- Tongue R. A new strategy to eliminate filariasis in Cameroon. Mediaterre.
- Nizeyimana JB, Mabée JM, Zongo JM. Study of the conditions of clinical screening and the psycho-socioeconomic effects of people with elephantiasis of the legs. Case of the district of guidiguis case of the far north region of Cameroon from 2017-2020.
- Kamgno J, Boussinesq M. Hyperendemicity of loiasis in the Tikar plain, shrub savannah region of Cameroon. Bull Soc Pathol Exot. 2001;94(4):342-346.
- Collomb H, Miletto G. Lymphatic filariasis. Rev Prat. 1956;6(8):839-850.
[PubMed]
- Laurent Y, Christian L. Thirty years of fighting onchocerciasis in West Africa. IRD Editions. 2003.
[Crossref]
- France in Cameroon. Understanding Filariasis in Loa loa in Congo Brazzaville and Cameroon: Launch of Preparations for the European Morlo Project. WHO. Annual technical report. 201:1-48.
- Annual Technical Report on Activities to Combat Neglected Tropical Diseases. 2017. 1-48.
- Kamgno J, Tsague Dongmo L, Gardon Jacques, Boussinesq Michel. Additional Data on the Loa loa Endemic in the Centre Province of Cameroon. OCEAC Liaison and Documentation Bulletin. 34(4):23-27.
- CDC. Onchocerciasis-Resources for Health Professionals. 2021.
- Kamgno J, Nana-Djeunga HC, Pion SD, Chesnais CB, Klion AD, Mackenzie CD, et al. Operationalization of the test and not treat strategy to accelerate the elimination of onchocerciasis and lymphatic filariasis in Central Africa. 2018;10:49–53.
- Nana-Djeunga HC, Domche A, Niamsi-Emalio Y, Moungui HC, Walker M, Basanez MG, et al. Situation analysis of onchocerciasis in Cameroon: A protocol for systematic review of epidemiological studies and impact of disease control interventions. Syst Rev. 2020;9(1):1-6.
[PubMed]
- Lepori A sophie. Onchocerciasis: Current data and new therapeutic horizon: The role of doxycycline in the treatment of onchocerciasis. 2018;107.
- Nana-djeunga HC, Tchatchueng-mbougua JB, Bopda J. Mapping of bancroftian filariasis in Cameroonâ?¯: Prospects for Elimination. PLoS Negl Trop Dis. 2015;9(9):1-19.
[Crossref]
- Duong TH, Richard-Lenoble D. Diagnosis of blood parasites. Rev Francoph des Lab. 2008;38(399):29-39.
- Et S, Recherche DELA. Sciences UDES, Technique, University of Science, Technology and Engineering of Bamako (Mali).
- Amaechi CB, haeri CC. Lymphatic filariasis: knowledge, attitude and practices among inhabitants of an irrigation project community, North Central Nigeria. Parasitol Res. 2016:709-713.
[Crossref]
- Manock RS, Nsue VU, Olutu A, Mpina M, Nyakurungu E, Raso J, et al.The impact of Loa loa microfilaraemia on research subject retention during a whole sporozoite malaria vaccine trial in Equatorial Guinea. Trans R Soc Trop Med Hyg. 2022;116(8):745-749.
- Wanji S, Esum ME, Njouendou AJ, Mbeng A, Ndongmo PWC, Abong RA, et al. Mapping of lymphatic filariasis in loiasis areas: A new strategy shows no evidence for Wuchereria bancrofti endemicity in Cameroon. PLoS Negl Trop Dis. 2019;13(3):1-15.
- Nana-djeunga HC, Tchatchueng-mbougua JB, Bopda J. Mapping of bancroftian filariasis in Cameroon: Prospects for elimination. PLoS Negl Trop Dis. 2015;9(9):1-19.
- M Lamontellerie. Results of surveys on the filariasis in western upper volta (Cercle de Banfora). Ann Parasitol Hum Comp. 1972;47(6):783-838.
- Camara MM. Evaluation entomologique dans les sites sentinelles du programme national d'elimination de la filariose lymphatique avant et apres le premier traitement de masse dans la region de sikasso au mali. Université de Bamako. 2012.
- Mathison BA, Couturier MR, Pritt BS. Diagnostic identification and differentiation of microfilariae. J Clin Microbiol. 2019;57(10):1-13.
[Crossref]
- Sarangi J, Arava S, Kumar H. Microfilaria in urine cytology: Report of three cases with review of literature. Diagn Cytopathol. 2020;48(7):675-678.
- Hausfater P. Procalcitonine and infection. Ann Fr Med d'Urgence. 2011;1(3):206-212.
[Crossref]
- Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38(2):59-68.
- De Carvalho FT, Rabello Filho R, Bulgarelli L, Serpa Neto A, Deliberato RO. Procalcitonin as a diagnostic, therapeutic, and prognostic tool: A critical review. Curr Treat Options Infect Dis. 2019;11(1):1-11.
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013;28(3):285-291.
- Wright CA, Path FRC, Burg M Van Der, Geiger D. Diagnosing mycobacterial lymphadenitis in children using fine needle aspiration biopsy: Cytomorphology, zn staining and autofluorescence-making more of less. Diagn Cytopathol. 2008;36(4):245-251.
- Azzini AM, Dorizzi RM, Sette P, Vecchi M, Coledan I, Righi E, et al. A 2020 review on the role of procalcitonin in different clinical settings: An update conducted with the tools of the Evidence Based Laboratory Medicine. Ann Transl Med. 2020;8(9):610-610.
- Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(2):3-20.
- Durand G, Beaudeux JL. Medical Biochemistry: Current Markers And Perspectives, Chapter 7: Biochemical Markers Of Inflammation, Medicine Sciences Publications. Lavoisier.
- Moreau J, Cuzon G, Pichon G, Outin-Fabre D, J. Lagraulet. The serum proteins of the lymphatic filarial Wuchereria bancrofti var. Pacifica. Blectrophoritic study and immunochemical assay of immunoglobulins a,m,g and e. Pathol Exot. 1972;456-463.
Citation: Fokou JBH, Biyong ST, Kouemo F, Akumwah FA, Reine FA, Fannang VS, et al. (2024). Tentative Analysis of Biomarkers Associated with Filariasis in Moungo Division, Littoral, Cameroon. Bio Med. 16:733.
Copyright: © 2024 Fokou JBH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.


