Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2021) Volume 0, Issue 0
Technology Update: Laser Ablation Technology for Transdermal Immunization
Devyani Joshi1, Mohammad N Uddin1, Martin J D’Souza1* and Rikhav P Gala22Department of Pharmaceutical Sciences, Fraunhofer USA Inc., Newark, USA
Received: 16-Sep-2021 Published: 07-Oct-2021, DOI: 10.35248/2157-7560.21.s15.001
Abstract
Vaccination is the modern technique to control the spread of the infectious diseases. Transdermal route of immunization has gained attention because of the large number of the antigen-presenting cells residing in the skin. However, the major challenge in transdermal immunization is to overcome the most superficial layer of the skin, stratum corneum, which acts as an impermeable barrier preventing the diffusion of the molecules across skin. A variety of methods to overcome the stratum corneum barrier have been developed to deliver the antigens across the skin, including microneedles, thermal ablation, tape stripping, laser ablation etc. The current review focuses on the use of the laser ablation technology as a means of creating the micropores on the surface of the skin for the transdermal immunization. Several groups have studied the effects of number of pores formed by the ablative laser as well as the intensity of the laser on the permeation of the antigens across the skin. The immune response generated by the ablative laser mediated transdermal immunization has been compared to the traditional routes of vaccination including subcutaneous and the intradermal routes, and the laser ablation has been shown to be an effective immunization strategy in the mouse model. The ablative laser mediated transdermal immunization is a non- invasive, convenient and safe immunization strategy, and has a potential to be used as an alternative immunization strategy for the mass vaccination in the near future.
Keywords
SALT; Langerhans cells; Laser ablation; Transdermal immunization; Microporation
Introduction
Immunization is the most effective and economical modern technique to control the spread of infectious diseases [1]. Vaccines are primarily administered into the subcutaneous or muscular tissue using a hypodermic needle. However, it is an invasive procedure for immunization and generates bio-hazardous sharps wastes. Moreover, many children have a needle phobia, resulting in non-compliance towards the vaccination regimen [2,3]. There has been a renewed interest in harnessing the Skin Associated Lymphoid Tissues (SALT) to generate an immune response, thus enabling easy self-administering vaccine delivery systems with the aid of micro needles, transdermal patches, etc.
Literature Review
The skin is the largest organ and houses vast immune machinery, including professional Antigen Presenting Cells (APCs), involving specialized Langerhans Cells (LCs) and dermal Dendritic Cells (dDCs) residing in the epidermal and dermal layers, respectively, that play a key role in skin immune responses [4]. They capture (uptake), process, and present the foreign antigens and closely interplay with keratinocytes, mast cells, and T-lymphocytes to elicit an effective immune response [5-7]. The recirculating properties of a subset of skin-seeking T lymphocytes equip them to migrate preferentially through dermal vessels and the lymph nodes that drain the skin, creating a network of surveillance and communication that ensures that appropriate immune effectors and regulators are produced and that threatening cutaneous pathogens are eliminated without compromise of skin function (Figure 1) [8].
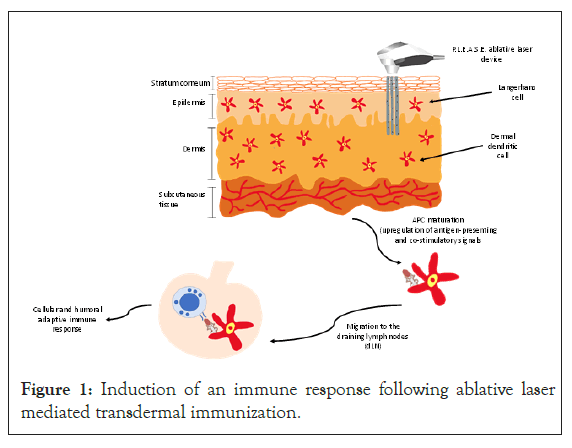
Figure 1: Induction of an immune response following ablative laser mediated transdermal immunization.
Vaccine antigens could be efficiently delivered via skin if an easily applicable, reliable, patient-friendly immunization device or system is available. The major challenge for transdermal immunization is to overcome the superficial layer of the skin, the stratum corneum, which acts as an impermeable barrier, preventing diffusion of molecules, particularly those of a molecular weight beyond 500 Da [9,10]. A variety of non or minimally invasive methods to enhance transdermal antigen delivery have been developed, including microneedles, thermal ablation, skin abrasion, tape stripping, liquid jet injection etc. [11-13]. However, the skin is a heterogeneous tissue, and its properties highly depend on body location, age, skin type, hydration level, and body weight and vary between individuals. Therefore, introducing a methodology to circumvent the stratum corneum in a reproducible and highly adaptable manner would be desirable [14,15].
Skin microporation via ablative laser technology is being developed as a means of transdermal immunization. Q-switched ruby lasers, Nd: YAG lasers, CO2 lasers have been used for the transdermal delivery of the high and low molecular weight drugs [16-18]. The Precise Laser Epidermal System (P.L.E.A.S.E.®) device developed by Pantec Biosolutions INC. offers the opportunity to create aqueous micropores via controlled fractional laser ablation of skin layers. This novel technique employs a diode-pumped Erbium: yttrium- aluminium-garnet (ER: YAG) laser, which emits energy at 2940 nm, a major absorption peak of water molecules. The excitation and evaporation leads to the formation of the aqueous micropores in the skin layers. In contrast to the CO2 laser, this leads to little or no thermal damage to the surrounding skin tissue. The number of micropores to be created can be varied by the user as well as the laser fluence (energy per unit area) determining the depth of the micropores [19]. This allows for selective targeting of different skin layers, rendering the P.L.E.A.S.E.® device adjustable to specific needs. The high pulse repetition rate of up to 1 kHz enables the sequential creation of an array of several hundred micropores within a few seconds. This has the advantage that the deeper cell layers can be targeted without generating ulcerous lesions, and complete wound healing is achieved within several days [20-23].
The laser microporation studies using uncharged molecules such as dextran or polyethylene glycol confirm that permeation rate increases with the number of micropores per area and decreases with the increasing molecular weight of the compound [24,25]. Chen, Shah, Kositratna, Manstein, Anderson, and Wu et al. observed slight differences in uptake of Ovalbumin (OVA) applied to laser-porated skin using laser energies of either 2.5 or 5 mJ, while increasing the number of pores showed a clear effect [26]. In contrast, Weiss, Hessenberger, Kitzmuller, Bach, Weinberger, Krautgartner, Hauser-Kronberger, Malissen, Boehler, Kalia, Thalhamer, and Scheiblhofer et al. reported a clear dependence of OVA uptake and subsequent stimulation of OVA-specific T-cell responses on the number of pulses per pore, which translates to an increase in the fluence. They also found that the uptake of the functional antibodies was proportional to the applied fluence and pore number [27].
The immunization using the laser-porated skin has only been performed in the murine model. Lee, Pan, Wang, Zhuo, Huang, and Fang et al. showed an induction of the antigen-specific antibodies upon administration of protein to the laser-porated skin. The transdermal immunization with lysozyme was enhanced by laser-poration but applying a higher fluence did not result in a further increase in the antibody levels [28]. Flow cytometric analysis of skin draining LNs 72 h after applying FITC-labeled dextran to the micropores revealed an increased number of DCs (carrying the fluorescent label) with an increased pore depth [27]. Joshi, Gala, Uddin and D’Souza et al. compared the induction of the serum antibodies after transdermal immunization via laser-porated skin to that of the traditional subcutaneous administration of the measles vaccine in the mouse model. The transdermal administration of the vaccine via laser-generated micropores elicited comparable levels of serum IgG as that of the subcutaneous vaccine administration, indicating that the ablative laser-mediated transdermal immunization was as effective as that of the subcutaneous immunization [22]. The laser ablation mediated transdermal immunization with the S1 subunit of the Spike protein of SARS-CoV-2 was shown to induce the serum IgG titers of 1:3200 in BALB/c mice. The sera were found to inhibit the binding of spike protein to its receptor ACE229. Weiss et al. showed that compared to an intradermal injection, T-cell proliferation following the laser- poration was equally efficient In vitro restimulation of splenocytes of vaccinated recipient mice and subsequent cytokine profiling showed that TH1, TH2, and TH17 cytokines increased with pore depth. Notably, IL-17 and GM-CSF, both involved in TH17 cell differentiation, displayed the most striking increase compared to subcutaneous injection and also between the groups treated with 1 to 4 laser pulses [2,27].
Another group of investigators assessed enhanced transcutaneous delivery of OVA, b-galactosidase, and a grass pollen allergen Phl p 5, following transdermal delivery via laser-microporated skin.Their study showed that laser-based delivery induced a Th2-biased immune response, which could be modulated by incorporating Th1-inducing adjuvants, like R848, poly I: C and CpG. Moreover, CpG-adjuvanted allergen immunization through laser-generated micropores gave rise to a therapeutic efficacy equivalent to that of subcutaneous injection [2,6,27-30].
Immunization against infectious diseases via the skin has only recently increased interest due to growing knowledge about skin immunology. The need for more immunogenic routes to reduce vaccine doses and increase efficacy in elderly or immunocompromised individuals has fueled the development of novel delivery modalities such as various devices for intradermal injection and transdermal immunization. The significant advantages anticipated for transdermal vaccination include the richness of the target tissue in antigen-presenting cells, efficient drainage by regional lymph nodes, the possibility of generating mucosal immune responses, and increased patient compliance, especially in children afraid of needle injections [3]. Factors such as application depth, application area, and the associated level of tissue damage, resulting in the release of Damage Associated Molecular Patterns (DAMPs), have been shown to influence the type of immune response induced substantially. In contrast to other barrier disruption methods such as tape stripping or microneedles, the easily adaptable parameters of fractional laser systems will allow us to investigate the underlying immunological mechanisms, a knowledge imperative for the rational design of safe and successful vaccination strategies [31,32]. The high diffusion rate of large molecular substances via laser-generated micropores additionally opens the field for application of more complex vaccine formulations, such as liposomes, nanoparticles, and microparticles, or even attenuated viruses or viral particles [2].
The major hurdles for the commercialization of laser-based vaccination approaches are the high cost associated with relatively complicated technology, limiting its use to specially equipped clinics. Although laser class 1 devices for self-administration at home (P.L.E.A.S.E.®, Pantec Biosolutions) are currently under development, self-administration of vaccines, in general, is not a realistic option especially not for allergen-specific immunotherapy, which has to be administered under close medical supervision [33- 35]. Potential side effects associated with laser-assisted transdermal administration may include local temporary hyperpigmentation and local side effects (itching, eczema, etc.) in case of allergen- specific immunotherapy. The latter may require the use of hypoallergens or allergoids with low IgE binding capacity [36]. One technical obstacle is the high variability of the skin depending on age, ethnicity, and body site, requiring special efforts to standardize pore depth. To address this issue, novel fractional laser devices continuously controlling ablation depth in real time are currently under development [2].
Conclusion
Taken together, laser-poration may turn out as a highly efficient method for painless and effective transcutaneous vaccination, in general, but also for immunotherapeutic strategies to treat allergic diseases. One remaining caveat is that up to now, only mouse data are available regarding the immune response induced by transcutaneous laser-assisted vaccination. First clinical trials will have to show if this novel approach can meet the expectations. Beyond the potential clinical application, defined, accurate, and reproducible laser-generated microporation of the skin offers a sophisticated, versatile, and beneficial technology for basic science investigating the principle mechanisms of skin immunity.
REFERENCES
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201-1214.
- Scheiblhofer S, Thalhamer J, Weiss R. Laser microporation of the skin: prospects for painless application of protective and therapeutic vaccines. Expert Opin Drug Deliv. 2013;10:761-773.
- Bal SM, Ding Z, van Riet E, Jiskoot W, Bouwstra JA. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release. 2010;148:266-282.
- Kashiwagi S. Laser adjuvant for vaccination. FASEB J. 2020;34:3485-3500.
- Streilein JW. Skin Associated Lymphoid Tissues (SALT): Origins and functions. J Invest Dermatol. 1983;80:12-16.
- Chen X, Wang J, Shah D, Wu MX. An update on the use of laser technology in skin vaccination. Expert Rev Vaccines. 2013;12:1313-1323.
- Ita K. Transdermal delivery of vaccines–Recent progress and critical issues. Biomed Pharmacother. 2016;83:1080-1088.
- Streilein JW. Skin-associated lymphoid tissue. Immunology series. 1989;46:73-96.
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165-169.
- Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008;364:227-236.
- Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: A review. Biomater Res. 2021;25.
- Szunerits S, Boukherroub R. Heat: a highly efficient skin enhancer for transdermal drug delivery. Front Bioeng Biotechnol. 2018;6:15.
- Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438-470.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261-1268.
- Mali AD. An updated review on transdermal drug delivery systems. International Journal of Advances in Scientific Research. 2015;1:1-244.
- Lee S, Kollias N, McAuliffe DJ, Flotte TJ, Doukas AG. Topical drug delivery in humans with a single photomechanical wave. Pharm Res. 1999;16:1717-1721.
- Lee WR, Shen SC, Wang KH, Hu CH, Fang JY. The effect of laser treatment on skin to enhance and control transdermal delivery of 5‐fluorouracil. J Pharm Sci. 2002;91:1613-1626.
- Gomez C, Costela A, García‐Moreno I, Llanes F, Teijón JM, Blanco D et al. Laser treatments on skin enhancing and controlling transdermal delivery of 5‐fluorouracil. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 2008;40:6-12.
- Scheiblhofer S, Drothler S, Braun W, Braun R, Boesch M, Weiss R. Laser facilitated epicutaneous peptide immunization using dry patch technology. BioRxiv. 2021.
- Scheiblhofer S, Strobl A, Hoepflinger V, Thalhamer T, Steiner M, Thalhamer J et al. Skin vaccination via fractional infrared laser ablation-Optimization of laser-parameters and adjuvantation. Vaccine. 2017;35:1802-1809.
- Weiss R, Hessenberger M, Kitzmüller S, Bach D, Weinberger EE, Krautgartner WD et al. Transcutaneous vaccination via laser microporation. J Control Release. 2012;162:391-399.
- Joshi D, Gala RP, Uddin MN, D'Souza MJ. Novel ablative laser mediated transdermal immunization for microparticulate measles vaccine. Int J Pharm. 2021;606:120882.
- pantec biosolutions: P.L.E.A.S.E.® technology < TECHNOLOGY. (Accessed: 13th February 2021)
- Haak CS, Bhayana B, Farinelli WA, Anderson RR, Haedersdal M. The impact of treatment density and molecular weight for fractional laser-assisted drug delivery. J Control Release. 2012;163:335-341.
- Lee WR, Shen SC, Al-Suwayeh SA, Yang HH, Li YC, Fang JY. Skin permeation of small-molecule drugs, macromolecules, and nanoparticles mediated by a fractional carbon dioxide laser: the role of hair follicles. Pharm Res. 2013;30:792-802.
- Chen X, Shah D, Kositratna G, Manstein D, Anderson RR, Wu MX. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J Control Release. 2012;159:43-51.
- Weiss R, Hessenberger M, Kitzmüller S, Bach D, Weinberger EE, Krautgartner WD et al. Transcutaneous vaccination via laser microporation. J Control Release. 2012;162:391-399.
- Lee WR, Pan TL, Wang PW, Zhuo RZ, Huang CM, Fang JY. Erbium: YAG laser enhances transdermal peptide delivery and skin vaccination. J Control Release. 2008;128:200-208.
- Scheiblhofer S, Drothler S, Braun W, Braun R, Boesch M, Weiss R. Laser-facilitated epicutaneous immunization of mice with SARS-CoV-2 spike protein induces antibodies inhibiting spike/ACE2 binding. Vaccine. 2021;39:4399-4403.
- Hessenberger M, Weiss R, Weinberger EE, Boehler C, Thalhamer J, Scheiblhofer S. Transcutaneous delivery of CpG-adjuvanted allergen via laser-generated micropores. Vaccine. 2013;31:3427-3434.
- Zehrung D, Kristensen D. Intradermal Delivery of Vaccines: A review of the literature and the potential for development for use in low-and middle-income countries. World Health Organization (WHO). Program for Appropriate Technology in Health (PATH). Batiment Avant Centre. 2009;13.
- Senti G, Von Moos S, Kündig TM. Epicutaneous allergen administration: is this the future of allergen‐specific immunotherapy? Allergy. 2011;66:798-809.
- Tripp CH, Voit H, An A, Seidl‐Philipp M, Krapf J, Sigl S, Romani N, Del Frari B, Stoitzner P. Laser‐assisted epicutaneous immunization to target human skin dendritic cells. Exp Dermatol. 2021;30:1279-1289.
- Kumar MN, Zhou C, Wu MX. Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy. J Control Release. 2016;235:82-90.
- Bauer M, Lackner E, Matzneller P, Al Jalali V, Pajenda S, Ling V et al. Phase I study to assess safety of laser-assisted topical administration of an anti-TNF biologic in patients with chronic plaque-type psoriasis. Front Med. 2021;8.
- Kakar P. Laser-Assisted Transdermal Drug Delivery and Vaccination. University of Rhode Island; 2019.
Citation: Joshi D, Uddin MN, D’Souza MJ, Gala RP (2021) Technology Update: Laser Ablation Technology for Transdermal Immunization. J Vaccines Vaccin. S15:001.
Copyright: © 2021 Joshi D, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

