Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2020) Volume 10, Issue 5
Synthesis of a Novel Composite Hydrous Titanium Oxide- Hydroxyapatite for Adsorbing Uranium from Waste Effluents
Amr H Ali*, Shaimaa MA Esmaeel, Salah A Zaki and Mohammed S HagagReceived: 16-Oct-2020 Published: 30-Nov-2020, DOI: 10.35248/2252-5211.20.10.390
Abstract
In this paper; a novel composite (HAP@HTO)derived from hydrous titanium oxide (HTO) extracted from Rosetta ilmenite mineral and hydroxyapatite (HAP) was formed by co-precipitation method,specified and used for uranium preconcentration from its solutions. Batch tests were performed to investigate its selectivity towards uranium; maximum adsorption efficiency reached at pH 2.5, 120 minutes contact time, 900 mg L–1 uranium concentration and adsorbent ratio (0.1g/75 mL). The equilibrium data fitted well with Langmuir adsorption isotherm. From Kinetics and thermodynamics data; process was fast, fitted well with pseudo-second order, spontaneous and exothermic. The percentage desorption of uranium reached its maximal at 15 minutes equilibrium time using 1 mol L-1 H2SO4. Thereby, (HAP@HTO)composite has promising potential applications in extracting U (VI) from its aqueous solutions in nuclear fuel field and environmental pollution cleanup.
Keywords
Ilmenite; Titanium hydroxide; Uranium; Langmuir; Elution
Introduction
One of the most popular methods of extraction and concentration of micro-quantities of elements is their sorption from its solutions with synthetic adsorbents. Therefore, obtaining adsorbents based on synthetic materials of organic and inorganic origin is one of the major problems of analytical chemistry. And the development on their basis of methods of concentration and the excretion of trace elements are always important. Methods of immobilization of reagents on the surface of various adsorbents and their use for concentrating metals in various objects are devoted to a number of works [1-4]. An important direction in the practice of using synthetic adsorbents is the targeted synthesis of new selective adsorbents and the improvement of the analytical characteristics of the already known by introducing functional analytical groups into the sorbent matrix that can interact with metal ions to form complexes, chelates, or ionic associates.
A set of technologies were developed for uranium removal and recovery from its ores, nuclear fuel effluents, mine tailings, seawater and other waste sources in consideration of the dual significance of uranium in nuclear power generation as well as its strategical importance [5-8]. Solid phase extraction (SPE) or solid–liquid extraction is more valuable technique compared with others due to the following advantages: insolubility of the applied solid in the aqueous phase, its low rate of physical degradation besides its high sorption capacity as well as its good flexibility, low costs, kinetic properties, absence of emulsion, safety with respect to hazardous samples and easier to operate in the automated analytical techniques [9-12].
Hydroxyapatite is an ideal material for long-term containment of contaminants because of its high sorption capacity for actinides and heavy metals, low water solubility, high stability under reducing and oxidizing conditions, availability, and low cost [13]. It was conducted in stabilization of a wide variety of metals (e.g., Cr, Co, Cu, Cd, Zn, Ni, Pu, Pb, As, Sb, U and V) by many investigators [14,15]. Among the adsorption materials, the hydrous titanium oxide has attracted attention as adsorbent due to its large surface area, high adsorption rate, heat and radiation resistance, free from the interference of other ions, insoluble in water and its photo catalytic action. It can be used for purification of ground water and industrial wastewater[16].
Additionally, preparation and design of composite particles consisting of cores covered by shells of different chemical compositions have attracted much interest due to their superior catalytic, optical, and electrical properties, which are different from those of single-component materials [17]. These interesting properties of core-shell materials are due to structure, size, morphology, composition of their shells and cores [18]. Among core-shell particles of various compositions, those made of core with inexpensive compounds and a reactive shell has received particular interest because of the functional and economic advantages that they can provide [15]. Arranging of reactive materials as thin shells on proper low cost cores greatly magnifies their use, and can also significantly enhances their properties via the so-called strain and ligand effects of the core substrate on the shell compound [19].
This study deals with the preparation of a core-shell structure composed of calcium hydroxyapatite hydrous titanium oxide. The fabricated composite will be subjected, in batch reactor, with uranyl ions waste aqueous solution to investigate its sorption characteristics in aqueous solution under different experimental conditions.
Materials and Methods
Chemicals and reagents
All chemicals used were of analytical grade purity and were used without further purification. Ammonia solution (NH4OH) was supplied from Readel, Germany. Calcium nitrate (Ca(NO3).4H2O) was supplied from Win Lab, UK while di-ammonium hydrogen phosphate ((NH4)2HPO4) was a product of VEB, Germany. Both hydrochloric acid and sodium hydroxide were obtained from Adwic, Egypt.
Preparation of titanium leach liquor from ilmenite processing
A sample of East Rosetta beach ilmenite concentrate (Table 1) was used in the present work to prepare the working Ti leach liquor by applying the optimum conditions previously determined and which have resulted in 95% leaching efficiency of titanium [20]. These conditions involved ilmenite leaching after grinding to -200 mesh size in concentrated HCl solution (12 mol L-1) in 1/8 S/L ratio at 80°C for 90 minutes and adding10% Fe powder. The ilmenite dissolution reactions can be represented as follows:
FeO.TiO2 + 4HCl ··TiOCl2 + FeCl2 + 2H2O
FeO.TiO2 + 6HCl ··TiCl4 + FeCl2 + 3H2O
Table 1: Chemical compositions of East Rosetta ilmenite concentrate.
| Oxide | TiO2 | Fe2O3 | FeO | MnO | V2O5 | CR2O3 | MgO | Al2O3 | CaO | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 44.01 | 21.41 | 28.50 | 1.15 | 0.18 | 0.29 | 0.80 | 0.90 | 0.44 | 0.75 |
After filtration and washing, the assay of the obtained leach liquor was found to be attaining 41.6 g TiO2 /L equivalent to 25 g Ti/L together with 60 g Fe2O3/L in hydrochloric acid concentration 5 mol L-1.
Composite preparation
The hydroxyapatite coated with hydrous titanium oxide (HAP@ HTO) was decided to be constructed by applying calcium hydroxyapatite as a core and hydrous titanium oxide as an active shell. They were prepared by firstly dropping a solution of 0.387 M (NH4)2HPO4 into an equal volume of 0.5 M Ca(NO3)2 solution with continuous stirring at room temperature. The mixture was stirred for 30 minutes at room temperature. After that, ammonium hydroxide was dropped into the mixed solution to precipitate hydroxyapatite (HAP) and the reaction was terminated at pH 11 which could produce the stoichiometric Ca/P ratio of 1.67. After further stirring for 30 minutes, HAP precipitate was filtered and washed repeatedly to remove the unwanted ions. Secondly, chloride solution bearing titanium was added to HAP precipitate at 5 mL minutes-1 rate with continuous stirring. A shell of hydrous titanium oxide was then precipitated on HAP surface by adding NH4OH till pH attained a value of 10. The mixture was left for settling wherein the supernatant was separated. The precipitate was then washed thoroughly with distilled water until constant pH value was sequentially attained. Finally, the composite was dried, pulverized, sieved and stored in a desiccator for further usage.
Batch mode adsorption and desorption studies
Batch adsorption experiments were carried out by stirring the aqueous solution with the prepared composite in glass beaker at 400 rpm for a period of 180 minutes using a magnetic stirrer. 0.1 g of composite was added to different volumes from aqueous sample (10-100 mL) containing various concentrations of U (VI) at temperatures of [25-55(±2)°C]. The pH of the working solutions was adjusted to 2.5. When the adsorption equilibrium was reached, it was filtered to separate the two phases. The quantity of uranium loaded on the composite was calculated using equation 1, i.e. by taking the difference between the initial and residual concentrations of uranium in solution and dividing it by the weight of the adsorbent. The removal or adsorption efficiency (Re) was defined as the U sorption percentage relative to the initial concentration equation 2:
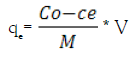 (1)
(1)
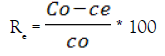 (2)
(2)
Where qe (mgg-1) is the amount of uranium loaded per unit weight of the adsorbent; Co and Ce are the initial and equilibrium (or at any time) uranium or interfering ions concentration (mg L-1), respectively; V is the volume per Liter of solution and M is the weight (g) of the adsorbent (HAP@HTO) composite. To desorb uranium from the (HAP@HTO) composite adsorbent, a variety of stripping agents were examined, including HCl, H2SO4, Na2CO3, double distilled H2O and HNO3.Uranium analyzed spectrophotometrically in the corresponding aqueous phases by Arsenazo (III) reagent using Lambada UV/VIS spectrophotometer (Perkin-Elmer, USA) [21]. Also, U (VI) was analyzed by redox titration method against ammonium metavanedate in the presence of diphenylamine sulfonate indicator prior to titration; proper reduction of U (VI) was performed using ferrous sulfate [22].
Instruments
The surface characteristic of the synthesized resin was characterized using Fourier Transform Infrared spectrophotometer (FTIR) model Thermo Scientific Nicolet IS10, Germany. The powder X-ray diffraction patterns was recorded withAP-Analytical X-Ray Diffraction equipment model X ׳Pert PRO with Monochromator, Cu-radiation (λ=1.542Å) at 45 K.V., 35 M.A. and scanning speed 0.03o/sec. were used. The reflection peaks between 2Ɵ =2o and 60o, corresponding spacing (d, Å) and relative intensities (I/Io) were obtained. The diffraction charts and relative intensities are obtained and compared with ICDD files. SEM model Philips XL 30 ESEM (25-30 k e V accelerating voltage, 1-2 mm beam diameter and 60-120 s counting time). Minimum detectable weight concentration is ranging from 0.1 to 1 wt % with realized precision lower than 1%. Inductive Coupled Plasma Optical Emission Spectrometry was employed for trace concentrations of metal ions (Prodigy Axial high dispersion ICP-OES-USA model).
Liquid waste sample
The used liquid waste sample in this study was obtained from disposal waste derived from ores products project at Anshas site, Nuclear Materials Authority, Egypt. The waste solution was collected from (washing, scrubbing, raffinate, solvent regeneration solutions, leaching and several precipitation tests). The waste solution precipitated by sodium hydroxide. The formed hydroxide precipitate washed several times with distilled water to remove the excess NaOH, then dried and about 60 g from the dried cake dissolved with HNO3. A sample from the wetted raffinate precipitated cake was analyzed against their constituents using (ICP–OES), it was indicated that the wetted cake contained about 1.45% U. The chemical composition of the hydrous cake is tabulated in (Table 2).
Table 2: Chemical composition of Heavy Metals and trace elements for the Precipitated Cake by (ICP–OES)
| Element | Conc., % | Element | Conc., % |
|---|---|---|---|
| U | 1.45 | Ge | UDL |
| As | 0.49 | Nb | UDL |
| Cd | 0.05 | Mo | 0.17 |
| Cs | 0.15 | Si | 1.24 |
| Pb | 0.86 | Ta | UDL |
| Se | UDL | Ti | 1.04 |
| Ag | UDL | W | 0.07 |
| Sr | 0.24 | Ce | UDL |
| Zn | 1.11 | Er | 4.5 |
| Cu | 10.07 | Eu | UDL |
| Cr | 4.67 | Gd | 0.5 |
| Al | 7.35 | Lu | 0.01 |
| V | 1.77 | UDL | UDL |
| Na | 4. 24 | Sc | UDL |
| K | 4.18 | Tm | 0.28 |
| Ca | 2.88 | Yb | 0.18 |
| Mg | 3.11 | Y | 0.13 |
| Fe | 20.50 | La | UDL |
| Ni | 0.53 | Dy | UDL |
| Mn | 0.41 | Ho | UDL |
| Ba | 1.53 | Pr | 4.57 |
| Co | 0.08 | Tb | UDL |
| B | UDL | Sm | 0.39 |
UDL=under detection limit
Results and Discussion
Composite characterization
Characterization of HAP@HTO composite with FT-IR: IR band changes from Figures 1 & 2 may reveal the adsorption of uranium ions onto (HAP@HTO) composite. The characteristic band of TiO2 appears at 745 cm-1 is slightly affected in intensity after uranium adsorption. The bands at 1000 cm-1 represented phosphate group. 1469.54 cm−1 indicates the presence of carbonate groups (CO32-) it can be identified as Ca10(PO4)6CO3. Broadband > 3300 cm-1 represented water stretching.
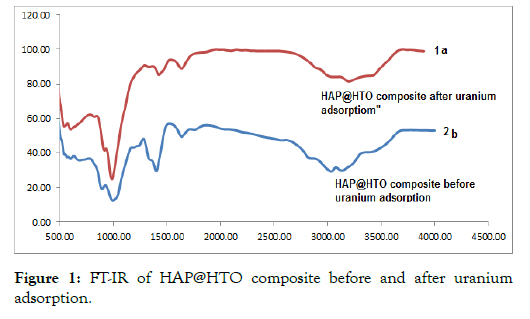
Figure 1: FT-IR of HAP@HTO composite before and after uranium adsorption.
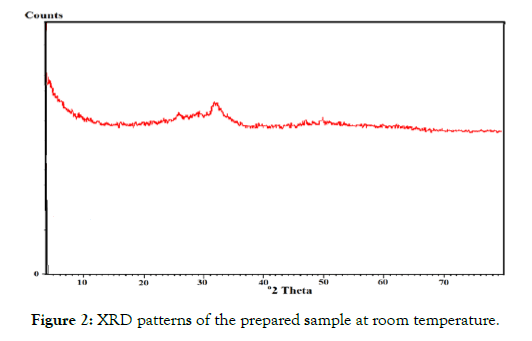
Figure 2: XRD patterns of the prepared sample at room temperature.
X-ray analysis: The phase structure of the synthesized sample was investigated by XRD and some of data are given in Figure 2, the shell structure of HAP@ HTO is HTO. So, the diffractogram clarified that the sample is amorphous.
Surface morphology studies
SEM images of the composite macrostructure before and after uranium adsorption Figures 3a & 3b showed the rudimentary on the surface (Figure 3a). After adsorption, these pores were occupiedwith uranium ions (Figure 3b).
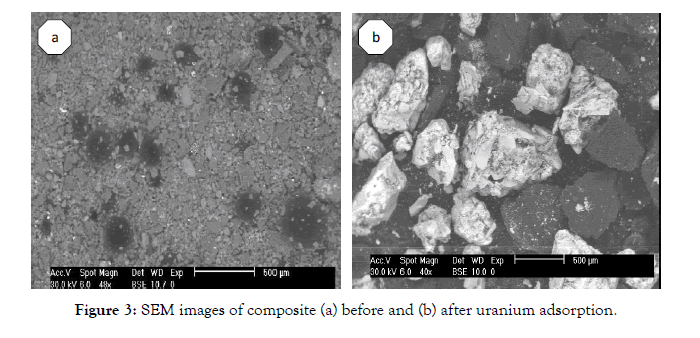
Figure 3: SEM images of composite (a) before and (b) after uranium adsorption.
Factors affecting the adsorption process
Effect of pH: The pH value of the aqueous solution is an important variable which control the adsorption process of the metal ion at the solid–liquid interface. Therefore, pH effect on uranium adsorption onto (HAP@HTO) composite was examined in pH range of 1 to 3.5, while the other experimental parameters were fixed. As can be seen in Figure 4, uranium adsorption efficiency and uptaking increased by pH increasing up to 2.5 then starts decreasing again. The pH solution could affect the mechanism of uranium adsorption through the hydrolysis of the uranyl ions. At (high acidity) lower pH below 2.5, adsorption efficiency was decreased due to competition occurs between the H+ ions, which are small and fast, and the uranium species, which are also positive, and adsorption is not favored. Thus, the presence of a higher concentration of H+ ions in the reaction mixture caused a reduction in the uranium uptake. In contrast, increasing solution pH resulted in the adsorbent surface becoming more negatively charged, which resulted in the more favorable adsorption of positively charged species [23]. At pH >2.5; showing gradual decrease of uranium adsorption, due to the domination of the negatively charged uranium species within the bulk solution.
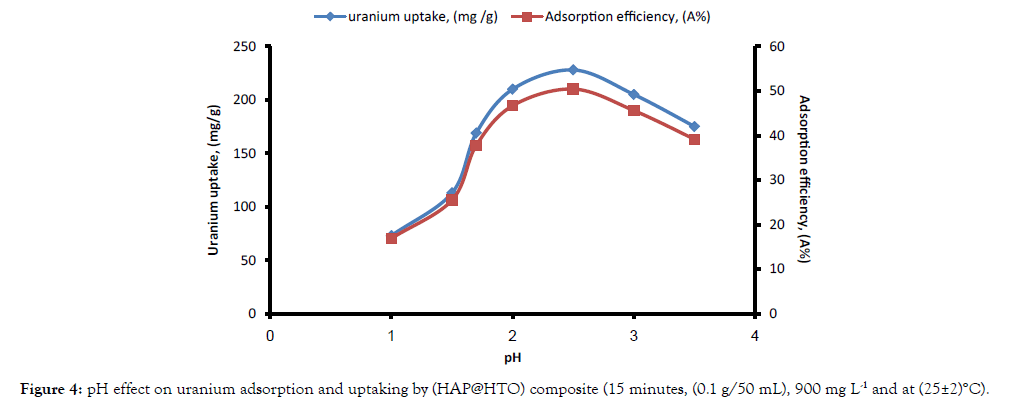
Figure 4: pH effect on uranium adsorption and uptaking by (HAP@HTO) composite (15 minutes, (0.1 g/50 mL), 900 mg L-1 and at (25±2)°C).
Effect of contact time: Contact time was studied in a range of 15- 180 minutes while other controlling factors were kept constant. The adsorption efficiency increased from 30.33% at 15 minutes till reached its maximum value 42.5% at 120 minutes, as shown by Figure 5. Furthermore, no significant increase in uranium adsorption took place, revealing that equilibrium state has been reached. The adsorption behavior shows that at the starting stage, adsorption takes place in fast way on the external surface of the adsorbent followed by a slower process. The trend in uranium adsorption gives an indication that the binding may be occurred due to conjugation with functional groups located on the surface of the composite. These results agree with [24] who found that, 120 minutes as contact time is efficient for lead adsorption on the surface of tobacco and also stated that at a fixed adsorbent dosage and at lower concentrations, the ratio of number of metal ions to the available adsorption sites is low and subsequently the fractional adsorption becomes independent of initial concentration. While at higher concentrations, the available sites of adsorption become fewer and subsequently the removal of metals depends on the initial concentrations.
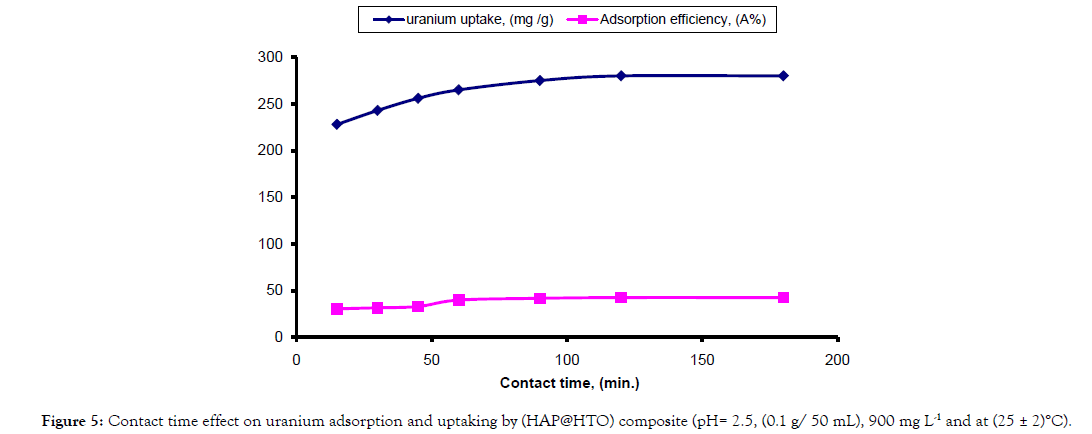
Figure 5: Contact time effect on uranium adsorption and uptaking by (HAP@HTO) composite (pH= 2.5, (0.1 g/ 50 mL), 900 mg L-1 and at (25 ± 2)°C).
Effect of adsorbent dose: Effect of adsorbent dose on U(VI) adsorption efficiency and uptaking onto (HAP@HTO) composite was studied under the following conditions: S/A varied from (0.1 g/10 mL) to (0.1 g/100 mL), initial uranium concentration 900 mg L–1, at room temperature (25±2)°C, 15 minutes., and pH 2.5. The results illustrated below in Figure 6; it is clear that; by increasing solution volume leads to increasing uranium uptake and decreasing adsorption efficiency till complete saturation of the adsorbent reached at (0.1 g/75 mL). Therefore, it was chosen for the subsequent experiments. Apparently, at this ratio uranium ions occupy most of the vacant adsorption sites.
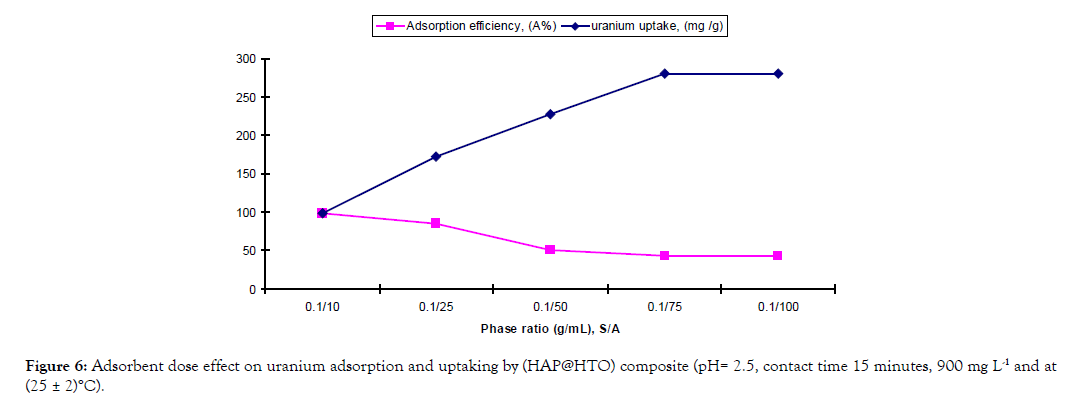
Figure 6: Adsorbent dose effect on uranium adsorption and uptaking by (HAP@HTO) composite (pH= 2.5, contact time 15 minutes, 900 mg L-1 and at (25 ± 2)°C).
Effect of uranium initial concentration: Uranium initial concentration was varied from 250 to 1500 mg L–1 under the following conditions: 0.1 g of adsorbent, 75 mL of solution, (25 ± 2) °C, contact time 45 minutes and pH 2.5. The results are shown below in Figure 7 which illustrated that maximal uranium uptake was reached at uranium concentration of 900 mg L–1 with 42.5% adsorption efficiency. This is attributed to higher mobility of uranyl ions (UO22+) in the dilute solutions and strong interaction of the ions with the adsorbent, in agreement with the data of Mezenner and Bensmaili [25]. At uranium concentrations higher than 250 mg L-1, the competition of uranium ions for free active sites becomes stronger, which negatively affects the adsorption efficiency. In this case, the adsorbed uranium amount increased further relative to the value obtained at 900 mg L–1. Therefore, it was chosen as optimum uranium concentration.
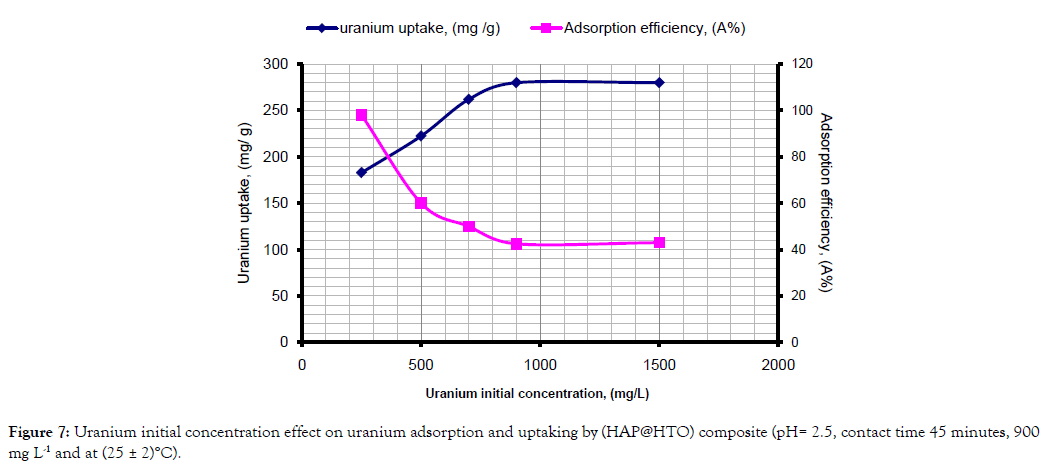
Figure 7: Uranium initial concentration effect on uranium adsorption and uptaking by (HAP@HTO) composite (pH= 2.5, contact time 45 minutes, 900 mg L-1 and at (25 ± 2)°C).
Effect of temperature: Temperature affect on adsorption efficiency and uranium uptaking by (HAP@HTO) composite was studied in temperature range of 25 to 55( ± 2)°C. Figure 8; showed that there is a sharp decrease in both uptaking and adsorption efficiency of U (VI) by temperature increasing. So, the temperature has a negative effect on U (VI) adsorption efficiency.
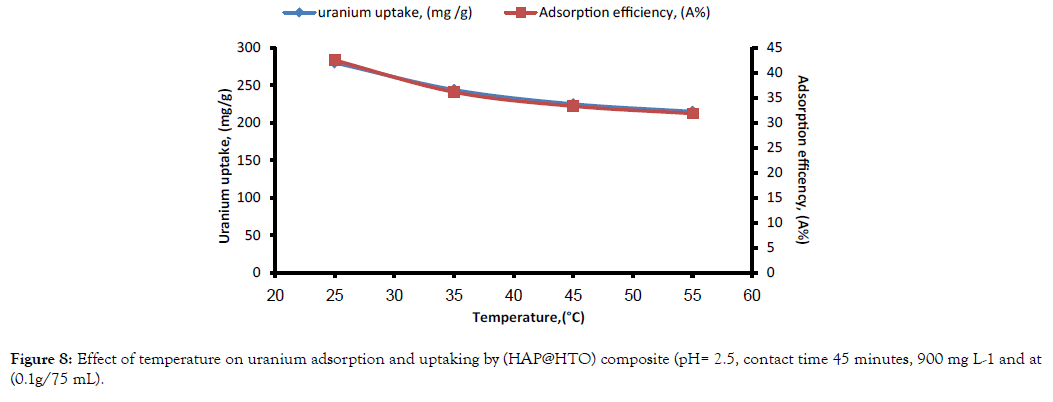
Figure 8: Effect of temperature on uranium adsorption and uptaking by (HAP@HTO) composite (pH= 2.5, contact time 45 minutes, 900 mg L-1 and at (0.1g/75 mL).
Adsorption kinetics and mechanism
Several experiments carried out at different temperatures (25– 55)°C were evaluated by using the simple Lagergren equation 3 to determine the rate of adsorbing interactions assuming pseudo first order kinetics:
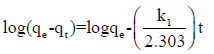 (3)
(3)
Where qt and qe are the amounts of uranium adsorbed (mg/g) at time t (minutes) and equilibrium time (60 minutes), respectively and (k1) is the pseudo first order Lagergren adsorption rate constant (minutes-1). The (k1) values could be obtained by plotting log (qe – qt) versus (t) for adsorption of uranium at different temperatures as shown in Figure 9; the values of the first order rate constant (kl) and correlation coefficient (R2) obtained from these plots are listed in (Table 3). The values of (kl) indicate that rate of adsorption process increased with temperature. The first order mechanism suffered from inadequacies when applied to uranium sorption on the prepared composite. The experimental (qe) values differed from the corresponding theoretical values. Thus, the interaction of uranium with the prepared composite does not follow the first order kinetics. In order to insure the description of the kinetics, second order kinetic equation was applied. The pseudo second order kinetics can be represented by the following linear equation 4:
Table 3: Data of kinetic parameters for uranium adsorption prepared composite.
| Temp, °C | Lagergren pseudo first-order | pseudo second-order | ||||||
|---|---|---|---|---|---|---|---|---|
| K1 (min -1) |
qecal (mg/g) |
qeexp (mg/g) |
R2 | K2 (min -1) |
qecal (mg/g) |
qeexp (mg/g) |
R2 | |
| 25 | 0.05596 | 171.9 | 280 | 0.91 | 1.4 x 10-3 | 285.7 | 280 | 0.99 |
| 35 | 0.03247 | 136.8 | 244 | 0.71 | 2.3 x 10-3 | 250 | 244 | 0.99 |
| 45 | 0.02947 | 192.9 | 225 | 0.72 | 1.6 x 10-3 | 238.1 | 225 | 0.99 |
| 55 | 0.02004 | 196.8 | 215 | 0.64 | 1.5 x 10-3 | 217.4 | 215 | 0.99 |
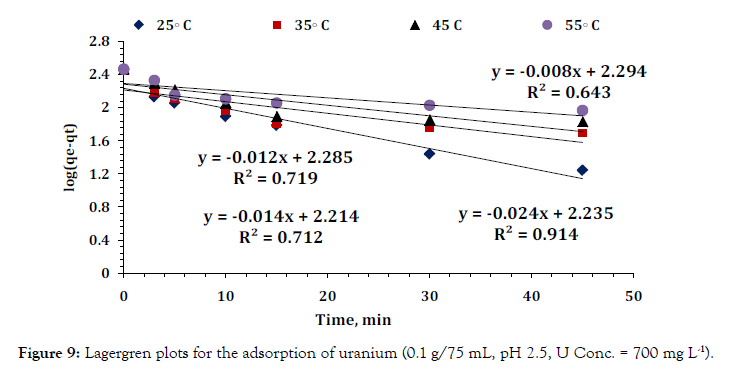
Figure 9: Lagergren plots for the adsorption of uranium (0.1 g/75 mL, pH 2.5, U Conc. = 700 mg L-1).
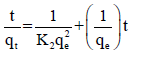 (4)
(4)
Where (k2) is the second order rate constant (g mg-1 minutes-1). The kinetic plots of (t/qt) versus (t) for uranium are shown in Figure 10. The plots showed straight lines with good linearity temperatures. The calculated correlation coefficients are closer to unity for the pseudo second order kinetic model. The calculated equilibrium adsorption capacity (qe) is consistent with the experimental data. The k2 values show the applicability of the above equation for the composite. Therefore the sorption reaction can be approximated more favorably by the pseudo second order sorption as the predominant mechanism.A comparative study for uptaking capacity (mgg-1) of different adsorbents towards uranium is shown in (Table 4) [26-34].
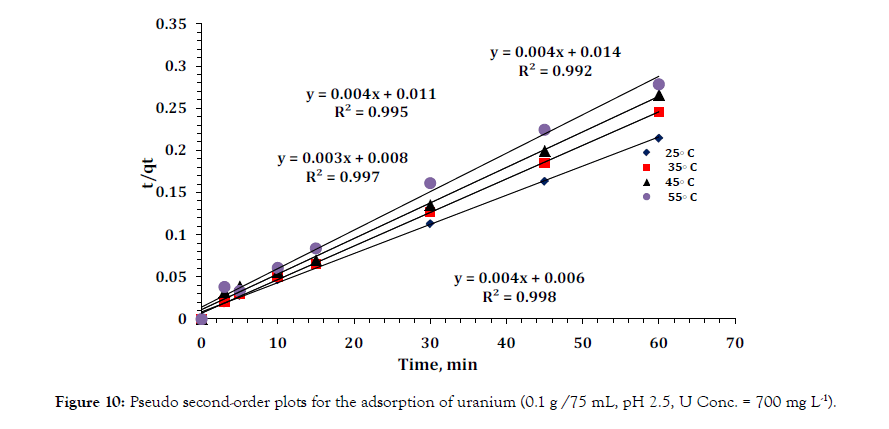
Figure 10: Pseudo second-order plots for the adsorption of uranium (0.1 g /75 mL, pH 2.5, U Conc. = 700 mg L-1).
Table 4: The experimental capacity of composite compared with the adsorption capacity of some resins and some composite.
| Type | adsorption capacity (mg/g) | Ref. |
|---|---|---|
| Activated carbon poly (acryl amide acrylic acid)-titanium silicate Amberlite XAD-4 functionalized with succinic acid natural clinoptilolite zeolite Ambersep 920U Cl 4-(2-Pyridylazo)resorcinol (PAR), Amberlite XAD-16 Amberlite IRA-910 RHA- Aluminium composite Zirconium phosphate Activated carbon with AFS Modified (MOCZ /amine) HAP@HTO composite |
28 64.1 12.33 28.98 0.7 81.75 115.5 85 283 142.5 99 280 |
[26] [27] [28] [29] [30] [31] [32] [8] [12] [33] [34] Present work |
Adsorption isotherms
To describe the results obtained, we used the Langmuir and Freundlich isotherms respectively [35,36]. Langmuir modelillustrated on Figure 11 assumes that the removal of metal ions occurs on an energetically homogenous surface by monolayer sorption and there are no interactions between the adsorbate species on adjacent sites. The linear form of the Langmuir equation is shown below by equation 5:
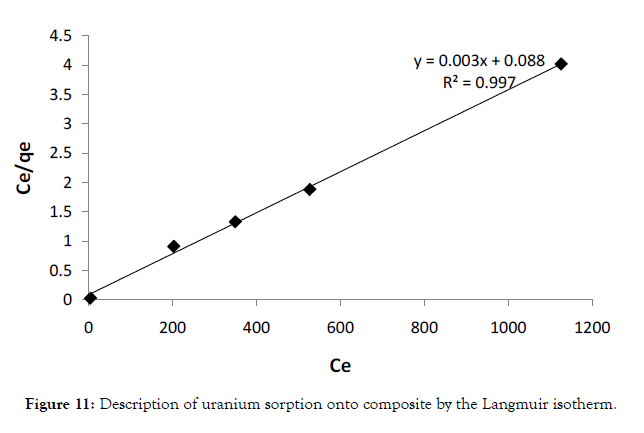
Figure 11: Description of uranium sorption onto composite by the Langmuir isotherm.
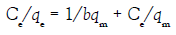 (5)
(5)
Freundlich isotherm Figure 12 is an empirical relationship describing the sorption of solutes from a liquid onto a solid surface. It assumes that different sites with several sorption energies are involved. The linear form of the equation is shown below by equation 6:
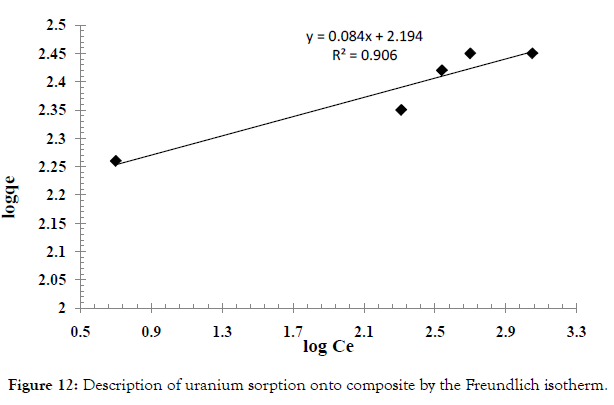
Figure 12: Description of uranium sorption onto composite by the Freundlich isotherm.
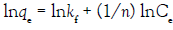 (6)
(6)
where, qe (mg g–1) and Ce (mg L–1) are the equilibrium concentrations of U(VI) in the solid and liquid phases, respectively, qm (mg g–1) is the monolayer capacity, b (Lmg–1) is the equilibrium constant, kf and n are characteristic constants related to the relative sorption capacity of the sorbent and sorption intensity, respectively. The correlation coefficients (R2) and corresponding parameters are presented below in (Table 5). Thus, comparing the isotherms applied with the experimental results, Langmuir isotherm describes the system more adequately than the Freundlich isotherm does i.e. Langmuir model suppose that, the adsorption occurs uniformly on the active sites of the sorbent, and once asorbate occupies a site, no further sorption can take place at this site.
Table 5: Langmuir and Freundlich model parameters of U (VI) sorption
| Metal | Adsorbent | Langmuir model parameters | Freundlich model parameters | ||||
|---|---|---|---|---|---|---|---|
| Qo (mg/g) | b(L/mg) | R2 | 1/n | Kf (mg/g) | R2 | ||
| Uranium | HAP@HTO | 285.71 | 0.0396 | 0.997 | 6.277 | 98.47 | 0.906 |
Elution studies
The reusability of(HAP@HTO) composite for preconcentrationseparation of uranium was evaluated by elution studies. For such studies, a weighted amount of the adsorbent was shaken at the optimum conditions previously obtained to obtain a loaded amount. Desorption of uranium ions from loaded (HAP@HTO) performed in a batch mode. These factors include type of eluent agent, concentration of eluent agent and shaking time.
Eluent agent type and its concentration effect
Different elution agents with 1 mol L-1concentration such as HCl, HNO3, H2SO4, Na2CO3 and DDW (double distilled water) were studied to recover adsorbed U (VI) from loaded (HAP@HTO). The obtained results in Figure 13; indicated that H2SO4 desorbs U (VI) more effectively than others used eluent agents. The required H2SO4 concentration to elute all adsorbed U(VI) from loaded composite was examined by varying its concentration from 0.25 – 1 mol L-1. Figure 14 indicated that about 100% of adsorbed U (VI) can be recovered using 1 mol L-1 of H2SO4.
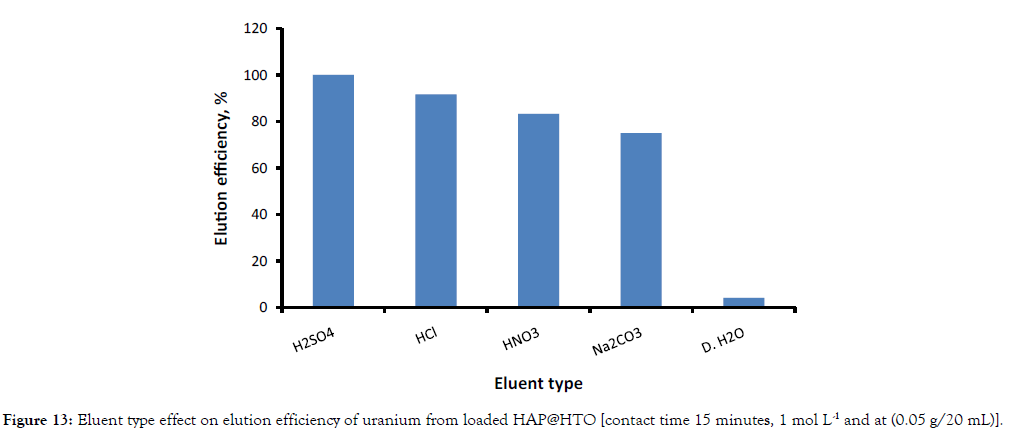
Figure 13: Eluent type effect on elution efficiency of uranium from loaded HAP@HTO [contact time 15 minutes, 1 mol L-1 and at (0.05 g/20 mL)].
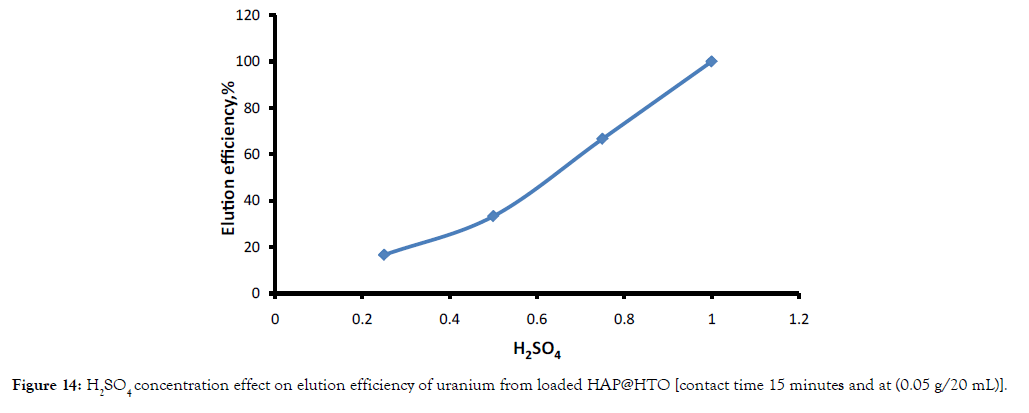
Figure 14: H2SO4 concentration effect on elution efficiency of uranium from loaded HAP@HTO [contact time 15 minutes and at (0.05 g/20 mL)].
Contact time effect
Elution experiments were carried out on loaded (HAP@HTO) with a fixed adsorbent dosage of 0.05g/ 20 mL and with 1 mol L-1 H2SO4 concentration. The results in Figure 15 showed that; elution of uranium increased with increasing contact time from 2-15 mins.
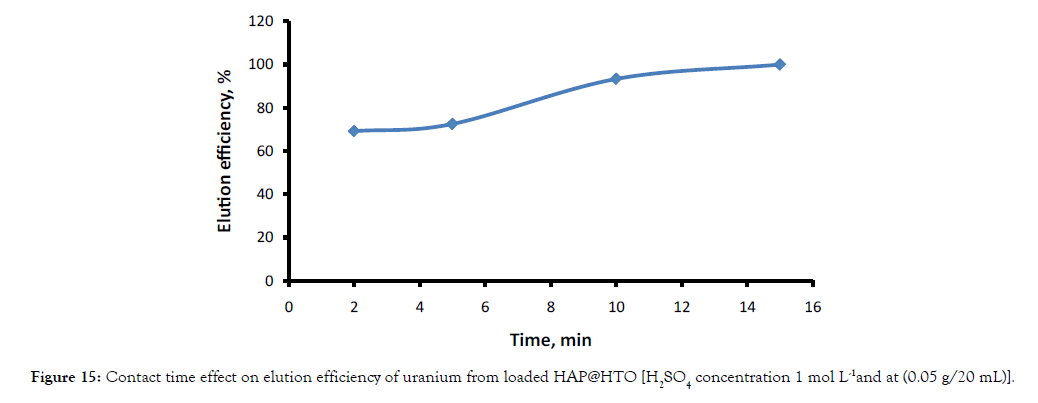
Figure 15: Contact time effect on elution efficiency of uranium from loaded HAP@HTO [H2SO4 concentration 1 mol L-1 and at (0.05 g/20 mL)].
Conclusion
A new sorbent HAP@HTO was successfully synthesized, characterized and its applicability for uptaking uranium from its solutions was investigated. Uranium adsorption process followed a traditional Langmuir adsorption isotherm. The synthesized HAP@HTO adsorbent material had strong structural stability and good adsorption capacity for U(VI). The prepared adsorbent has been successfully applied for uranyl ions removal form waste sample solution. Thus, HAP@HTO exhibits the characteristics of a promising adsorbent in the area of separation science.
REFERENCES
- Fan FL, Qin Z, Bai J, Rong WD, Fan FY, Tian W, et al. Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@ SiO2 composite particles. J Environ Radioact. 2012;106:40-6.
- Li X, Hu HY, Yu JY, Zhao WY. Selection of suitable microalgal species for sorption of uranium in radioactive wastewater treatment. Huanjingkexue. 2016;37(5):1858.
- Metilda P, Sanghamitra K, Gladis JM, Naidu GR, Rao TP. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium (VI). Talanta. 2005;65(1):192-200.
- Zhang J, Guo Z, Li Y, Pan S, Chen X, Xu J. Effect of environmental conditions on the sorption of uranium on Fe3O4@ MnO2 hollow spheres. J Mol Liq. 2016;223:534-40.
- IAEA meeting, Proceedings of an advisory group meeting organized by the International Atomic Energy Agency Production of yellow cake and uranium fluorides 1979, 5–8 June. Paris.
- El-Ansary AL, Abd El-Wahab GM, Bayoumi EE, Nouh ES. Purification of Abu-Zenima wet crude yellow cake using alkaline leaching of U (VI). Egypt J Pet. 2018;27(4):523-30.
- Morsy AM, Ali AH. Sorption of uranium from waste effluent solutions by mesoporous carbon impregnated with trioctylamine. Radiochemistry. 2017;59(2):152-9.
- Youssef WM, Hagag MS, Ali AH. Synthesis, characterization and application of composite derived from rice husk ash with aluminium oxide for sorption of uranium. Adsorpt Sci Technol. 2018;36(5-6):1274-93.
- PrasadaRao T, Preetha CR. Naphthols as reagents for solid phase preconcentrative separation of inorganics. J Separation & Purification Reviews. 2003; 32:1-17.
- Junker-Buchheit A, Witzenbacher M. Pesticide monitoring of drinking water with the help of solid-phase extraction and high-performance liquid chromatography. J Chromatogr A. 1996;737(1):67-74.
- Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Essling AM, Graczyk D. Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta. 1992;266(1):25-37.
- Ali AH. Potentiality of zirconium phosphate synthesized from zircon mineral for uptaking uranium. Sep Sci Tech. 2018;53(14):2284-96.
- Šćiban M, Klašnja M. Wood sawdust and wood originate materials as adsorbents for heavy metal ions. HolzalsRoh-und Werkstoff. 2004;62(1):69-73.
- Dou X, Mohan D, Pittman Jr CU, Yang S. Remediating fluoride from water using hydrous zirconium oxide. Chem Eng J. 2012;198:236-45.
- Chen X, Wright JV, Conca JL, Peurrung LM. Effects of pH on heavy metal sorption on mineral apatite. Environ Sci Tech. 1997;31(3):624-31.
- Shahid M, McDonagh A, Kim JH, Shon HK. Magnetised titanium dioxide (TiO2) for water purification: preparation, characterisation and application. Desalin Water Treat. 2015;54(4-5):979-1002.
- Zhong CJ, Maye MM. Core–shell assembled nanoparticles as catalysts. Adv Mater. 2001;13(19):1507-11.
- Lin SC, Chen SY, Chen YT, Cheng SY. Electrochemical fabrication and magnetic properties of highly ordered silver–nickel core-shell nanowires. J Alloys Compd. 2008;449(1-2):232-6.
- Kalele S, Gosavi SW, Urban J, Kulkarni SK. Nanoshell particles: synthesis, properties and applications. Curr sci. 2006:1038-52.
- El-Hazek N, Lasheen TA, El-Sheikh R, Zaki SA. Hydrometallurgical criteria for TiO2 leaching from Rosetta ilmenite by hydrochloric acid. Hydrometallurgy. 2007;87(1-2):45-50.
- Marczenko Z. Spectrophotometric determination of elements. E. Horwood; 1975.
- Ritcey GM, Ashbrook AW. Solvent Extraction. Principles and Applications to Process Metallurgy. Part I. 1984: 739.
- Han R, Zou W, Wang Y, Zhu L. Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. J Environ Radioactivity. 2007;93(3):127-43.
- Li W, Zhang L, Peng J, Li N, Zhang S, Guo S. Tobacco stems as a low cost adsorbent for the removal of Pb (II) from wastewater: Equilibrium and kinetic studies. Industrial crops and products. 2008;28(3):294-302.
- Mezenner NY, Bensmaili A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem Eng J. 2009;147(2-3):87-96.
- Abbasi WA, Streat M. Adsorption of uranium from aqueous solutions using activated carbon. Sep Sci Technol. 1994;29(9):1217-30.
- Haggag ES, Abdelsamad AA, Masoud AM. Potentiality of uranium extraction from acidic leach liquor by polyacrylamide-acrylic acid titanium silicate composite adsorbent. Int J Environ Anal Chem. 2020;100(2):204-24.
- Metilda P, Sanghamitra K, Gladis JM, Naidu GR, Rao TP. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium (VI). Talanta. 2005;65(1):192-200.
- Camacho LM, Deng S, Parra RR. Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazardous Mat. 2010;175(1-3):393-8.
- Cheira MF, El-Didamony AM, Mahmoud KF, Atia BM. Equilibrium and kinetic characteristics of uranium recovery by the strong base Ambersep 920U Cl resin. IOSR J Appl Chem. 2014;7:32-40.
- Cheira MF. Synthesis of pyridylazo resorcinol–functionalized Amberlite XAD-16 and its characteristics for uranium recovery. J Environ Chem Eng. 2015;3(2):642-52.
- Rahmati A, Ghaemi A, Samadfam M. Kinetic and thermodynamic studies of uranium (VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy. 2012;39(1):42-8.
- Esmaeel SM. Sorption of uranium after carbonate leaching by low cost activated carbon–aluminumferrisilicate composite. Int J Environ Anal Chem. 2020:1-20.
- Yousef LA, Bakry AR, Ahmad AA. Uranium (VI) recovery from acidic leach liquor using manganese oxide coated zeolite (MOCZ) modified with amine. J Radioanal Nucl Chem. 2020;11:1-3.
- Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc. 1916;38(11):2221-95.
- Freundlich HM. Over the adsorption in solution. J Phys Chem. 1906;57(385471):1100-7.
Citation: Ali AH, Esmaeel SMA, Zaki SA, Hagag MS (2020) Synthesis of a Novel Composite Hydrous Titanium Oxide- Hydroxyapatite for Adsorbing Uranium from Waste Effluents. Int J Waste Resour 10: 390. doi: 10.35248/2252-5211.20.10.390
Copyright: © 2020 Ali AH, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.