Indexed In
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 7, Issue 2
Synchronous Differential Independent Lung Ventilation Scenario: A Clinical Challenge
Reda El Bayoumy1* and Mathieu Besnard22Department of Thoracic Surgery, Ajaccio Teaching Hospital, Ajaccio, France
Received: 02-Apr-2023, Manuscript No. JSA-23-20558; Editor assigned: 05-Apr-2023, Pre QC No. JSA-23-20558(PQ); Reviewed: 20-Apr-2023, QC No. JSA-23-20558; Revised: 27-Apr-2023, Manuscript No. JSA-23-20558(R); Published: 04-May-2023, DOI: 10.35248/2684-1606.23.7.205
Abstract
Perioperative complications following lung resection are common and pose massive challenges to anesthesiologists in terms of mechanical ventilation management.
Continuous air leak can arise following lung decortication and resection which could mimic clinically a Broncho- Pleural Fistula (BPF) albeit it is not true BPF; however, clinically it resembles BPF in terms of sudden loss of positive end-expiratory pressure and tidal volume associated with severe refractory hypoxemia following the end of single-lung ventilation.
Here we present a 62-year-old man who experienced septic shock and acute respiratory failure due to massive left empyema and lung collapse. The patient underwent surgical left empyema drainage and lung decortication.
Keywords
Lung empyema; Broncho-pleural fistula; Double-lumen endobronchial intubation; Single-lung ventilation; Synchronous differential independent lung ventilation; Lung resection
Abbreviations
BPF: Bronchopleural Fistula; PEEP: Positive End-Expiratory Pressure; DLTs: Double Lumen Endobronchial Tube; V-P: Ventilation-Perfusion; O2 Sat: oxygen Saturation; IV: Intravenous; FiO2: Fractional inspired Oxygen; PaCO₂: Partial arterial Carbon Dioxide Pressure; PaO₂: Partial arterial Oxygen Pressure; HFJV: High-Frequency Jet Ventilation.
Introduction
Mechanical ventilation for thoracic surgery procedures is generally challenging because of the single-lung ventilation method, either via a Double-Lumen Endobronchial Tube (DLTs) or bronchial blocker [1,2]. The presence of a major air leak following lung resection or decortication can potentially create several important clinical problems [3,4]. If the lung fails to completely re-expand, atelectasis and Ventilation-Perfusion (V-P) mismatching may worsen hypoxemia; this is especially a major problem with very large leaks (e.g. several hundred milliliters per breath), or underlying lung pathology causing decreased elastic lung recoil [4,5]. Loss of a large proportion of each delivered tidal volume via major air leak may impair the expansion and ventilation of other lung areas, thus contributing to V-P mismatching [4,5]. Hypoxemia may also be worsened if a large air leak prevents the effective application of Positive End- Expiratory Pressure (PEEP) in patients with acute lung pathology [3-5].
Depending on the mode employed and the manner in which the ventilator is set, a large air leak can interfere with effective ventilatory support [4,5]. In assist-controlled mode, high pleural suction pressures can be transmitted to the central airways and cause factitious triggering. Conversely, depending on the ventilator brand and inspiratory flow cut-off threshold used in the pressure-support mode, a large air leak may prevent the inspiratory phase from terminating [3].
The use of differential independent lung ventilation method; either synchronous or asynchronous via DLTs and two ventilators improve gas exchange has generally been achieved [5,6].
The settings of the two ventilators should be based on the pathology of the ventilated lung. Each lung may be ventilated in a similar manner, but with lower pressures and volumes to the affected lung or with continuous PEEP alone to the affected lung. The volume of the air leak, as well as hemodynamic and gas exchange stability, are the key variables used to determine the adequacy of the ventilator settings [5,6].
"Written consent has been obtained from the patient for the publication of this case report”
Case Presentation
A 62-year-old man (160 cm, 58 kg) presented to the emergency department with an earthy look, fever, corporal temperature of 38.6°C, Atrial Fibrillation (AF), heart rate 128 beats/min, low blood pressure 86/42 mmHg, diffuse chest pain, severe dyspnea at rest, hypoxemia, and oxygen saturation (O2 Sat) 91% on room air. The arterial blood gases showed pH 6.91 PaCO2 29 PaO2 59 HCO3 19.3 lactate 8.6 mmol/L. Blood work was Hb 10.9 g%, WBCs 14.27, Platelets 548000, CRP 245.2, Troponine T 11, normal coagulation profile and renal functions.
The patient was a heavy smoker (110 packs/year) with a history of excessive alcohol consumption. Initial history taking revealed that the patient initially suffered from left chest pain for three weeks which was intermittently relieved by over-the-shelf painkillers and eventually a recent weight loss of 12 kg. The patient was initially diagnosed with septic shock. The patient was rushed to a computed tomography scan that showed massive left empyema with a collapsed left lung and shifted mediastinum and trachea to the right side (Figures 1 and 2).
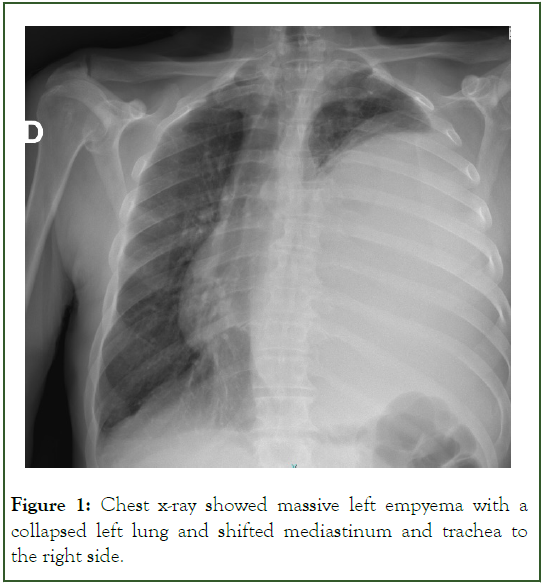
Figure 1: Chest x-ray showed massive left empyema with a collapsed left lung and shifted mediastinum and trachea to the right side.
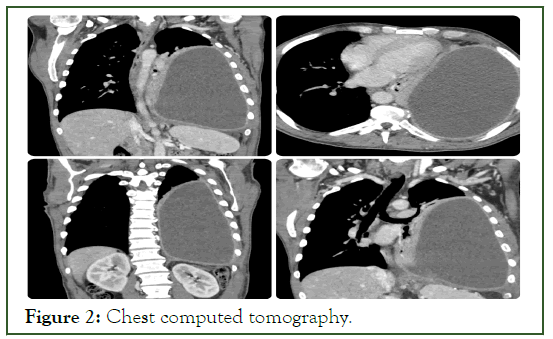
Figure 2: Chest computed tomography.
Prompt surgical intervention was decided upon for drainage of the left empyema and left lung decortication via video-assisted thoracoscopy.
G14 peripheral venous catheter was inserted, followed by Intravenous (IV) general crash anesthetic induction with 20 mg etomidate, 10 mcg sufentanil, 50 mg ketamine, 2 mg midazolam and 100 mg suxamethonium, patient was easily intubated via 37- French left Carlens DLT with immediate exclusion of the collapsed left lung and permissive hypercapnic protective pressure-controlled ventilation to the right lung.
Arterial and central venous lines were inserted under ultrasonography guidance. A urinary catheter was inserted using a thermal probe. The DLT position was confirmed by bronchoscopy.
The surgeon managed to suction approximately 2800 ml of purulent pus from the left pleural cavity via thoracoscopy and then performed extensive left lung decortication via open thoracotomy. The surgeon inserted 2 thoracic drains in situ. Operation lasted for three hours.
Intraoperative events were remarkable; the patient was in septic shock and hypotensive with rapid AF. We managed to maintain his blood pressure and slow down his AF via judicial fluid management guided by transesophageal echocardiography and vasopressor use. The patient had acceptable gas exchange, and his O2 saturation was maintained at around 94% with 100% Fractional inspired Oxygen (FiO2).
At the end of the surgical procedure, surgeon asked to expand the left lung, we declamped the left bronchial lumen and suddenly, we were confronted by massive air leak which led to the loss of tidal volume, plateau airway pressure and consequently the patient started to desaturate down to O2 Sat of 76% with 100% FiO2. We clamped the left bronchial lumen again and immediately regained our optimal ventilatory parameters via right-sided selective ventilation. We tried again to declamp the left bronchial lumen; we were immediately confronted with the same massive air leak and loss of optimized mechanical ventilation. We immediately established the diagnosis of BPF-like clinical manifestations by extensive left lung decortication and resection. We decided to revert to synchronous differential independent lung ventilation in both lungs using a DLTs and two ventilators titrated to optimize the patient's oxygenation and ventilation and minimize ventilatorinduced lung injury (Figure 3). We initially left the right lung to be ventilated via the anesthetic machine ventilator and connected the left lung to another portable ventilator (Drager Oxylog 3000 plus); we used a very small tidal volume (3 ml/kg) to expand the left lung with a respiratory rate of 22 breaths/min and PEEP of 5 cm H2O; we adjusted the same Ventilatory settings for the right lung during the surgical procedure (Vt 4 mL/kg and PEEP of 5 cm H2O) and adjusted the same respiratory rate as the left lung (22 breaths/min) to synchronize ventilation of both lungs to avoid the creation of high intraalveolar auto-PEEP and consequent hemodynamic instability. We used a high inspiratory flow rate of >7 L/min.
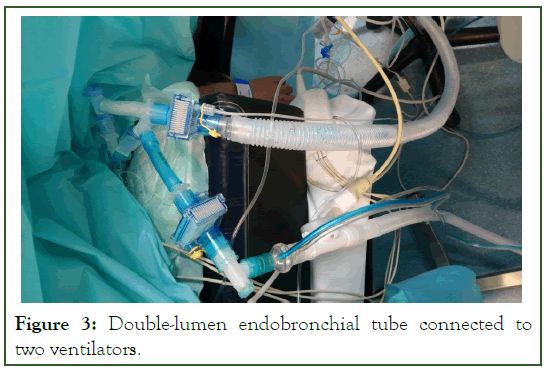
Figure 3: Double-lumen endobronchial tube connected to two ventilators.
The patient was immediately stabilized with lung ventilation, gas exchange, and hemodynamics following the initiation of synchronous differential independent lung ventilation (Figure 4). Thereafter, we connected the right lung to a second portable ventilator (Drager Oxylog 3000 plus) with the same ventilatory parameters as above. The patient was hemodynamically stable with an O2 saturation of 95% on 100% FiO2.
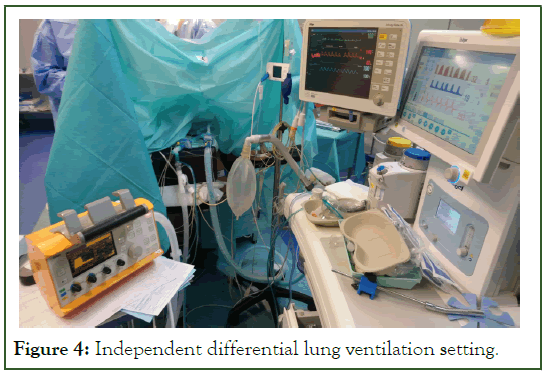
Figure 4: Independent differential lung ventilation setting.
The patient was immediately transferred to ICU for further management. The patient was ventilated uneventfully for 24 hours via synchronous differential independent lung ventilation until vasopressor weaning. The next day, the air leak nearly resolved, the differential lung compliance improved, and chest radiography showed that the left lung was reasonably reexpanded; consequently, the patient was successfully extubated.
The patient was maintained on high-flow O2 via Optiflow 30 L/ min for two days. The patient was later discharged from ICU to the surgical ward with 2 L/min of O2 via the nasal prongs.
The patient was discharged from the hospital five days following the removal of two left thoracic drains, with no complications.
Microbiology results showed the pathogen implicated was streptococcus pneumoniae.
Results and Discussion
Positive pressure ventilation in patients with massive air leak or BPF poses significant challenges to intensive care and anesthetic physicians, as ventilations delivered to the lung pass directly to the pleural space, causing loss of tidal volume and lung atelectasis [1,2].
Major air leaks greater than 500 mL/breath are usually associated with a significant risk of high morbidity and mortality [3,4].
When the ventilatory needs of both the lungs differ, treating them as a single unit is detrimental [3,4].
Ventilatory waveforms enable the rapid diagnosis of air leaks in mechanically ventilated patients, where the expiratory limb of the volume tracing fails to return to zero baseline. It is important to distinguish an air leak in the pleural cavity from elsewhere in the ventilatory circuit or the LDT tube cuff [3,4].
Split lung ventilation is used to manage respiratory failure when there is marked difference in the pulmonary mechanics of the right and left lung due to unilateral lung pathology [5,6].
Differential independent lung ventilation permits individual titration of tidal volume, inspiratory flow, PEEP and FiO2 to each lung protecting the injured lung until the compliance and tidal volume nearly equalize [5,6].
Strategies aimed at decreasing peak inspiratory pressure and minute volume using permissive hypercapnia (potentiates hypoxic pulmonary vasoconstriction, helps to reduce shunt and improves perfusion of the ventilated lung) while minimizing tidal volumes (3-5 mL/kg), PEEP (0-5 cm H2O), shortening the inspiratory time (0.5-0.8 seconds), reducing the respiratory rate, plateau pressure <30 cm H2O and limiting negative intrapleural pressure would help in mechanical ventilation [4-6].
High-Frequency Jet Ventilation (HFJV) with permissive hypercapnia is another alternative strategy for improving gas exchange and avoids the need for a DLT or single lumen tube with a bronchial blocker [7,8]. HFJV avoid barotrauma to the unaffected lung and decrease air leakage. If HFJV is not available, a major air leak or BPF is a strict indication for splitlung ventilation. DLTs are preferred because it allows physiologic separation of both lungs if an air leak becomes severe enough to require varied ventilator modes or settings for each lung [3,4].
Some patients with major air leaks postoperatively may fail to ventilate or oxygenate despite the strategies being deployed, including differential independent lung ventilation and HFJV, which might lead to severe refractory hypoxemia and eventually necessitate Veno-Venous Extracorporeal Membrane Oxygenation (VV-ECMO) [9].
Conclusion
Mechanical respiratory support in the setting of unilateral lung pathology offers a unique challenge. The difference in compliance between the two lungs if exacerbated could lead to selective ventilation of the more compliant healthy lung and gradual collapse of the contralateral pathologic lung. This results in increased ventilation-perfusion mismatch and worsening shunt fraction which lead to hypoxemia. Attempts at rectifying the situation by increasing tidal volume or the application of PEEP may further aggravate the situation. As the more compliant healthy lung becomes hyperinflated, the increased airway pressure results in compression of the vascular bed and preferential redistribution of the pulmonary blood flow to the collapsed pathologic lung, the end result of which would be worsening ventilation-perfusion mismatch and hypoxemia.
Furthermore, cyclic hyperexpansion of the unaffected healthy lung may result in barotrauma. Differential selective independent lung ventilation has been advocated to avert this vicious cycle.
Historically, differential selective lung ventilation was introduced by Bjork and Carlens in 1949. Trew, Warren, and Potter described the use of two synchronized ventilators in the postoperative care after massive bleeding of a thoracic aneurysm into the left lung in 1977. Powner, Eross, and Grenvik were first to adopt this approach to the care of a patient with unilateral pneumonia in 1977. Parish, Gracey, Southorn, Pairolero and Wheeler described the successful use of synchronous differential lung ventilation in a patient with a large bronchopleural fistula after decortication of the right lung in 1984.
In an attempt to circumvent the need for two ventilators and a synchronization device, differential lung ventilation was achieved using one ventilator. PEEP was adjusted to the need of each lung through the use of separate PEEP valves connected to each exhalation port. An inline retardation device to the inspiratory circuit of the healthy lung was adjusted to redistribute more volume to the contralateral side. This method, however, might limit the respiratory rate and tidal volumes if a favorable inspiratory : expiratory ratio is to be maintained because of the prolonged inspiratory time created by the inline retardation device and the increased resistance to flow.
Another useful application of differential selective independent lung ventilation has been in the management of patients with bronchopleural fistulas. Mechanical respiratory support in this group of patients is often very difficult because of excessive air leak might prevent effective ventilation. Attempts at compensation by increasing minute ventilation might further exacerbate air leak. Because of excessive air leak using conventional ventilation and inability to maintain adequate gas exchange, a trial of differential independent lung ventilation was carried out using lower tidal volumes to the affected side and more conventional volumes to the contralateral healthy side. Adequate gas exchange and hemodynamic stability were achieved. The use of differential independent lung ventilation in combination with high frequency jet ventilation or application of continuous positive airway pressure to the involved side has also been described in this setting.
The use of two ventilators allows greater flexibility in adjusting the respiratory settings to each lung. Although some modern ventilators have built-in capacity for synchronous use, the theoretic concern of hemodynamic instability associated with asynchronization could be encountered. The key to avoid asynchronization and hemodynamic instability is to set similar respiratory rate on both ventilators and the patient should be adequately sedated and relaxed.
Resumption of conventional mechanical ventilation depends upon the resolution of the underlying pathologic process and the reduction of compliance differential between both lungs.
In summary, differential selective independent lung ventilation is a useful tool in the management of unilateral lung pathology. The limitations of this technique are primarily related to the use of the double-lumen tube. Indeed, the narrow internal diameter might lead to accumulation of secretions with prolonged use and limits its application in children. The second major concern relates to the potential for bronchial mucosal ischemia and future risk of bronchial stenosis. Bronchial rupture could be resulted from DLTs cuff overinflation. A useful approach entails the use of minimal bronchial cuff inflation pressure by gradual inflation of the cuff until the exhaled volume measured by an inline spirometer, equals the measured tidal volume. Even when such method is applied, it is advocated that bronchoscopy should be carried out after resumption of conventional mechanical ventilation to spot any complications. In addition to this, malposition of the endobronchial tube could easily obstruct the ipsilateral upper lobe bronchus, ongoing monitoring by bronchoscopy is therefore necessary.
References
- Berg S, Bittner EA, Berra L, Kacmarek RM, Sonny A. Independent lung ventilation: implementation strategies and review of literature. World J Crit Care Med. 2019; 8(4):49.
[Crossref] [Google Scholar] [PubMed]
- Grotberg JC, Hyzy RC, de Cardenas J. Bronchopleural fistula in the mechanically ventilated patient: a concise review. Crit Care Med. 2021; 49(2):292-301.
[Crossref] [Google Scholar] [PubMed]
- Pierson DJ, Luks AM, Parsons PE, Hollingsworth H. Management of bronchopleural fistula in patients on mechanical ventilation. 2012.
- Luks AM, Pierson DJ. Barotrauma and bronchopleural fistula. Principles and practice of mechanical ventilation. 23rd ed. Edited by Tobin MJ: New York, McGraw-Hill. 2012:943-961.
- Cheatham ML, Promes JT. Independent lung ventilation in the management of traumatic bronchopleural fistula. Am Surg. 2006; 72(6):530-533.
[Crossref] [Google Scholar] [PubMed]
- Darwish RS, Gilbert TB, Fahy BG. Management of a bronchopleural fistula using differential lung airway pressure release ventilation. J Cardiothorac Vasc Anesth. 2003; 17(6):744-746.
[Crossref] [Google Scholar] [PubMed]
- Mao R, Ying PQ, Xie D, Dai CY, Zha JY, Chen T, et al. Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: Experience of consecutive 13 cases in 9 years. J Thorac Dis. 2016; 8(7):1577.
[Crossref] [Google Scholar] [PubMed]
- Ha DV, Johnson D. High frequency oscillatory ventilation in the management of a high output bronchopleural fistula: A case report. Can J Anaesth. 2004; 51(1):78.
[Crossref] [Google Scholar] [PubMed]
- Garlick J, Maxson T, Imamura M, Green J, Prodhan P. Differential lung ventilation and venovenous extracorporeal membrane oxygenation for traumatic bronchopleural fistula. Ann Thorac Surg. 2013; 96(5):1859-1860.
[Crossref] [Google Scholar] [PubMed]
Citation: Bayoumy RE, Besnard M (2023) Synchronous Differential Independent Lung Ventilation Scenario: A Clinical Challenge. J Surg Anesth. 7:205.
Copyright: © 2023 Bayoumy RE, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
