Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 2
Survival, Growth Performance and Changes in Gonads of Oreochromis niloticus (Linnaeus, 1758) Fed with Mango (Mangifera indica) Leaves Meal Diet
Mutlen Melvin*, Zango Paul, Fotio Tchouppe Franck Juve, Kenne Sockeng Yannick and Tomedi Eyango MinetteReceived: 03-Feb-2024, Manuscript No. JARD-24-24812; Editor assigned: 05-Feb-2024, Pre QC No. JARD-24-24812 (PQ); Reviewed: 19-Feb-2024, QC No. JARD-24-24812; Revised: 26-Feb-2024, Manuscript No. JARD-24-24812 (R); Published: 04-Mar-2024, DOI: 10.35248/2155-9546.24.15.846
Abstract
The aim of the present study was to explore the possible use of Mangifera indica leaf powder as a reproductive inhibitor in Oreochromis niloticus. To this end, 450 juveniles with an average weight of 11.11 g ± 0.61 g were randomly distributed in 15 happas measuring 0.7 m × 0.7 m × 1 m placed in a tarpaulin tank with a usable volume of 12 m3, at a density of 30 juveniles per happa and subjected to natural temperature and light conditions and fed 4 experimental diets, formulated from a control diet to contain Mangifera indica leaf powder at doses of 2 g/kg, 4 g/kg, 6 g/kg and 8 g/kg feed respectively. After 60 days post-treatment, survival and zootechnical growth parameters were assessed. A descriptive examination of the gonads made it possible to determine the impact of the treatment on gonadal development at the end of this phase. The results show that the different treatments had a similar effect on survival (p˂0.05), i.e. an average value of 98.97% ± 0.69%. Comparative analysis of the growth characteristics of control group and group treated with different doses of Mangifera indica leaf powder reveals a significantly greater effect of the dose of 8 g/kg Mangifera indica leaf powder compared with the other treatments applied. On the other hand, analysis of the gonado somatic index at 60 days post-treatment of group treated with different doses of Mangifera indica leaf powder revealed a significantly higher effect of the 4 g/kg dose on the gonado somatic index in both males and females with respective mean values of 0.64 ± 0.08 (males) and 2.47 ± 0.12 (females). With regard to the morphological characteristics of the male and female gonads, gonad atrophy was observed in the entire group treated with the different doses of Mangifera indica powder, which justifies the low gonado somatic index values observed in these different treated batches compared with the control batches. These gonad atrophies observed in the treated group reflect the impact of the different treatments on gonad inhibition. Analysis of the weight and size of the male gonads shows a significantly greater effect of the control batches compared with the treated group, with mean values of 0.54 g ± 0.07 g and 4 mm ± 0.16 mm. In the females, fecundity and egg size were higher in the control group than in those treated with the different doses of Mangifera indica powder, with respective averages of 284 mm ± 3.05 mm and 3 mm ± 0.14 mm. Observations on egg colour showed that the normal olive green colour of Oreochromis niloticus eggs was not maintained in all treatments. The results of this study indicate that Mangifera indica leaves could be used as an alternative ecological method for inhibiting reproduction in Oreochromis niloticus.
Keywords
Oreochromis niloticus; Hibiscus rosa sinensis; Survival; Growth; Gonadal development
Introduction
The tilapia Oreochromis niloticus, Linnaeus, 1758, commonly known as “Nile tilapia”, is the most common fish farmed in tropical Africa. A warm-water, farmed fish, it is the mainstay of freshwater fish farming in the world’s intertropical belt [1,2]. Worldwide, tilapia is the second most farmed and produced group of fish with 3.49 Million tonnes (Mt) well after carp (24 Mt), followed by clarids with 2.97 Mt and salmonids with 2.36 Mt [3-7]. The nutritional value, which is rich in essential amino acids and fatty acids of good nutritional quality, tilapia O. nilotocus is very edible, with flesh that is much appreciated by consumers, making it a highly commercialised fish [8,9]. Tilapias possess all the valuable characteristics desirable of a good culture fish species, such as adaptability to environments, hardiness and acceptance of wide range of feed. However, because of its very high reproduction and precocity, tilapia O. niloticus is exposed to frequent cases of dwarfism and close inbreeding. This could have a negative impact on farm production yields [10,11].
To circumvent these constraints linked to anarchic reproduction and improve yields by producing high-growth individuals, various practices exist and have been developed, including manual sexing, polyculture with predatory species, culture of monosex male populations (by hormonal inversion either by administering androgens via the diet or by balneation, masculinisation via thermal shocks, hybridisation, genetic approaches to producing YY males or YY supermales); sterilization (through the use of irradiation, chemosterilants and other reproductive inhibitors), intermittent/ selective harvesting, the use of slow maturing tilapia species, among others [10,12-14]. However, all these population control methods have their limitations. It is therefore necessary to examine a less costly and appropriate technology to solve the problem of uncontrolled tilapia breeding using biological inhibitory agents. The search for alternative methods for control of reproduction has led to the consideration of the use of medicinal plants that have been successfully used to induce sterility in laboratory animals [15,16]. Indeed plant extracts contain various bioactive principles such as alkaloids, flavonoids, pigments, phenolics, terpenoids, steroids, essential oils which have been reported to promote various activities such as anti-stress, growth stimulation, appetite stimulation, tonicity and immunostimulant and antimicrobial properties during fish production. Therefore, plant extracts could be used as safe alternative agents to control tilapia early maturity and prolific reproduction in production systems by impairing fertility through gonads (testes and ovaries) destruction [17]. A study by Jegede et al, reported swollen spermatids nuclei, increased interstitial cells and focal necrosis in testes; and hydropic degeneration, ruptured follicles, granulomatous inflammation in the interstitium and necrosis ovaries when neem (Azadirachta indica) leaves were incorporated in Tilapia zilli basal diet at 2.0 gkg-1 [18]. Similar findings were reported in O. niloticus fed Carica papaya, Hibiscus rosa-sinensis, and Aloe vera as well as in O. mossambicus fed dietary Carica papaya and Moringa oleifera respectively [19-22].
Mangifera indica L., which belongs to the Anacardiaceae family, is a large evergreen tree of tropical and subtropical regions. Mangifera indica grows in the tropical and subtropical region and its parts are commonly used in folk medicine for a wide variety of remedies [23]. Different parts of mango have a broad range of medicinal properties, such as antimicrobial, antiviral, antifungal, anti-inflammatory, anti-diarrhoeal, antioxidant activity, antitumor, as well as immunomodulatory [24-31]. Mangoes leave exhibit exceptional biological, medicinal, and metabolic properties. Mangoes leaves are otherwise considered as a waste material generated mainly through the pruning of mango plants; in reality, they are a most significant resource containing a wide variety of bioactive compounds (phenolics and essential oils), crude protein, dietary fiber, minerals, and vitamins [32]. The various bioactive compounds present in the Mangoes leaves include phenolic compounds, flavonoids, benzophenones, sesquiterpenes, saponins, xanthones, tannins, terpenoids, and alkaloids [32]. Saponins occur in higher concentration compared to the other bioactive compounds, hence believed to be responsible for the antifertility effects of M. indica extracts [33]. Inclusion of M. indica leaf powder in Nile tilapia diets at dosages of 0.5 g-8.0 gkg-1 for 56 days reduced the number of spawned hatchlings, with complete inhibition of spawning observed at 2.0 gkg-1 of diet [34]. However, the work carried out by Obaroh et al, does not provide information on the action of the different treatments on gonadal development as well as on the growth and survival performance of O. niloticus, hence the interest of this study, which aims to assess the effect of the inclusion level of Mangifera indica leaf powder on the survival, growth and inhibition of gonadal development of juveniles of Oreochromis niloticus (Linn., 1758) [34].
Materials and Methods
Experimental site
The experiment took place from 14 February to 12 July 2022 at the Agro-aqua fish farm in the Lendi district of Douala V, Wouri Department, Littoral-Cameroon Region, with the following geographical coordinates: latitude 04°07’-04°03’N, longitude 09°37’–09°41’E and an average altitude of 60 m [35]. Douala has a humid equatorial coastal climate, influenced by the sea. There are two main seasons: A long rainy season lasting around 9 months (from March to November) and a short dry season lasting 3 months (from December to February). The average annual temperature hovers around 27.5°C, with 25.5°C in August and 28.9°C in February [35].
Collection and selection of animal material: 450 Oreochromis niloticus juveniles with an average weight of 11.11 ± 0.61 g from an initial stock from the Agro-aqua fish farm were used to carry out this work. Acclimatisation took place over 14 days. During this phase, the fish were fed ad libitum 3 times a day with a commercial feed name Gouessant containing 46% protein and 10% lipids until juveniles were produced. The photoperiod was 12L/12D. The physico-chemical parameters were monitored on a daily basis in order to resolve any problems that might affect the development or growth of the fish.
Harvesting and packaging of plant material: 3 kg of fresh Mangifera indica leaves were harvested in their natural habitat in the vicinity of the Agro-aqua fish production farm located in the Lendi district in the Douala V district. The collected leaves were cleaned and then dried for 21 days away from the sun, as drying in the sun could cause photoreactions that could alter the molecules of certain active ingredients [36]. The dried leaves were then ground to a powder using a mechanical mill. The powder obtained was stored in a hermetically sealed jar.
Preparation of experimental diets: Five experimental diets corresponding to the different treatments were prepared using a basal diet with the same crude protein composition (38%), prepared from specific ingredients including maize meal, fish meal, soybean meal, cottonseed meal, wheat bran, soybean oil and vitamin pre- mix. The proportions of the ingredients are given in Table 1. The protein composition was based on the protein requirements of O. niloticus juveniles as recommended by Lim et al [37]. One of the experimental feeds served as a control for this experiment. The other three were formulated from a control diet to contain Mangifera indica leaf powder at doses of 2 g/kg, 4 g/kg, 6 g/kg and 8 g/kg of feed respectively. The ingredients were mixed manually, and two-thirds of the total mass was wetted with warm water. The resulting mixture was granulated using a manual granulator to sizes of between 3 mm and 3.5 mm. The pellets obtained were dried in the shade at room temperature (around 28°C to 35°C) for 3 days. After complete drying, the pelleted feed was kept in labeled plastic containers and stored in the feed room in a dry place.
| Ingredients | Composition of experimental diets (g/kg of diet) | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| Fish meal | 26 | 26 | 26 | 26 | 26 |
| Soybean meal | 23 | 23 | 23 | 23 | 23 |
| Cotton cake | 22 | 22 | 22 | 22 | 22 |
| Wheat milling | 10 | 10 | 10 | 10 | 10 |
| Corn flour | 10 | 8 | 6 | 4 | 3 |
| Bone and shellfish meal | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Soya oil | 2 | 2 | 2 | 2 | 2 |
| Premix1 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Mangifera indica leaves powder | 0 | 2 | 4 | 6 | 8 |
| Total quantity in Kg | 100 | 100 | 100 | 100 | 100 |
| crude protein (%DM) | 38% | 38% | 38% | 38% | 38% |
Note: Premix1: A vit. (15 million I.U.), E vit. (15 mg), B1 vit. (1.0 mg), B12 vit. (5.0 mg), K3 vit. (2.5 mg), B6 vit. (2.0 mg), Pantothenic acid (10.0 mg), Folic acid (1.2 mg), Biotin (0.05 mg) and D3 vit. (3.0 million I.U.). Copper (7.0 mg), Manganese (100.0 mg), iodine (0.4 mg), Iron (40.0 mg), Zinc (50.0 mg), Selenium (0.15 mg) and anti- oxidant (125.0 mg).
Table 1: Centesimal composition of experimental diets.
Experimental procedure
450 Oreochromis niloticus juveniles with an average weight of 11.11 g ± 0.61 g were placed in 15 happas measuring 0.7 m × 0.7 m × 1 m placed in a 12 m3 tarpaulin tank at a density of 30 juveniles per happa and subjected to natural temperature and light conditions (Figure 1). A 50W RS electric aerator was installed in the tank to oxygenate the water. The quantity of feed distributed was set according to the average biomass of juveniles per week. The juveniles were fed 5% of their Ichtyo-biomass for the first four weeks of experimentation, and 4% for the last three weeks according to Mareck’s rationing table. The daily ration was divided into 3 meals, from 07:00 to 17:30 with an interval of 5.5 hours and adjusted each week according to the results of weekly population samples. The happas were washed every 3 days to prevent clogging by pond sediment [38]. Every morning and evening at 8 am and 4 pm respectively, the physico-chemical parameters of the water (temperature, pH, and dissolved oxygen) were taken. These parameters, which provide information on water quality, were monitored regularly to ensure optimum rearing conditions for Oreochromis niloticus juveniles. After 60 days post-treatment, a sample of 10 individuals (five males and five females) with respective mean weights of 43.28 g ± 0.5 g and 34.28 g ± 0.5 g was taken at random from one of the group subjected to each treatment in order to determine the sex ratio and the impact of the treatment on gonadal development. The sex ratio was assessed using the manual sexing method described by Pelebe et al [39]. For this purpose the subjects were previously euthanised by an overdose of benzocaїne (400 mg.L-1). After dissection, the gonads (testes and ovaries) were removed, and separated by treatment in order to avoid mix-ups and confusions of interpretation. These different organs were weighed in order to determine the gonadosomatic index of the different subjects according to the treatments.

Figure 1: Experimental set-up: made up of 15 happas (3 happas per treatment) measuring 0.7 m × 0.7 m × 1 m each randomly arranged in a 12 m3 tarpaulin tank. Notes: (T0;T0’;T0’’)=Control Treatment (Treatment with Mangifera indica powder at a dose of 0 g/kg of feed); (T1;T1’;T1’’): Treatment with Mangifera indica powder at a dose of 2 g/kg of feed; (T2;T2’;T2’’): Treatment with Mangifera indica powder at a dose of 4 g/kg of feed; (T3;T3’;T3’’)=Treatment with Mangifera indica powder at a dose of 6 g/kg of feed; (T4;T4’;T4’’)=Treatment with Mangifera indica powder at a dose of 8 g/kg of feed.
The gonadosomatic index was determined by the following formula:
Gonadosomatic Index (GSI)=(mass of the whole organ (g)/mass of the animal (g))*100
The various observations aimed at evaluating the impact of the treatments on gonadal development were made using an EduBlue binocular magnifying glass from pieces of gonad previously cut using a scalpel blade and then placed on graph paper. These observations were compared with those of the control group to determine the level of observable alterations.
The survival and growth of the fishes were monitored from the second week of experimentation, respectively by counting the dead individuals counted and by weighing a sample of 30 individuals taken at random from each of the treatments, at the end of the treatments and then every fortnight until the end of the experiments. The growth performance of Oreochromis niloticus juveniles at the end of this experiment (in terms of Average Weight Gain (AWG), Daily Weight Gain (DWG), Specific Growth Rate (SGR), Total Fish Length (TL), Condition Factor (CF) and Survival Rate (SR) were determined using the following formulae borrowed from various authors [20,40-44]. These various parameters were calculated at the end of the experiment.
• Average Weight Gain (AWG): AWG (g)=(Average Final Weight- Average Initial Weight) (g);
• Specific Growth Rate (SGR in%.day)=100(ln FAW-ln IAW). t-1 with IAW: Initial Average Weight(g); FAW: Final Average Weight (g);
• Food Conversion Ratio (FCR)=Rd. (Bf-Bi)-1 with Bi: Initial Biomass (g) Bf: Final Biomass (g) and Rd: Ration or quantity of feed consumed or distributed (g);
• Condition factor (K)=W × 100/ LT3 with W: weight (g), LT: Total length (cm).
• Survival Rate (%)=100 × (final number of individuals/initial number of individuals).
Statistical analysis
Results are expressed as mean ± standard deviation. The homoscedacity and normality of the data sets were first checked using Hartley’s test. Once the conditions of normality and homoscedacity had been met, a one-way analysis of variance (one- factor ANOVA) was used to analyse the differences between the treatments. 2 to 2 comparisons were made using Duncan’s multiple tests. Differences were considered significant at p˂05. Statistical tests were performed using SPSS version 18.0 software.
Results
Survival and growth characteristics of O. niloticus juveniles treated with different doses of Mangifera indica leaf powder
A comparative analysis at the end of the experimental phase of the different survival rates of Oreochromis niloticus juveniles in control group and group treated with different doses of Mangifera indica leaf powder (2 g/kg,4 g/kg, 6 g/kg and 8 g/kg) did not show a significant difference (p˂0.05) between treatments (Table 2). In fact, group treated with different doses of Mangifera indica and control group had a similar effect on the survival of Oreochromis niloticus juveniles, with an average survival rate of 98.97% ± 0.69%. These results show that the level of inclusion of Mangifera indica in the diet of Oreochromis niloticus juveniles does not significantly affect juvenile mortality.
| Dietary Mangifera indica powder g.kg-1 of diet | ||||||
|---|---|---|---|---|---|---|
| Parameters | 0(control) | 2 | 4 | 6 | 8 | P-value |
| IBW (g) | 11,11 ± 0.61 | 11,11 ± 0.61 | 11,11 ± 0.61 | 11,11 ± 0.61 | 11,11 ± 0,41 | - |
| FBW(g) | 31,23 ± 0,8bc | 36,54 ± 0,46a | 28,71 ± 0,2c | 34,7 ± 0,49b | 37,8 ± 0,34a | 0.032 |
| WG (g) | 20,12 ± 7,45c | 24,26 ± 8,88b | 17,9 ± 6,5d | 24,2 ± 8,27b | 27 ± 9,26a | 0.045 |
| ADG (g.day-1) | 0,75 ± 0,42c | 0,89 ± 0,53b | 0,66 ± 0,36d | 0,83 ± 0,5bc | 0,93 ± 0,54a | 0.036 |
| SGR (g.day-1) | 0,91 ± 0,47bc | 0,92 ± 0,38bc | 0,86 ± 0,47c | 0,98 ± 0,36b | 1,07 ± 0,52a | 0.013 |
| FCR | 1,72 ± 0,14a | 1,63 ± 0,23a | 1,67 ± 0,37a | 1,54 ± 0,12a | 1,41 ± 0,21a | 0.181 |
| CF (%g/cm3) | 1,67 ± 0,16a | 1,74 ± 0,23a | 1,75 ± 0,94a | 1,73 ± 0,14a | 1,87 ± 0,12a | 0.071 |
| SR (%) | 98.5 ± 1.04a | 98.61 ± 1.62a | 100 ± 0;0a | 97,77 ± 0,83a | 100 ± 0.0 | 0.414 |
Note: Data are expressed as means ± standard deviations. a,b,c,d: Values with the same superscripts of the same row are not significantly different (p>0.05). Where, IBW=Initial Body Weight; FBW=Final Body Weight; WG=Weight Gain; ADG=Average Daily Gain; SGR=Specific Growth Rate; FCR=Food Conversion Ratio; FL=Fish Length; CF=Condition Factor; SR=Survival Rate.
Table 2: Survival and growth parameters of Oreochromis niloticus juveniles treated with different doses of Mangifera indica leaf powder, compared with untreated group.
A comparative analysis of the main growth characteristics of the control group and group treated with different doses of Mangifera indica (2 g/kg, 4 g/kg, 6 g/kg and 8 g/kg) of the different progeny shows a significant difference (p˂0.05) between the treatments (Table 2). The group treated with 8 g/kg Mangifera indica leaf powder had a significantly greater effect than the other treatments in terms of final average weight (with an average of 37.8 g ± 0.34 g), average weight gain (with an average value of 27 g ± 9.26 g), average specific growth rate (with an average of 1.07%/d ± 0.52%/d) and average daily gain (with an average of 0.93 g/d ± 0.54 g/d). The poorest growth performance was recorded with the treatment containing 4 g/kg of Mangifera indica leaf powder in view of the values obtained for Average Weight Gain (an average of 17.9 g ± 6.5 g), Specific Growth Rate (with an average value of 0.86%/d ± 0.47%/d) and Average Daily Gain (0.66 g/d ± 0.36 g/d). These results show that the treatment with 8 g/kg of Mangifera indica leaf powder gave the best growth performance of the offspring compared with the other treatments. However, it should be noted that the different treatments had a similar effect (p˃0.05) on the feed conversion ratio and the Condition Coefficient (K).
Effect of the level of inclusion of Mangifera indica leaf powder on the inhibition of reproduction of O. niloticus juveniles
An analysis of the gonado somatic index at 60 days post-treatment of males and females from group treated with different doses of Mangifera indica leaf powder compared with control group shows a significant difference (p˂0.05) between treatments (Table 3). Male and female subjects treated with different doses of Mangifera indica leaf powder showed significantly (p<0.05) lower gonad/ mass weight ratios compared with subjects from control group.
| Dietary Mangifera indica Powder g.kg-1 of diet | GSI (%) | Testis average weight (g) | Testis average length (mm) | observations |
|---|---|---|---|---|
| 0(control) | 0.67 ± 0.19a | 0,54 ± 0.07a | 4 ± 0.16a | Testis atrophy |
| 2 | 0.61 ± 0.21ab | 0,38 ± 0.05b | 3,6 ± 0.24b | Testis atrophy |
| 4 | 0.64 ± 0.08a | 0,47 ± 0.03ab | 4,1 ± 0.12a | Testis atrophy |
| 6 | 0.54 ± 0.23b | 0,28 ± 0.09c | 3,6 ± 0.60ab | Testis atrophy |
| 8 | 0.47 ± 0.28c | 0,31 ± 0.01bc | 3,8 ± 0.17ab | Testis atrophy |
| p-value | 0.042 | 0.038 | 0.025 | - |
Note: Data are expressed as means ± standard deviations. a,b,c,d : Values with the same superscripts of the same column are not significantly different (p˂0.05).
Table 3: Gonado somatic index and characteristics description of testis of Oreochromis niloticus fed Mangifera indica diets.
This is reflected in the higher gonado somatic index values in the untreated subjects. Mean values were 0.67% ± 0.19% (males) and 2.54% ± 0.16% (females) respectively. However, analysis of group treated with different doses of Mangifera indica leaves revealed a significantly higher effect of the 4 g/kg dose on the gonado somatic index in both males and females, with mean values of 0.64 ± 0.08 (males) and 2.47 ± 0.12 (females) respectively. The lowest values were observed in individuals from group treated with the 8 g/kg dose, with mean values of 0.47 ± 0.28 (males) and 2.11 ± 0.14 (females) respectively.
Descriptive analysis of the male and female gonads and gametes from the various treated group compared with the control group at 60 days post-treatment reveals differences between the treatments (Table 3). Comparative analysis of the weight and size (length) of the gonads in samples from treated group and those from control group revealed higher averages for these two parameters in samples from control group, with respective averages of 0.54 g (weight) and 4 mm (length). The lowest averages were observed in samples from group treated with a dose of 6 g/kg, with respective values of 0.28 g (weight) and 3.6 mm (length). Morphological analysis of the gonads showed atrophy in all group treated with the different doses of Mangifera indica powder. Observation of female gonadal structures revealed a difference between treatments (Table 3). Subjects from the control groups showed two well-developed oocyte envelopes. However, in subjects treated with different doses of Mangifera indica, gonad atrophy was observed in all treated group. In addition to this gonad atrophy, malformation of the second ovarian envelope was also observed in embryos from group treated with 6 g/kg Mangifera indica powder. However, in fishes treated with 8 g/kg, this second oocyte envelope was non-existent. Analysis of the number of oocytes, oocyte diameter and oocyte colour also revealed differences between treatments. There was a significant difference between treatments (p˂0.05) in the number of oocytes. Samples from the control group had a higher average fecundity (284 ± 3.05), while those from the samples treated with 8 g/kg had the lowest, with an average value of 107 ± 1.32. Egg size analysis also showed a difference between treatments (p˂0.05). Samples from the control group had the largest mean egg size (33 mm ± 0.14 mm), while those from the samples treated with 8 g/kg had the smallest mean diameter, with a mean value of 1 mm ± 0.03 mm. Observations on oocyte colour revealed a difference between treatments. The samples from the control group and those from the group treated with 2 g/kg and 4 kg respectively showed oocytes with a yellowish colour. On the other hand, samples from group treated with doses of 4 g/kg and 8 g/kg respectively showed whitish-coloured oocytes, indicating that the treatment dose had an impact on oocyte quality (Figure 2).
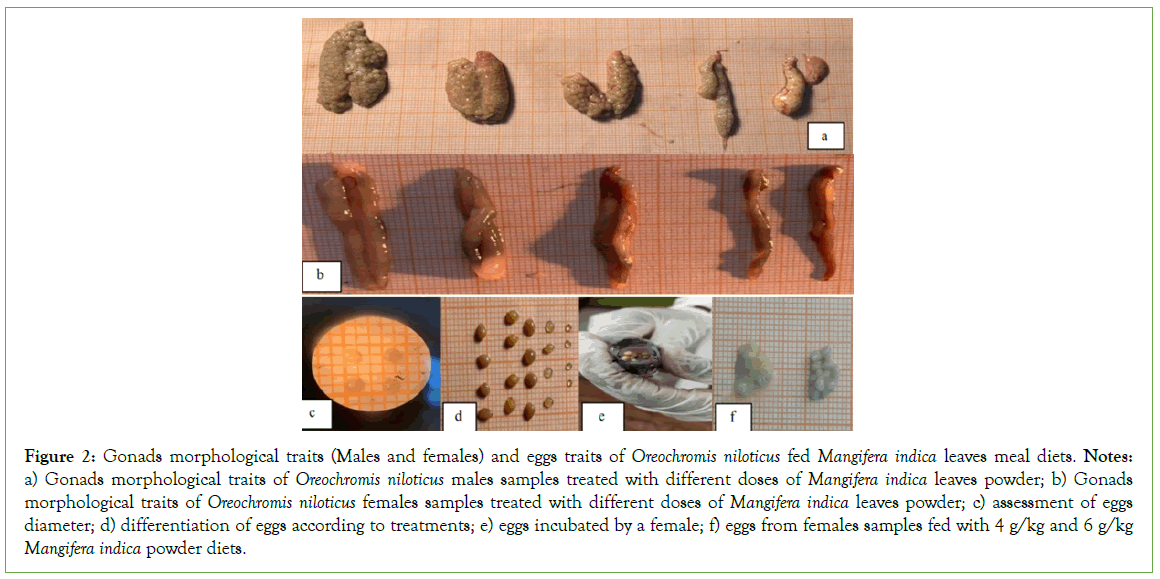
Figure 2: Gonads morphological traits (Males and females) and eggs traits of Oreochromis niloticus fed Mangiferaindica leaves meal diets. Notes: a) Gonads morphological traits of Oreochromis niloticus males samples treated with different doses of Mangifera indica leaves powder; b) Gonads morphological traits of Oreochromis niloticus females samples treated with different doses of Mangifera indica leaves powder; c) assessment of eggs diameter; d) differentiation of eggs according to treatments; e) eggs incubated by a female; f) eggs from females samples fed with 4 g/kg and 6 g/kg Mangifera indica powder diets.
Discussion
The main physical and chemical parameters measured during this experiment were temperature, pH and dissolved oxygen. The average values for temperature, pH and dissolved oxygen remained relatively stable during the experimental period. In fact, the temperature values oscillated in the average range of 27°C ± 0.25°C and 28.1°C ± 0.45°C, while the pH values ranged from 6.9 ± 03 to 8.1 ± 0. The average dissolved oxygen values varied from 5.03 mg/l ± 0.8 mg/l to 5.41 mg/l ± 0.72 mg/l. These main temperature and dissolved oxygen values presented are within the acceptable norms for rearing O. niloticus as reported by Omitoyin et al, since the optimum temperature for growth of O. niloticus is between 24°C and 28°C, while the pH is between 7-8. The optimum dissolved oxygen concentration is 5 mg/l [45,46].
Analysis of the different survival rates at 60 days post-treatment of Oreochromis niloticus juveniles from control group and group treated with different doses (i.e. 2 mg/kg, 4 mg/kg, 6 mg/kg and 8 mg/kg respectively) of Mangifera indica leaf powder showed no significant difference (p˂0.05) between treatments. The mean value of the survival rate obtained was 98.97 ± 0.69%. These high values of the survival rates in the entire group treated with different doses of Mangifera indica leaf powder as well as the control group show that these treatments could not have a deleterious effect on the survival of the various offspring. These results are superior to those obtained by Diyaware et al, whose work aimed to evaluate the effect of graduated levels of Faldhabia albida leaf meal on the growth and reproduction indices of Oreochromis niloticus, i.e. survival rate values ranging from 90.00% ± 0.00% to 96.66% ± 3.33% [47]. This difference could be associated with various factors, in particular the plant material used (Mangifera indica leaves for our experiments, Faldhabia albida leaves for Diyaware et al); the difference in age of the subjects used, but also the types of treatments applied, which would have a differential effect on the survival of the offspring [47].
Comparative analysis of the growth characteristics of control group and group treated with different doses of Mangifera indica leaf powder revealed a significant (p˂0.05) greater effect of the 8 g/ kg dose of Mangifera indica leaf powder compared with the other treatments applied. In view of the mean values of the growth parameters obtained, in particular 37.8 g ± 0.34 g for the mean final weight, 27 g ± 9.26 g for the mean weight gain, 1.07%/d ± 0.52%/d for the mean specific growth rate and 0.93 g/d ± 0.54 g/d for the mean daily gain. These different growth parameter values are higher than those obtained by Ugonna et al, in O. niloticus fry fed a diet supplemented with Carica papaya seed meal at similar doses [48]. The respective averages were 0.021 g/d ± 0.001 g/d and 3.56%/d ± 0.02%/d for Average Weight Gain (AWG) and Specific growth Rates (SGR). This difference could be associated with the types of treatments applied, which would have a differential effect on the growth performance of the offspring. But also to the age of the subjects (juveniles for our experiments and fry for Ugonna et al) and the duration of the treatment (60 Days for our experiments and 28 Days for Ugonna et al.) [48]. However, the work of Ugonna et al. revealed a significantly greater effect of control group on growth performance compared with group treated with Carica papaya seed meal [48].
An analysis of the somatic gonad index at 60 days post-treatment of males and females from group treated with different doses of Mangifera indica leaf powder compared with control group showed a significant difference (p˂0.05) between treatments. Male and female samples treated with different doses of Mangifera indica leaf powder showed significantly (p<0.05) lower gonad/mass weight ratios compared with subjects from control batches. The respective mean values were 0.67% ± 0.19% (males) and 2.54% ± 0.16% (females). However, analysis of the group treated with different doses of Mangifera indica leaves revealed a significantly higher effect of the 4 g/ kg dose on the gonado somatic index in both males and females, with mean values of 0.64 ± 0.08 (males) and 2.47 ± 0.12 (females) respectively (Table 4). This lower ratio in individuals from group treated with different doses of Mangifera indica leaf powder reflects an inhibition of gonadal development in male and female individuals. These results indicate a dose-dependent effect depending on the treatment and the gonad weight/mass ratio. Although these results are superior to those obtained by Yadave et al, in O. niloticus fry fed a diet supplemented with Carica papaya seed powder (i.e. mean gonado somatic index values ranging from 0.1% ± 0.00% to 0.2% ± 0.01%) are in agreement with his hypothesis that as the extract dose increases, the gonado somatic index value decreases [49]. This difference could be associated with various factors, in particular the plant material used (Mangifera indica leaves for our experiments, Carica papaya seeds for Yadave et al, the age of the subjects (juveniles for our experiments and fry for Yadave et al, but also to the different doses applied, which would have a different effect on the gonad weight/mass ratio [49].
| Dietary Mangifera indica Powder g.kg-1 of diet | GSI (%) | Fecundity | Egg size (mm) | Eggs colour and Shape | observations |
|---|---|---|---|---|---|
| 0 (control) | 2.54 ± 0.16a | 284 ± 3.05a | 3 ± 0.14a | Yellowish eggs, Oval shape | Two well-developed bilateral lobes ovaries |
| 2 | 2.28 ± 0.31c | 173 ± 2.41b | 2 ± 0.06b | Yellowish eggs, Oval shape |
Ovaries atrophy |
| 4 | 2.47 ± 0.12b | 154 ± 2.01bc | 2 ± 0.04b | Yellowish eggs, Oval shape |
Ovaries atrophy |
| 6 | 2.19 ± 0.34d | 179 ± 1.96b | 1,5 ± 0.02bc | Whitish eggs, Oval shape | Malformation of the second bilateral lobes ovaries; ovaries atrophy |
| 8 | 2.11 ± 0.14e | 107 ± 1.32c | 1 ± 0.03c | Whitish eggs, Oval shape |
Absence of the second bilateral lobes; ovaries atrophy |
| p-value | 0.046 | 0.033 | 0.031 | - | - |
Note: Data are expressed as means ± standard deviations. a,b,c,d,e: Values with the same superscripts of the same column are not significantly different (p˂0.05).
Table 4: Gonado somatic index and characteristics description of testis of Oreochromis niloticus fed Mangifera indica diets.
Descriptive analysis of the male and female gonads of the samples from the different treated group compared with the control group reveals a difference between the treatments. Analysis of the weight and size of the male gonads shows a significantly greater effect of the control group compared with the treated group, with mean values of 0.54 g ± 0.07 g and 4 mm ± 0.16 mm. Analysis of the morphological characteristics of the male and female gonads revealed atrophy of the gonads in all the group treated with the different doses of Mangifera indica powder. These observations are similar to those of Kushwaha et al, on Oreochroms niloticus juveniles fed diets supplemented with different doses of Aloe vera latex [50]. The gonad atrophies observed in the treated group reflect the impact of the various treatments on gonad inhibition. In addition to the atrophy, other morphological differences were observed in the females. Subjects from the control group showed two well- developed oocyte envelopes. However, subjects treated with 6 g/ kg Mangifera indica powder showed malformation of the second oocyte envelope, whereas subjects treated with 8 g/kg Mangifera indica powder showed no second oocyte envelope. Analysis of fertility and egg size showed a significant difference between treatments (p˂0.05). Subjects from control group showed higher fertility and average egg size than those from treated group, with respective averages of 284 mm ± 3.05 mm and 3 mm ± 0.14 mm. These results, although lower than those obtained by Kushwaha et al, on O. niloticus juveniles fed diets supplemented with different doses of Aloe vera latex (i.e. respective averages of 258.0 mm ± 2.12 mm (fecundity) and 2.33 mm ± 1.77 mm (oocyte size)), are in line with his hypothesis that as the dose increases, fecundity decreases [50]. This result is in agreement with Coward et al who reported that eggs produced by incubating females of O. niloticus normally exceed 2 mm in diameter and that fecundity is generally less than 350 in these females [51]. However, morphological observations of eggs from the various treatments did not reveal any deleterious changes in egg shape. In fact, a normal oval egg shape was observed. This observation corroborates the study carried out on Tilapia [52]. Analysis of oocyte colouration showed a difference between treatments. Subjects from control group as well as those treated at doses of 2 g/kg and 4 g/kg showed a yellowish coloration. On the other hand, oocytes from group treated with 4 g/kg and 8 g/ kg respectively were whitish in colour. These observations show that the normal olive green colour of O. niloticus eggs was not maintained as stipulated by Kushwaha et al [50]. This difference in color reveals an impact of the treatment dose on the quality of the oocytes.
Conclusion
The aim of the present study was to evaluate the effect of the inclusion level of Mangifera indica leaf powder on the survival, growth performance and inhibition of gonad development of O. niloticus juveniles. This work showed that the dose of extract did not significantly affect juvenile mortality. Comparative analysis of the growth characteristics of control group and group treated with different doses of Mangifera indica leaf powder revealed a significantly greater effect of the dose of 8 g/kg of Mangifera indica leaf powder compared with the other treatments applied. However, analysis of the gonado somatic index at 60 days post-treatment of batches treated with different doses of Mangifera indica leaf powder revealed a significantly higher effect of the 4 g/ kg dose on the gonado somatic index in both males and females, with mean values of 0.64 ± 0.08 (males) and 2.47 ± 0.12 (females) respectively. With regard to the morphological characteristics of the male and female gonads, gonad atrophy was observed in all group treated with the different doses of Mangifera indica powder, which justifies the low gonado somatic index values observed in these different treated batches compared with the control batches. These gonad atrophies observed in the treated batches reflect the impact of the different treatments on gonad inhibition. Analysis of the weight and size of the male gonads showed a significantly greater effect of the control group compared with the treated group, with mean values of 0.54 ± 0.07 g and 4 ± 0.16 mm. In females, fecundity and egg size were higher in subjects from control group compared to those treated with different doses of Mangifera indica powder, with respective mean values of 284 ± 3.05 and 3 ± 0.14 mm. Observations on egg colour show that the normal olive green colour of O. niloticus eggs was not maintained in all treatments. This difference in colour reveals an impact of the treatment dose on the quality of the oocytes.
Acknowledgement
The authors would like to thank Mr. Kenne Sockeng Yannick, promoter of the Agro-aqua fish farm, for making the facilities of his farm available to us for this research work. We would also like to thank Mr. Fotio Tchouppe Franck Juve, Halieute Design Engineer, for his contribution to this activity.
Author Contributions
• Conceptualization: L.V. and M.S.A
• Methodology: A.S, V.R.A.S, and R.A.C
• Software: A.S
• Validation: C.A.B.L, R.L.V, M.S.A
• Formal analysis, L.V
• Investigation: M.S.A
• Resources: L.V, A.S, V.R.A.S
• Data curation: B. K
• Writing-original draft preparation: L.V
• Writing-review and Editing: K.W, B. K. and M.S.A
• Supervision: B.K. and M.S.A
Conflicts of Interest
There are no conflicts of interest.
Funding
We confirm that there was no funding for this study. It was self- financed by the authors.
References
- Meyer C. Dictionnaire des sciences animales. CIRAD, Montpellier, France. 2013.
- Sayed E, Abdel-Fattah M. Tilapia culture. Cabi Publishing, London, UK. 294.2006
- Burel C, Médale F. Quid de l’utilisation des protéines d’origine végétale en aquaculture?. OCL. 2014;21(4):D406.
- Trosvik KA, Rawles SD, Thompson KR, Metts LA, Gannam A, Twibell R, et al. Growth and body composition of Nile tilapia, Oreochromis niloticus, fry fed organic diets containing yeast extract and soybean meal as replacements for fish meal, with and without supplemental lysine and methionine. J World Aquac Soc. 2012;43(5):635-647.
- FAO (Food and Agricultural Organization of the United Nations). La situation mondiale des pêches et de l’aquaculture. Rome, Italie. 2012.
- FAO (Food and Agricultural Organization of the United Nations). Globefish. FAO, Rome, Italy. 2010.
- El-Sayed AF. Alternative dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture. 179(1-4):149-168.
- Lovell, T. Opportunities in aquaculture nutrition: Practical considerations in making tilapia feeds. Feed Management 46:13-22.
- Lovell T. Nutrition and feeding of fish. New York: Van Nostrand Reinhold. 1989.
- Lim C. Practical feeding-tilapias. Nutrition and feeding of fish. 1989:163-183.
- Mair GC, Abucay JS, Beardmore JA, Skibinski DO. Growth performance trials of genetically male tilapia (GMT) derived from YY-males in Oreochromis niloticus L.: On station comparisons with mixed sex and sex reversed male populations. Aquaculture. 1995;137(1-4):313-323.
- Hickling CF. The Malacca tilapia hybrids. J Genet. 1960;57:1-0.
- Baroiller JF, D'Cotta H. Environment and sex determination in farmed fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;130(4):399-409.
[Crossref] [Google Scholar] [PubMed]
- Akin-Obasola BJ, Jegede T. Reproduction control of male Oreochromis niloticus (Nile tilapia) using Gossypium herbaceum (cotton) root bark meals as fertility inhibitor. Eur Sci J. 2016;12(12).
- Bodhankar SL, Garg SK, Mathur VS. Antifertility screening of plants. Part IX. Effect of five indigenous plants on early pregnancy in female albino rats. Indian J Med Res. 1974;62(6):831-837.
[Crossref] [Google Scholar] [PubMed]
- Das RP. Effect of papaya seed on the genital organs & fertility of male rats. Indian J Exp Biol. 1980;18(4):408-409.
[Google Scholar] [PubMed]
- Gabriel NN, Qiang Jun QJ, Kpundeh MD, Xu Pao XP. Use of herbal extracts for controlling reproduction in tilapia culture: Trends and prospects-A review.
- Jegede T, Fagbenro O. Histology of gonads in Oreochromis niloticus (Trewavas) fed pawpaw (Carica papaya) seed meal diets. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture. 2008;1135-1141.
- Abdelhak ME, Madkour FF, Ibrahim MA, Sharaf MS, Sharaf MM, Mohammed DA. Effects of pawpaw, Carica papaya seeds meal on the productive performance and histological characters of gonads in Nile tilapia, Oreochromis niloticus. International Journal of Aquaculture Research. 2013;3(12):34-37.
- Jegede T. Control of reproduction in Oreochromis niloticus (Linnaeus 1758) using Hibiscus rosa-sinensis (Linn.) leaf meal as reproduction inhibitor. J Agric Sci. 2010;2(4):149.
- Jegede T. Effects of Aloe vera (Liliaceae) on the gonad development in Nile tilapia, Oreochromis niloticus (Linnaeus 1758). 2011.
- Ampofo-Yeboah A. Effect of phytogenic feed additives on gonadal development in Mozambique tilapia (Oreochromis mossambicus). PhD (Agric) thesis), University of Stellenbosch, South Africa. 2013.
- Coe FG, Anderson GJ. Screening of medicinal plants used by the Garifuna of Eastern Nicaragua for bioactive compounds. J Ethnopharmacol. 1996;53(1):29-50.
[Crossref] [Google Scholar] [PubMed]
- Kabuki T, Nakajima H, Arai M, Ueda S, Kuwabara Y, Dosako SI. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000;71(1):61-66.
- Keita Y, Kone O, Karim LYA, Hakkinen V. Chemical and antibacterial activity of some Guinean mango varieties distillates. Comptes Rendus Chimie. 2004;7(10e11):1095e100.
- Cojocaru M, Droby S, Glotter E, Goldman A, Gottlieb HE, Jacoby B, et al. 5-(12-Heptadecenyl)-resorcinol, the major component of the antifungal activity in the peel of mango fruit. Phytochemistry. 1986;25(5):1093-1095.
- Garrido G, González D, Lemus Y, Garcıa D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG®). Pharmacol Res. 2004;50(2):143-149.
[Crossref] [Google Scholar] [PubMed]
- Sairam K, Hemalatha S, Kumar A, Srinivasan T, Ganesh J, Shankar M, et al. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J Ethnopharmacol. 2003;84(1):11-15.
[Crossref] [Google Scholar] [PubMed]
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71(1-2):23-43.
[Crossref] [Google Scholar] [PubMed]
- Anila L, Vijayalakshmi NR. Antioxidant action of flavonoids from Mangifera indica and Emblica officinalis in hypercholesterolemic rats. Food Chem. 2003;83(4):569-574.
- Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78(2-3):133-137.
[Crossref] [Google Scholar] [PubMed]
- Kumar M, Saurabh V, Tomar M, Hasan M, Changan S, Sasi M, et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10(2):299.
[Crossref] [Google Scholar] [PubMed]
- Obaroh IO, Nzeh GC, Oguntoye SO. Control of reproduction in Oreochromis niloticus (L) using crude extract of Azadirachta indica saponin. Adv Environ Biol. 2012;6(4):1353-1356.
- Obaroh IO, Nzeh GC. Antifertility effect of some plant leaf extracts on the prolific breeding of Oreochromis niloticus. Acad J Interdiscip Stud. 2013;2(12):87.
- Njueya AK, Likeng JD, Nono A. Hydrodynamique et qualité des eaux souterraines dans le bassin sédimentaire de Douala (Cameroun): Cas des aquifères sur formations quaternaires et tertiaires. Int J Biol Chem Sci. 2012;6(4):1874-94.
- Diarra MN. Etude phytochimique d'une plante antipaludique utilisée au Mali: Spilanthes oleracea Jacq (Asteraceae). Doctoral dissertation, Université de Bamako.
- Lim CE, Webster CD, Li MH. Feeding Practices: Tilapia Biology, Culture, and Nutrition, Food Products Press, New York. 2006:547-559.
- Eduardo Ferrari Sanches L, Hayashi C. Effect of feeding frequency on Nile tilapia, Oreochromis niloticus (L.) fries performance during sex reversal in hapas. Acta Sci Anim Sci. 2001:871-876.
- Pelebe Orobiyi ER. Analyse histologique des gonades, du foie, du rein et de l’état physiologique du tilapia du Nil Oreochromis niloticus exposé aux pesticides agricoles dans les retenues d’eau du Nord-Bénin. Biol. Anim. 2016. ffdumas-02878839f.
- Akinwande A, Dada A, Moody F. Effect of dietary administration of the phytochemical “Genistein”(3,5,7,3,4 Pentahydroxyflavone) on masculine tilapia. Oreochromis niloticus. 2011:2231-2233.
- Khalil WK, Hasheesh WS, Marie MA, Abbas HH, Zahran EA. Assessment the impact of 17 α -methyltestosterone hormone on growth, hormone concentration, molecular and histopathological changes in muscles and testis of Nile tilapia; Oreochromis niloticus. J. Life Sci. 2011;8(3):329-343.
- Kefi AS, Kang’ombe J, Kassam D, Katongo C. Growth, reproduction and sex ratios in Oreochromis andersonii (Castelnau, 1861) fed with varying Levels of 17 α -methyl testosterone. J Aquac Res Dev. 2012;3:130-137.
- Ogunji JO, Wirth M. Partial replacement of fish meal with some alternative protein sources in the diet of Tilapia Oreochromis niloticus (Linn). Isr. J. Aquac.-Bamidgeh. 2001;53(1):34-43.
- Ahmad M, Abdel-Tawwab M, Shalaby A, Khattab Y. Effects of 17 α -methyltestosterone on growth performance and some physiological changes of Nile tilapia, Oreochromis niloticus L.) Fingerllngs. Egypt J Aquat Biol Fish. 2002;6(2):1-23.
- Omitoyin BO. Introduction to fish farming in Nigeria. Ibadan University Press; 2007.
- Malcolm C, Beveridje H, Andrew BJ Mc. Tilapias: Biologie and exploitation. Institute of aquaculture. University of stirling, Scotland.
- Diyaware MY. Effect of graded levels of Faldhabia albida leave meal on growth and reproductive indices of the Nile tilapia (Oreochromis niloticus, Linnaeus 1758). 2018
- Ugonna BO, Solomon SG, Olufeagba SO, Okomoda VT. Effect of Pawpaw Carica papaya seed meal on growth and as a natural sex‐reversal agent for Nile tilapia. N Am J Aquac. 2018;80(3):278-285.
- Yadav CN, Pandit NP, Jha DK, Gharti K. Study of effect of papaya seed on reproductive performance in Nile tilapia (Oreochromis niloticus). Int J of Agricu and App Sci. 2021;2(1):151-158.
- Kushwaha MP. Effects of Aloe vera (Liliaceae) on Gonad development in Nile tilapia, Oreochromis niloticus (L.) during intensive aquaculture. Int J Fish Aquat. 2013;1(2):56-60.
- Coward K, Bromage NR. Reproductive physiology of female tilapia broodstock. Rev Fish Biol Fish. 2000;10:1-25.
- Jegede T. Effects of Aloe vera (Liliaceae) on the gonad development in Nile tilapia, Oreochromis niloticus (Linnaeus 1758). Better Science, Better Fish, Better Life. 2011.
Citation: Melvin M, Paul Z, Juve FTF, Yannick KS, Minette TE (2024) Survival, Growth Performance and Changes in Gonads of Oreochromis niloticus (Linnaeus, 1758) Fed with Mango (Mangifera indica) Leaves Meal Diet. J Aquac Res Dev. 15:846.
Copyright: © 2024 Melvin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : We confirm that there was no funding for this study. It was self financed by the authors

