Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2024) Volume 12, Issue 4
Surgical Treatment of Symptomatic Large Aortic Aneurysm with Horseshoe Kidney
Milton Sérgio Bohatch Júnior1*, Alexandre Maiera Anacleto1, Beatriz Camelini Moreno2, Andressa Hellen Nora da Silva2, Fernando Reis Neto2, Marcia Maria Morales1 and Jose Maria Pereira de Godoy22Department of Vascular Surgery, Invase Vascular Surgery Institute, Sao Jose do Rio Preto, Sao Paulo, Brazil
Received: 11-Jun-2024, Manuscript No. JVMS-24-25965; Editor assigned: 14-Jun-2024, Pre QC No. JVMS-24-25965 (PQ); Reviewed: 28-Jun-2024, QC No. JVMS-24-25965; Revised: 05-Jul-2024, Manuscript No. JVMS-24-25965 (R); Published: 12-Jul-2024, DOI: 10.35248/2329-6925.24.12.561
Abstract
The association between Abdominal Aortic Aneurysm (AAA) and Horseshoe Kidney (HK) is rare, found in approximately 0.1% of aneurysm correction surgeries. The open surgical approach represents a challenge requiring consideration of three critical technical aspects, given each patient's unique anatomy: Type of exposure to AAA, the need to section the renal isthmus and whether to preserve the Accessory Renal Artery (ARA). We describe an open transperitoneal repair of a giant AAA with preservation of the isthmus and ARA.
Keywords
Horseshoe kidney; Abdominal aortic aneurysm; Anatomical pathological condition
Introduction
Horseshoe Kidney (HK) is the most common congenital abnormality of the urologic system, with a population incidence of up to 0.25% and a male preponderance of 2:1 [1,2]. HK is characterized by three anatomical anomalies (ectopia, malrotation and variation in vascular supply) [3-5]. The association between Abdominal Aortic Aneurysm (AAA) and HK is rare, found in approximately 0.1% of AAA surgeries [2]. The position of the isthmus over the aneurysm, the anatomical anomalies of the urinary collector system, and renal vascularization make AAA repair challenging [4,6]. Bibliographic data are limited to mainly case reports or small case series [1]. In the present case, we describe an open transperitoneal repair of a giant AAA with preservation of the isthmus and ARA.
Case Presentation
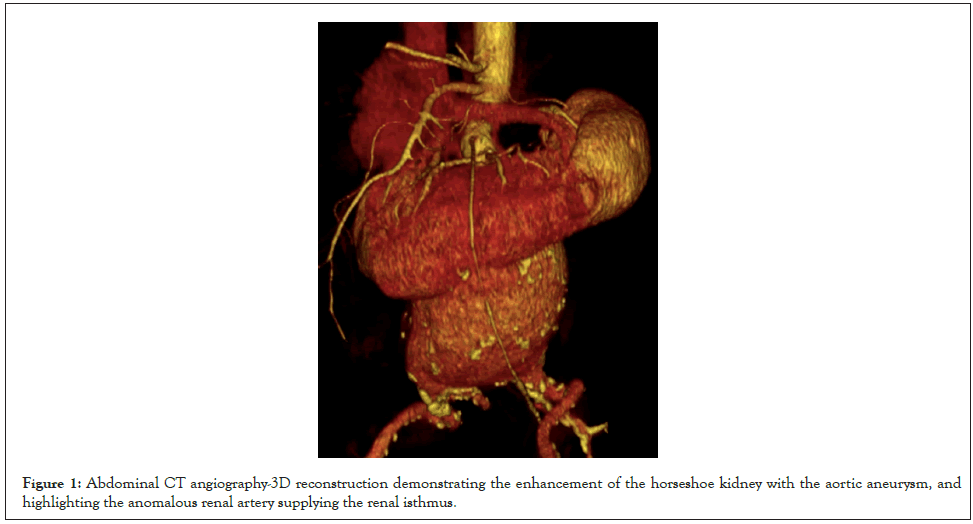
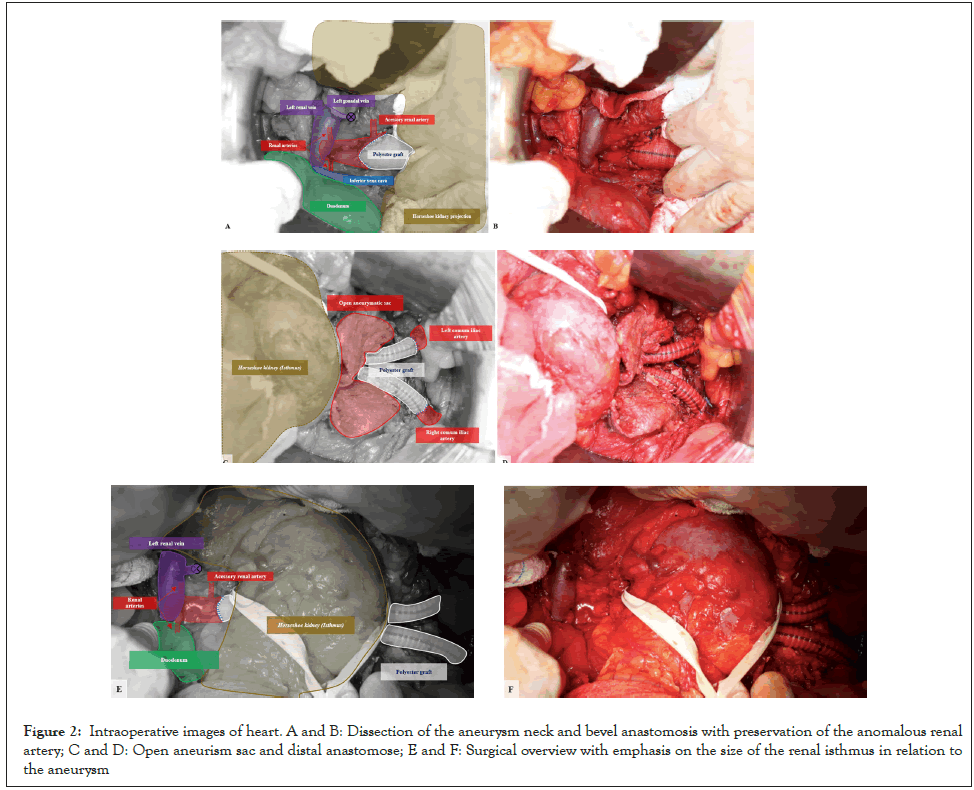
Male, 64 years old, with a history of hypertension, coronary artery disease, and smoking. Patient was referred with a history of AAA and abdominal pain. On examination, a painful pulsatile mass on palpation of the abdomen was observed. The CT angiography showed a 10 cm infrarenal AAA and HK (Figure 1). The patient underwent surgical correction with a transperitoneal approach and interposition of an aortoiliac graft with an 18 mm × 9 mm polyester graft. The supply of the ARA was ensured with a bevel anastomosis of the aorta (Figures 2A and 2B). For adequate access to the aneurysm, it was necessary to dissect and repair the renal isthmus, enabling its mobilization (Figures 2C-2F). The surgical time was 6 hours, and a transfusion of 3 red blood cell concentrates, and two plasmas was performed. A total bleeding of 2900 ml was collected in the cell saver, and there was no need for vasoactive drugs. On the second postoperative day, an exploratory laparotomy was performed due to ischemic colitis, but no visceral resection was necessary. An acute kidney injury was resolved within four days (creatinine 2.4 mg/dL; baseline creatinine 1.4 mg/dL). After five days in the ICU, the patient was discharged on the eighth postoperative day. The patient authorized the case description and signed the consent form.
Figure 1: Abdominal CT angiography-3D reconstruction demonstrating the enhancement of the horseshoe kidney with the aortic aneurysm, and highlighting the anomalous renal artery supplying the renal isthmus.
Figure 2: Intraoperative images of heart. A and B: Dissection of the aneurysm neck and bevel anastomosis with preservation of the anomalous renal artery; C and D: Open aneurism sac and distal anastomose; E and F: Surgical overview with emphasis on the size of the renal isthmus in relation to the aneurysm.
Results and Discussion
The first accounts of HK date back to 1521 autopsy reports by Jacobo Berengario da Carpi [7,8]. When treating patients with a AAA and concurrent HK, the classification developed by Eisendrath, et al., in 1925 is most frequently employed (Table 1) [8,9]. The coexistence of HK and the AAA was first published in 1956. In the same year, Phelan, et al., performed the first successful AAA repair with mobilization of the isthmus and ligation of the ARA [10].
| Eisendrath’s classification | |
|---|---|
| Type I | One renal artery can be found on each side of the HK |
| Type II | In addition to type I, there is an auxiliary aortic branch to the renal isthmus |
| Type III | Inserts one more renal artery to each side of type II |
| Type IV | Two renal arteries exist on each side and at least one comes from the isthmus branch or the iliac arteries |
| Type V | Multiple renal arteries originating from the iliac, mesenteric and aortic arteries are present |
Table 1: The classification of Eisendrath, et al., HK: Horseshoe Kidney [9].
One of their most notable traits is their diverse range of aberrant renal vasculature, with ARA originating from the mesenteric, iliac, or aorta arteries [3]. Three-dimensional CT angiography offers precise information about the HK's vascularization, which is crucial to determining the optimal therapeutic approach [11]. Renal artery anatomy is normal in just 21.9% of people [1]. In this case, the patient was classified as a type II by Eisendrath, et al., with a single 7 mm diameter ARA located 8 mm away from the aneurysm neck. The ARA irrigated at least two-thirds of the left kidney and the lower pole of the right kidney, making an attempt at endovascular repair difficult [9].
Until today, there is no recognized gold standard for handling an AAA linked to HK [12]. The open surgical approach represents a challenge requiring consideration of three critical technical aspects, given each patient's unique anatomy: Type of exposure to AAA, the need to section the renal isthmus, and whether to preserve the ARA. These characteristics all present various challenges [3,8].
Most vascular surgeons are familiar with the transperitoneal approach, which provides optimal exposure to the vascular and renal architecture. It also enables fast proximal and distal vascular management in emergencies of ruptured aortic aneurysm [3,13]. Additionally, it allows for assessing intestinal vitality, provides access to the suprarenal aorta and the iliac bifurcation, and can be converted into retroperitoneal access with a left medial visceral rotation [1,8,12,13]. The procedure's drawbacks include the risk of postoperative ileus and its potential unsuitability for individuals with a hostile abdomen or an inflammatory aneurysm [13]. In these cases, the retroperitoneal technique can assist in seeing the AAA [1]. Additional benefits of the retroperitoneal technique include avoiding kidney dissection, preventing involvement with the urinary tract, and reducing the likelihood of iatrogenic harm [2,4,8]. Nevertheless, this method is restricted to reaching the right renal artery and iliac bifurcation, and the denervation of the lateral abdominal wall muscles may result in heightened postoperative discomfort and a bulging flank [11,13].
The second significant technical challenge is the presence of a centrally located renal isthmus, which forms due to an atypical movement of metanephric cells during the fourth and sixth weeks of embryonic development [11]. The isthmus may contain either functional parenchymal tissue (85%) or fibrous tissue (15%) [8,11]. If the isthmus consists of fibrotic, non-functional tissue, sectioning is considered the optimal choice when using a transperitoneal technique since maintaining it might hinder proper exposure to the AAA [2]. Nevertheless, it is advisable to refrain from using it if the tissue is functional or vascularized. Although performing the isthmus transection may result in maximum visibility, there are potential risks, such as bleeding, urine leakage, infection, and kidney failure [3,14]. Alternatively, the isthmus can be carefully separated and moved away from the aneurysm wall, as detailed in this article [1,3].
It is essential to preserve kidney function to reduce illness and mortality [1,15]. The collateral blood flow between renal segments is frequently insufficient, and each of these ARAs supplies a specific area of the renal parenchyma. Therefore, ARA ligation may impair renal function [2,12]. In order to prevent renal ischemia, studies have indicated that ARA with a diameter greater than 2 mm (>3 mm) or that supply more than 30% of renal parenchyma should be preserved [1,14]. The risk of postoperative renal dysfunction is minimal when small ARAs (<2 mm) are ligated [3,12]. Revascularization of abnormal branches can be achieved by surgically reattaching an aortic patch containing the vessels' sources or by directly reattaching the arteries to the prosthesis using a Carrel patch [2,8,12]. In this case, the revascularization of ARA was obtained by reimplanting an aortic patch in an anastomosis suture.
Conclusion
The decision on the surgical approach is complex and requires careful consideration of the benefits and risks specific to the patient’s anatomy. In this case, the choice for open treatment was made based on the need to preserve the ARA and the urgency due to symptoms of acute expansion. The transperitoneal approach provides optimal vascular and renal exposure, but the access route must be decided based on the surgeon's experience and the renal system's anatomical variations. Large parenchymal isthmuses can be mobilized, allowing transperitoneal access without needing a section. Regardless of the surgical technique, one should always seek to preserve the anomalous renal artery, except when it is smaller than 2 mm.
Declaration
The authors have no competing interests.
References
- Christoforou P, Kapoulas K, Bekos C. Open surgical repair of abdominal aortic aneurysm and horseshoe kidney: A strange relationship. Int J Surg Case Rep. 2022;93:106971.
[Crossref] [Google Scholar] [PubMed]
- Melmer PD, Patel A, Biswas S, Borowicz MR. Horseshoe kidney isthmus infarction after percutaneous endovascular aortic aneurysm repair. Cureus. 2020;12(3):7279.
[Crossref] [Google Scholar] [PubMed]
- Bowden S, Nagle G R. Fenestrated endovascular abdominal aortic aneurysm repair with concomitant horseshoe kidney. BMJ Case Rep. 2021;14(1):236755.
[Crossref] [Google Scholar] [PubMed]
- Hajibandeh S, Hajibandeh S, Johnpulle M, Perricone V. Transperitoneal repair of a juxtarenal abdominal aortic aneurysm and co-existent horseshoe kidney with division of the renal isthmus. J Surg Case Rep. 2015;2015(10):134.
[Crossref] [Google Scholar] [PubMed]
- Kin K, Takano H, Nakagawa T, Shirakawa Y. Hybrid repair of an abdominal aortic aneurysm: debranching with endovascular aneurysm repair in a patient with horseshoe kidney. Ann Vasc Dis. 2017;10(1):41-43.
[Crossref] [Google Scholar] [PubMed]
- Handa K, Sakamoto T, Kakizawa Y, Kitahara M, Fukui S, Shirakawa Y, et al. Endovascular aneurysm repair for a patient with horseshoe kidney and the importance of watershed sign and volumetry by preoperative contrast-enhanced computed tomography. Ann Vasc Dis. 2021;14(4):396-399.
[Crossref] [Google Scholar] [PubMed]
- Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. J Pediatr Urol. 2016;12(5):275-280.
[Crossref] [Google Scholar] [PubMed]
- Sachsamanis G, Charisis N, Maltezos K, Galyfos G, Papapetrou A, Tsiliggiris V, et al. Management and therapeutic options for abdominal aortic aneurysm coexistent with horseshoe kidney. J Vasc Surg. 2019;69(4):1257-1267.
[Crossref] [Google Scholar] [PubMed]
- Eisendrath DN, Phifer FM, Culver HB. Horseshoe kidney. Annals of surgery. 1925;82(5):735.
- Phelan JT, Bernatz PE, Deweerd JH. Abdominal aortic aneurysm associated with a horseshoe kidney: report of a case. Proc Staff Meet Mayo Clin. 1957;32(4):77-81.
[PubMed]
- Bounssir A, Bakkali T, Taghi H, Sefiani Y, Lekehal B. Best strategy in managing the association of Horse-shoe-Kidney and Abdominal Aortic Aneurysm: Case report. Int J Surg Case Rep. 2020;75:11-15.
[Crossref] [Google Scholar] [PubMed]
- Caridi GD, Massara M, Greco M, Mastrojeni C, Serra R, Salomone I, et al. Surgical treatment of a voluminous infrarenal abdominal aortic aneurysm with horseshoe kidney: Tips and tricks. Ann Vasc Dis. 2015;8(4):324-327.
[Crossref] [Google Scholar] [PubMed]
- Buckarma E, Beckermann J, Gurrieri C, Frodl B, Saran N, Carmody T, et al. A midline retroperitoneal approach for complex abdominal aortic repair: Case description and operative technique. J Vasc Surg Cases Innov Tech. 2022;8(4):678-687.
[Crossref] [Google Scholar] [PubMed]
- Ikeda A, Tsukada T, Konishi T, Matsuzaki K, Jikuya T, Hiramatsu Y, et al. Open surgical repair for a ruptured abdominal aortic aneurysm with a horseshoe kidney. Ann Vasc Dis. 2015;8(1):52-55.
[Crossref] [Google Scholar] [PubMed]
- Yesildal C, Yavuzsan AH, Kirecci SL, Koramaz I, Yilmaz M, Albayrak A, et al. The rupture of an abdominal aortic aneurysm at the level of horseshoe kidney: A case report. Urol Case Rep. 2020;30:101110.
[Crossref] [Google Scholar] [PubMed]
Citation: Júnior MSB, Anacleto AM, Moreno BC, Silva AHND, Neto FR, Morales MM, et al. (2024) Surgical Treatment of Symptomatic Large Aortic Aneurysm with Horseshoe Kidney. J Vasc Surg. 12:561.
Copyright: © 2024 Júnior MSB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.