Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 4
Surgical Skills and Techniques in Robot-Assisted QM-C2 Radical Hysterectomy
Ya Li, Xinyou Wang, Shichao Han* and Jun Wang*Received: 23-May-2024, Manuscript No. JBDT-24-25877; Editor assigned: 27-May-2024, Pre QC No. JBDT-24-25877 (PQ); Reviewed: 12-Jun-2024, QC No. JBDT-24-25877; Revised: 19-Jun-2024, Manuscript No. JBDT-24-25877 (R); Published: 26-Jun-2024, DOI: 10.4172/2155-9864.24.15.587
Abstract
Objective: To investigate robotic radical hysterectomy with the goal of standardizing and optimizing surgical techniques, thus enhancing the learning curve for this procedure.
Design: Surgical techniques are guided by the anatomical structure of embryonic compartments, serving to not only mitigate tumor dispersion caused by disruption of these compartments but also enhance the ability to achieve adequate resection margins.
Subjects: Patients with stage IB2, IIA1, and certain specific pathological types of IB3 and IIA2 cervical cancer (FIGO 2018).
Results: Performing radical hysterectomy with robotic assistance, alongside the principles of membrane anatomy, not only facilitates a bloodless surgical approach but also enhances procedural efficiency and precision through streamlined and simplified techniques.
Conclusion: Employing robotic technology in radical hysterectomy can result in a more meticulous and refined surgical outcome. The implementation of precise surgical techniques aids in standardizing and optimizing procedures, thereby facilitating the learning curve.
Keywords
Cervical cancer, Radical hysterectomy, Robot, Hysterectomy, Robotic surgery
Introduction
Cervical cancer is among the prevalent gynecological malignancies globally. The primary treatment for early-stage cervical cancer is radical hysterectomy and pelvic lymph node dissection [1,2]. This procedure can be performed either as open surgery or minimally invasive surgery. Retrospective studies indicate reduced intraoperative blood loss, shorter hospitalization duration, and decreased postoperative complications associated with the minimally invasive approach [3-5]. Several retrospective studies have demonstrated comparable disease- free survival and overall survival rates between minimally invasive procedures (conventional laparoscopy and robot-assisted laparoscopy) and open surgery [6,7].
Robot-assisted laparoscopic hysterectomy has demonstrated superior outcomes, including reduced intraoperative blood loss, lower complication rates, and shorter hospital stays, compared to the traditional open laparotomy approach in patients with early cervical cancer [8,9].
Performing a radical hysterectomy demands a higher level of technical skill from the surgeon due to its intricacy and complexity. With the assistance of steady three-dimensional vision, instruments equipped with articulating tips, and the capability to scale down the surgeon's movements without tremor, radical trachelectomy can be executed with greater effectiveness and efficiency.
The procedure of radical hysterectomy involves separating the uterus, cervix, and vagina from the bladder, ureters, and rectum. Therefore, performing robotic radical hysterectomy poses significant challenges. Although the overall surgical procedures closely resemble those of open and laparoscopic radical hysterectomy, our objective is to illustrate the surgical process of radical hysterectomy to ensure procedural consistency.
Materials and Methods
Indications
• International Federation of Gynecology and Obstetrics (FIGO 2018) Patients with stage IB2, IIA1, and certain specific pathological types of IB3 and IIA2 cervical cancer.
• No evidence of lymph node metastasis, as determined by Magnetic Resonance Imaging (MRI)/Computed Tomography (CT).
Contraindications
• (FIGO) stage higher than IB3 and IIA2.
Perioperative considerations
Before surgery, patients with cervical cancer often experience prolonged vaginal bleeding, vaginal discharge, tumor ulceration, and other factors that contribute to a compromised vaginal environment, increasing the risk of inflammation. To address this, vaginal flushing with povidone-iodine solution for at least 3 days helps maintain vaginal cleanliness and reduce congestion in the parametrium and paracolpium. This not only facilitates surgical procedures but also aids in preventing postoperative infections.
During surgery, for obese patients or those with significant ureteral exposure, the placement of a D-J tube may be considered to reduce the risk of postoperative ureteral fistula complications.
Surgical steps
Uterine suspension: With 1-0 absorbable suture, two "8" stitches are placed on the posterior wall near the uterine fundus. A 5 mm incision is created above the pubic symphysis, ensuring avoidance of the bladder. The uterus is manipulated using a laparoscopic needle holder, which grasps the uterine sutures (Figure 1).
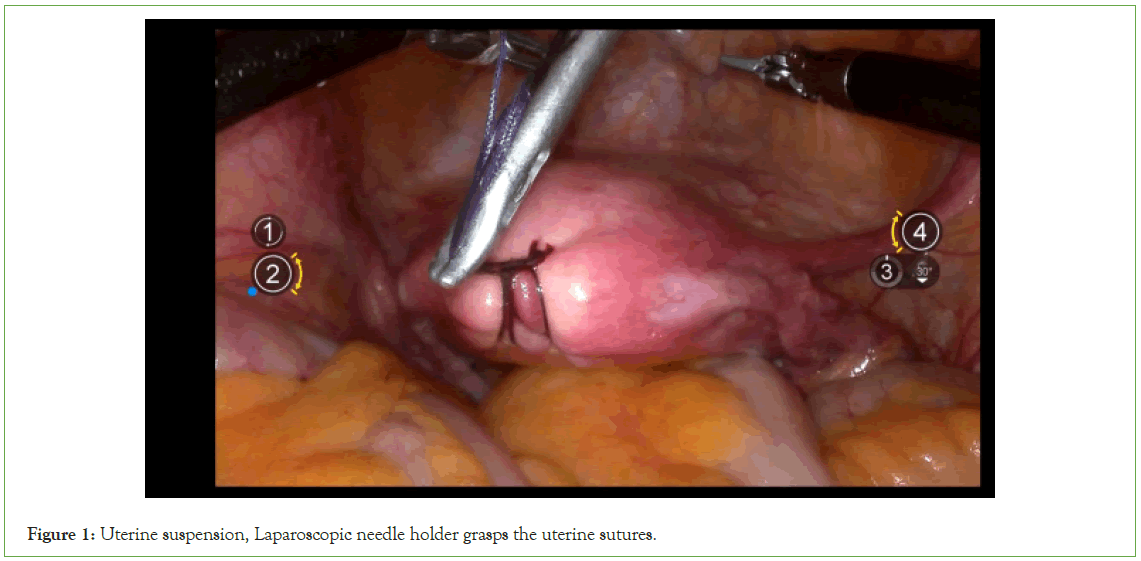
Figure 1: Uterine suspension, Laparoscopic needle holder grasps the uterine sutures.
Lymphrectomy:
Expose the lateral retroperitoneal space: Incise the lateral peritoneum along the junction of the ovarian suspensory ligament and the iliac vessels, extending towards the caudal side until reaching the convergence with the round ligament (Figures 2 and 3).
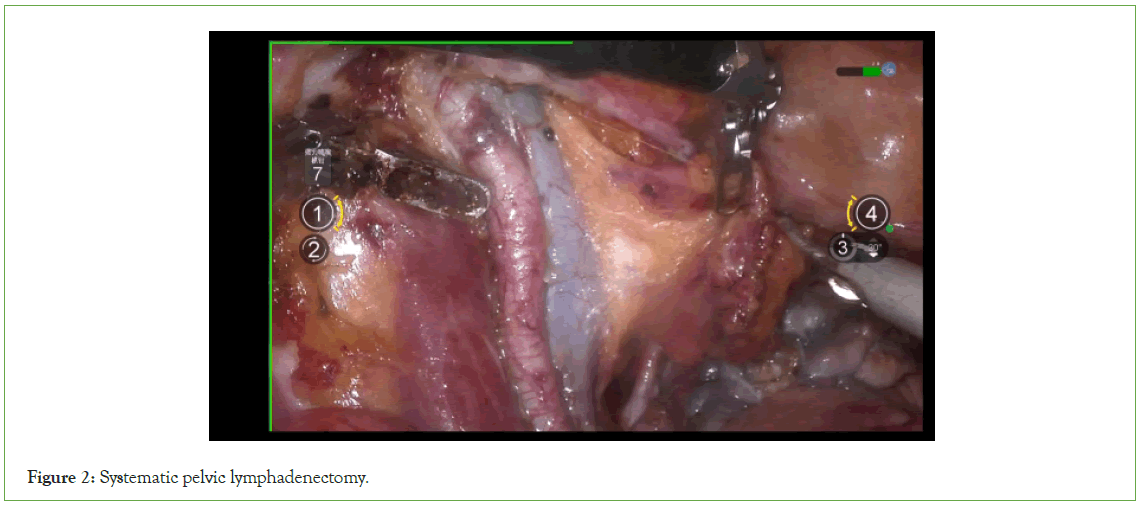
Figure 2: Systematic pelvic lymphadenectomy.
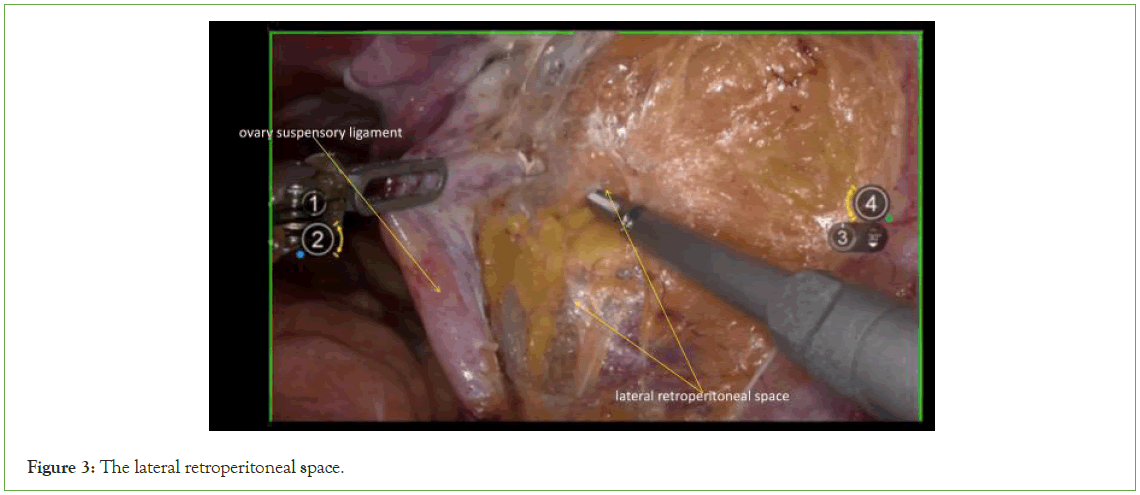
Figure 3: The lateral retroperitoneal space.
Resection of the adnexa: Fully expose the suspensory ovary ligament, visually identify the ureter, and then cut the suspensory ovary ligament (Figure 4).
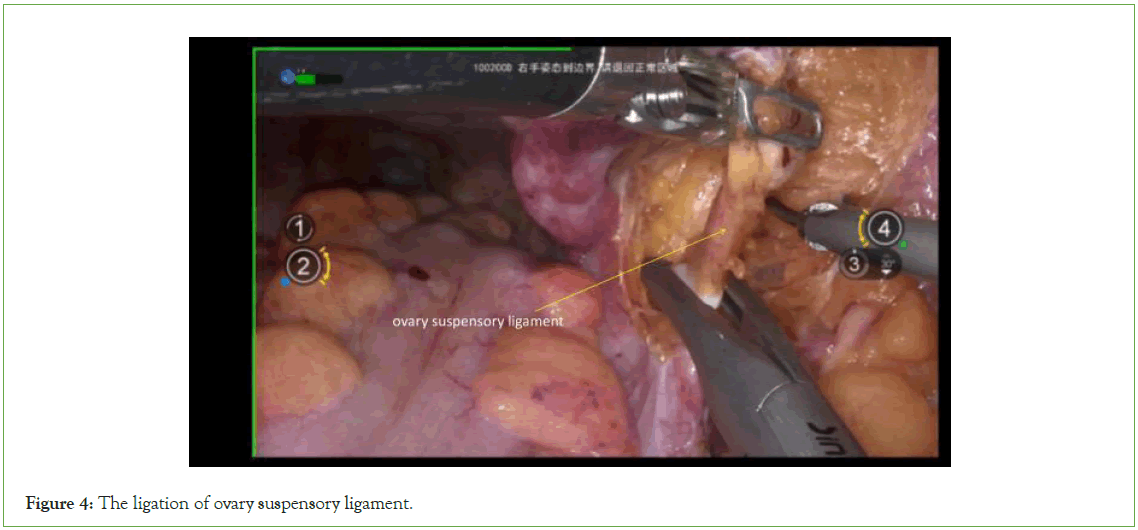
Figure 4: The ligation of ovary suspensory ligament.
Expose the obturator space: Sharp dissection of the lateral retroperitoneal space reveals the internal iliac artery and lateral umbilical ligament. Subsequently, precise dissection is conducted adjacent to the lateral aspect of the internal iliac artery and lateral umbilical ligament to expose the obturator space. Proper exposure of the obturator space facilitates adequate traction of the internal iliac artery and lateral umbilical ligament outward during the dissection of the lateral parametrial space (Figure 5).

Figure 5: The obturator space, Dissection of the lateral parametrial space.
Resection of lateral parametrium
Expose the Latzko’s pararectal space and the paravesical space: Medially, traction is applied to the adnexa and peritoneum, while laterally, traction is exerted on the internal iliac artery and lateral umbilical ligament. Sharp dissection is carried out between the ureter and the internal iliac artery and lateral umbilical ligament until the uterine artery is exposed. Cranially to the uterine artery, Latzko’s pararectal space is exposed, while caudally to the uterine artery, the paravesical space is exposed (Figure 6).
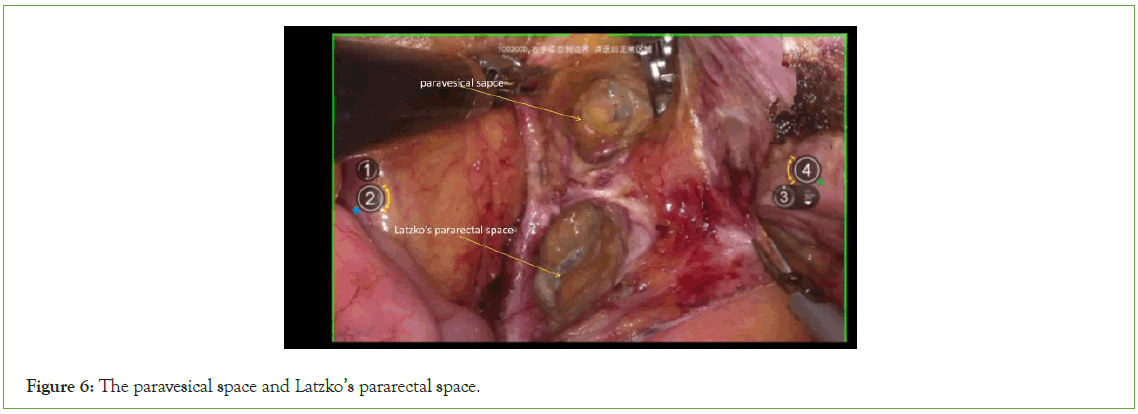
Figure 6: The paravesical space and Latzko’s pararectal space.
Resection of uterine artery and deep uterine vein: Following vascular clamping between the Latzko’s pararectal and paravesical spaces, the uterine artery, superficial uterine vein, and deep uterine vein are sharply dissected (Figure 7).
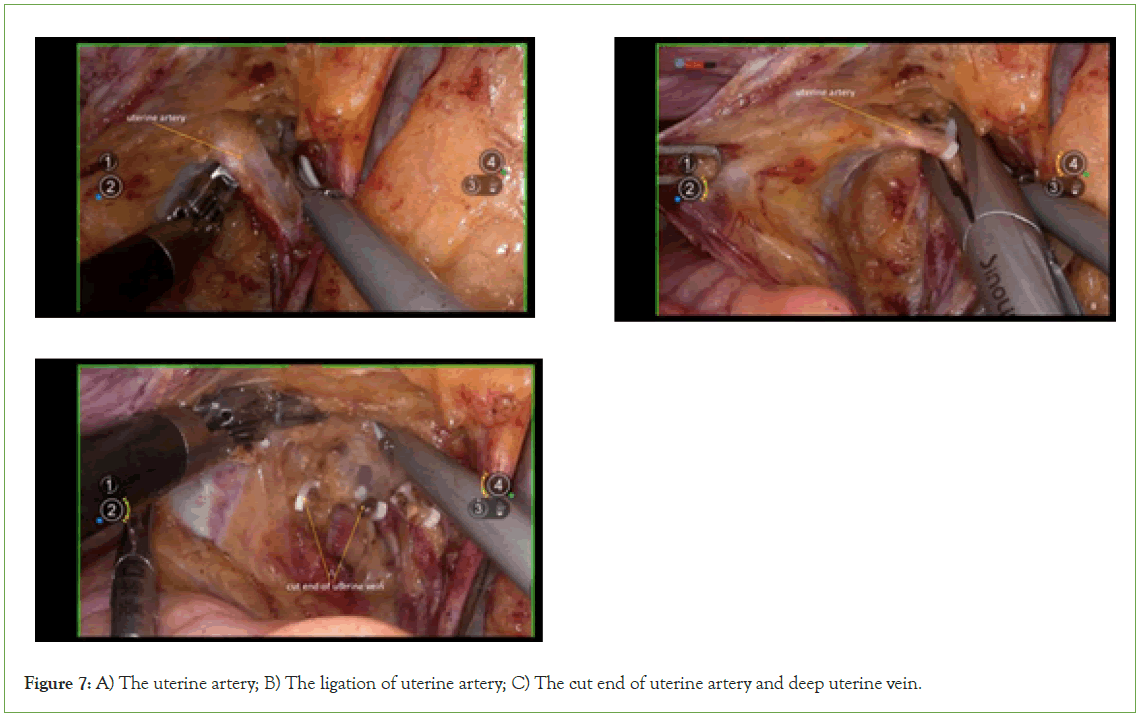
Figure 7: A) The uterine artery; B) The ligation of uterine artery; C) The cut end of uterine artery and deep uterine vein.
Expose Okabayashi’s pararectal space
Externally, traction is applied to the ureter, while internally, traction is applied to the lateral peritoneum. Sharp dissection is performed between them to create space, followed by the opening of Okabayashi's pararectal space (Figure 8).
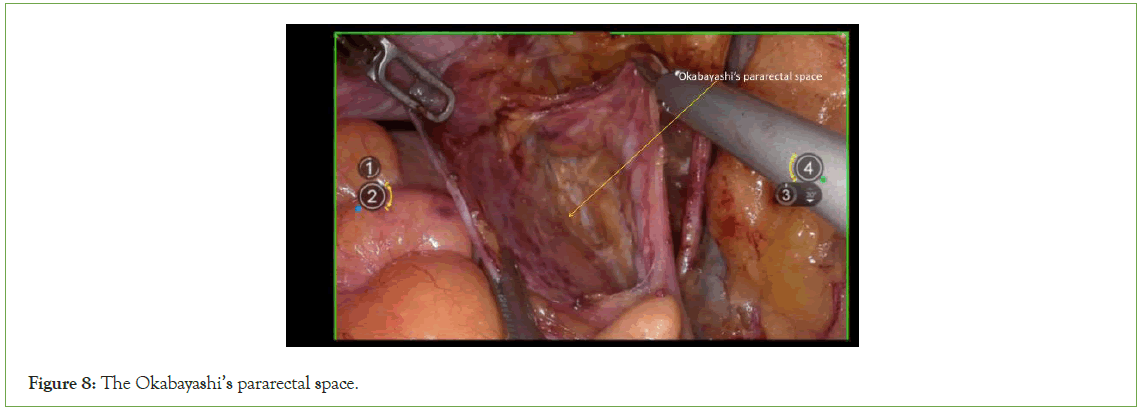
Figure 8: The Okabayashi’s pararectal space.
Dorsal parametrium
Initially dissect the space between the rectal mesentery and the uterosacral ligament, then proceed to expose the rectovaginal space. In QM-C2 radical trachelectomy, the exposure of the uterosacral ligament should extend to the level of the sacral fascia. The dissection of the dorsal parametrium serves as a comprehensive illustration of membrane anatomy and embryonic compartmentalization. The dorsal parametrium consists of only two embryonic compartments, facilitating relatively straightforward dissection and allowing for bloodless separation of the embryonic compartment and expose the uterosacral ligament (Figure 9).
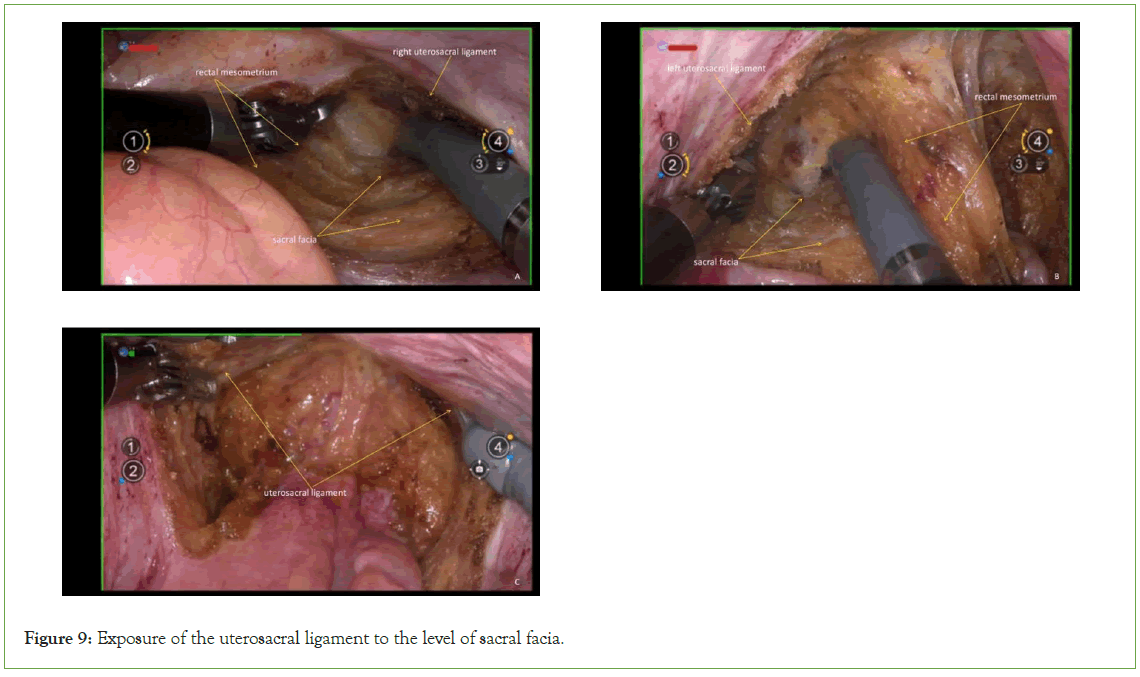
Figure 9: Exposure of the uterosacral ligament to the level of sacral facia.
Transect the uterosacral ligament: Transect the uterosacral ligament at the level of the sacral facia (Figure 10).
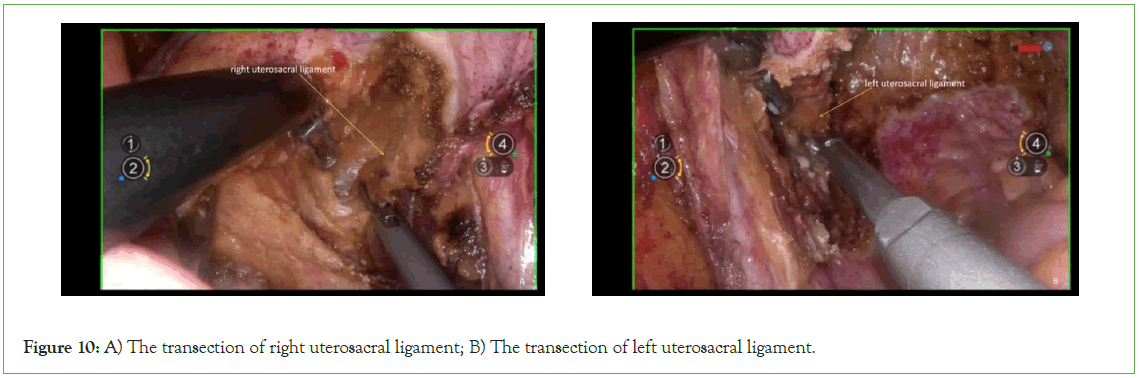
Figure 10: A) The transection of right uterosacral ligament; B) The transection of left uterosacral ligament.
Ventral parametium
Expose the vesicocervical space and the vesicovaginal space: Sharply dissect the space between bladder and cervix, vagina (Figure 11).
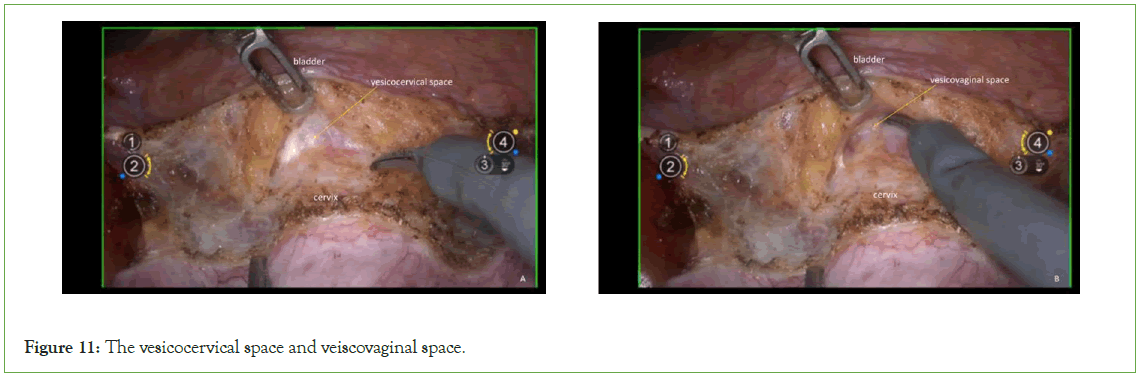
Figure 11: The vesicocervical space and veiscovaginal space.
Ureter: Sharp dissection with an ultrasonic scalpel separates the relationship between the uterine artery and the ureter, proceeding to free it up to the entrance of the ureteral 'tunnel (Figure 12).
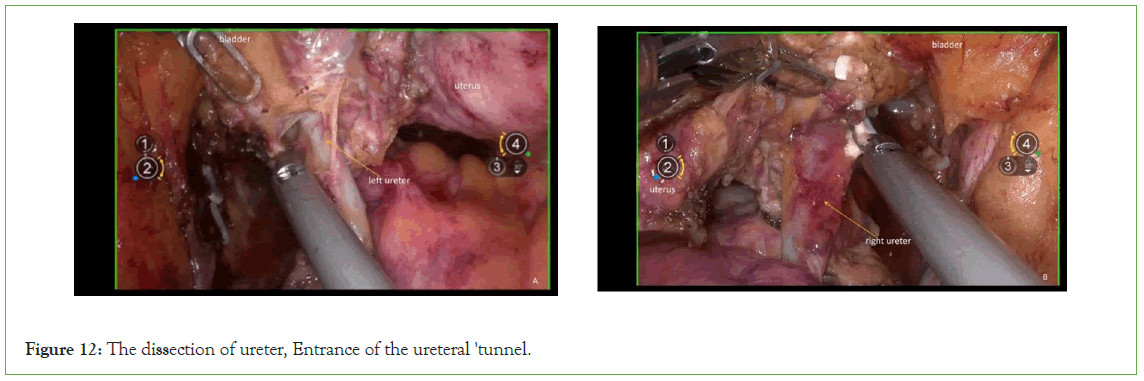
Figure 12: The dissection of ureter, Entrance of the ureteral 'tunnel.
Expose the fourth space: The fourth space corresponds to the exit point of the ureteral "tunnel". To expose this space, gently draw the bladder toward the ventral side, and then laterally widen the vesicovaginal space (Figure 13).
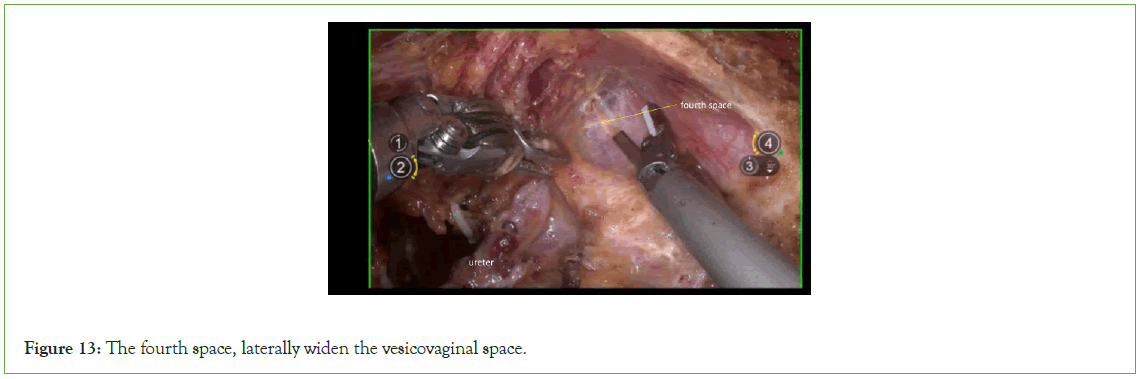
Figure 13: The fourth space, laterally widen the vesicovaginal space.
Transection of the vesicocervical ligament: The vesicocervical ligaments lie between the ureteral tunnel's entrance and exit. When dissecting this ligament using energy-based devices like ultrasonic scalpels or bipolar instruments, it is significant to ensure direct visualization of the ureter and maintain a safe distance from it. Flushing with saline can help cool the energy device and prevent inadvertent injury to the ureter (Figure 14).
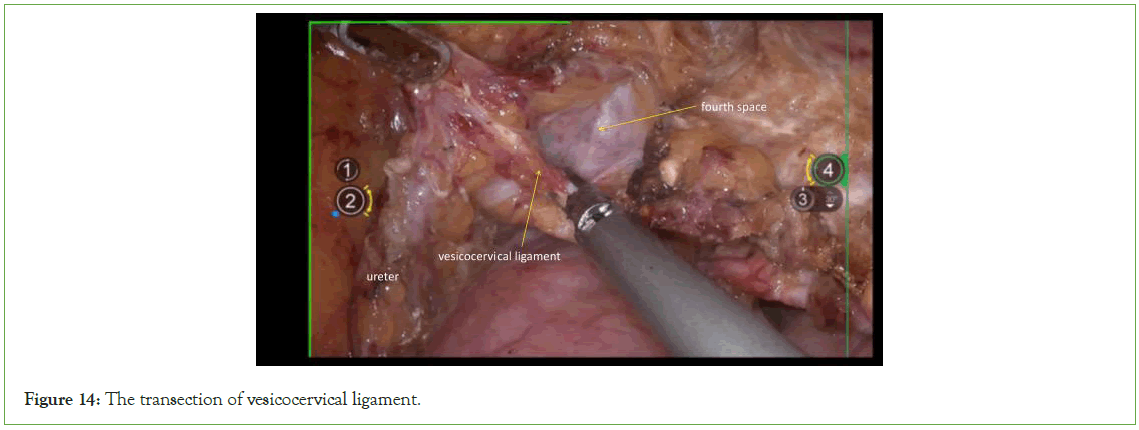
Figure 14: The transection of vesicocervical ligament.
Expose the paravaginal space
By gently pulling the ureters and bladder downward, sharply dissect the connective tissue between the paracolpium and the posterior bladder wall in a caudal direction (Figure 15).
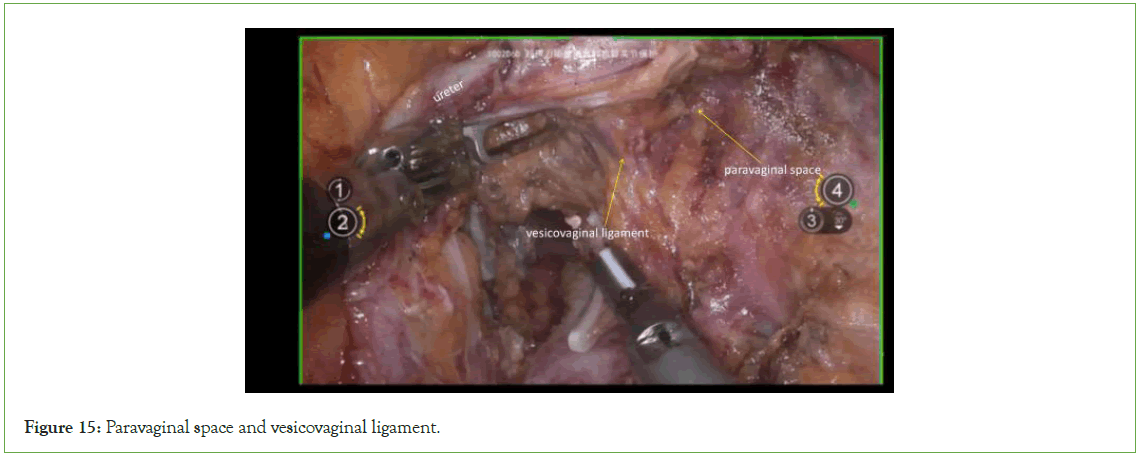
Figure 15: Paravaginal space and vesicovaginal ligament.
Transection of the vesicovaginal ligament: The vesicovaginal ligament, located between the paravaginal and paravesical spaces, should be carefully transected. Due to the presence of the vesical venous plexus within the ligament, it is advisable to first clamp and ligate the vesicovaginal ligament before proceeding with transection (Figure 16).
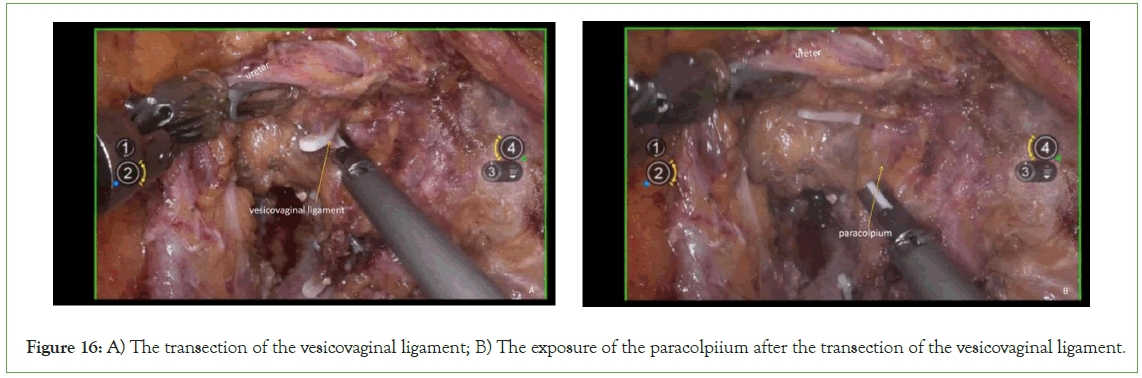
Figure 16: A) The transection of the vesicovaginal ligament; B) The exposure of the paracolpiium after the transection of the vesicovaginal ligament.
Transection of the paracolpium
Transect the paracolpium perpendicular to the direction of the vagina after bipolar coagulation (Figure 17).
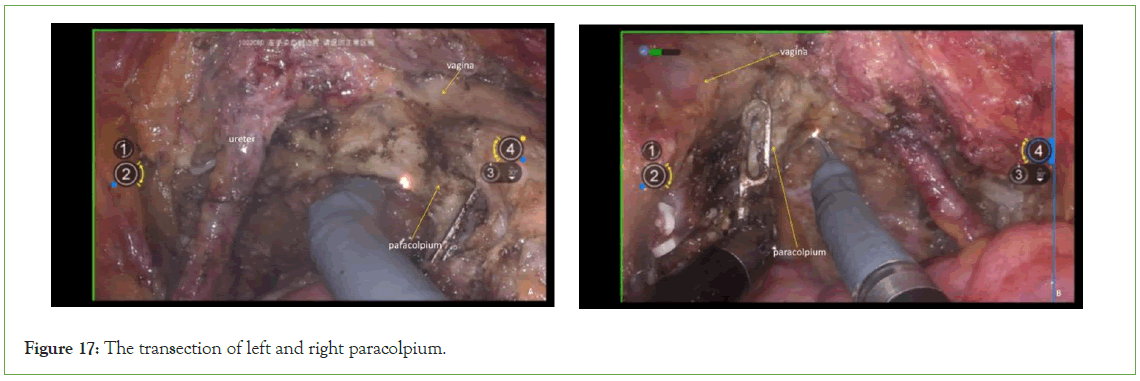
Figure 17: The transection of left and right paracolpium.
Closure of the vagina
Employ continuous circular suturing of the vaginal wall using 1-0 barbed suture for vaginal closure. Ensure that the sutures do not penetrate through the vaginal wall, achieving a tight closure to prevent tumor spillage (Figure 18).
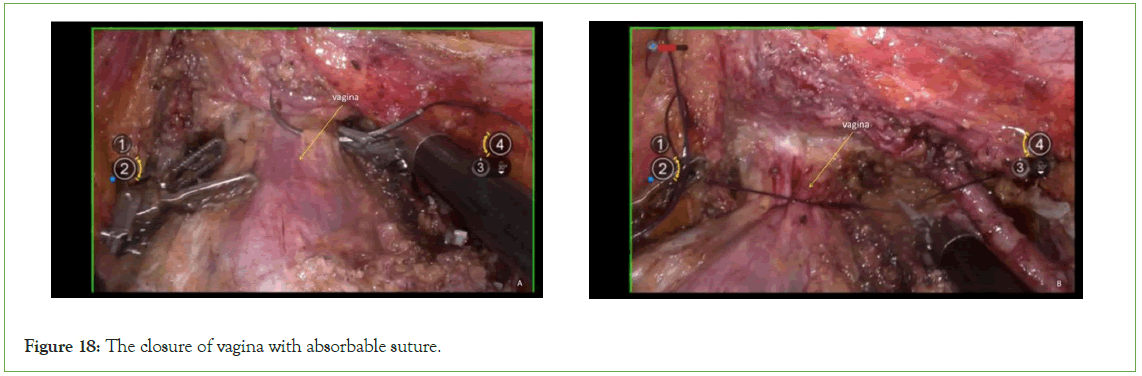
Figure 18: The closure of vagina with absorbable suture.
Flush the vagina
Repeatedly irrigate the vagina with sterile distilled water at 42°C to dislodge any tumor cells adhering to the vaginal wall, thus minimizing the risk of tumor shedding and subsequent implantation at the surgical site within the vagina or pelvis after vaginal incision (Figure 19).

Figure 19: The flush of vagina, Vagina or pelvis after vaginal incision.
Incision and suturing of the vagina
Incise the vagina at the level of the paracolpium and suture it with 1-0 absorbable suture (Figure 20).
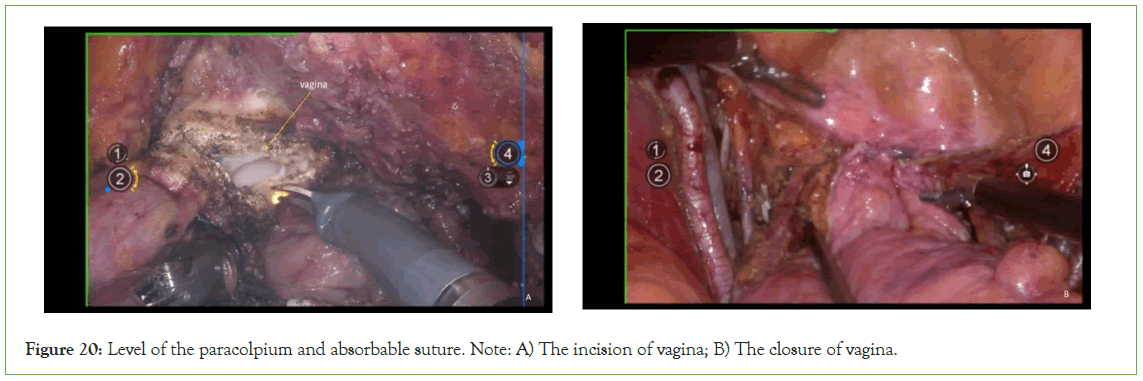
Figure 20: Level of the paracolpium and absorbable suture. Note: A) The incision of vagina; B) The closure of vagina.
Results and Discussion
In addition to conventional laparoscopy, robot-assisted laparoscopic surgery for gynecological indications received approval from the Food and Drug Administration in 2005. Subsequently, its adoption in gynecologic oncology has increased significantly. Numerous studies have underscored the safety and effectiveness of robotic surgery in the treatment of cervical cancer [10,11]. The findings from the Laparoscopic Approach to Cervical Cancer (LACC) trial contradicted previous research results and raised concerns about the safety of minimally invasive surgery [12]. Many retrospective studies, including those rebutting or following the LACC trial, have been reported [13,14]. However, no significant difference in oncological outcomes between the two surgical methods.
A comparative study evaluating patients with early-stage cervical cancer who underwent either open or minimally invasive radical trachelectomy revealed that the 4-year overall survival rates were 95.7% (95% CI, 88.7-98.4) for the Minimally Invasive Surgery (MIS) group and 92.3% (95% CI, 83.5-96.5) for the open surgery group, with a median follow-up duration of 37 months [15].
The choice of approach for radical hysterectomy remains at the discretion of the individual surgeon. Currently, abdominal surgery is the recommended approach for early-stage cervical cancer treatment following findings from the LACC trial. Retrospective data consistently show lower pregnancy rates following abdominal radical trachelectomy compared to vaginal radical trachelectomy or minimally invasive methods. Notably, the highest pregnancy rates have been observed after robot-assisted radical trachelectomy, though larger studies are warranted.
Robotic radical hysterectomy has demonstrated several advantages over the laparotomy approach in early-stage cervical cancer patients, including reduced blood loss, decreased need for blood transfusions, fewer complications, and shorter hospital stays, despite longer operating times.
Conclusion
The integration of robotics in radical hysterectomy enhances visualization of the surgical field with three-dimensional (3D) vision. This technology enables precise instrument manipulation without tremor, improving surgical accuracy and enabling intricate suturing and tissue dissection. Additionally, it reduces surgeon fatigue, especially during prolonged and complex procedures. As a result, the incorporation of robotics into radical hysterectomy procedures can lead to a more meticulous and refined surgical outcome. The adoption of precise surgical techniques helps standardize and optimize procedures, thereby facilitating the learning process.
Conflict of Interest
None declared.
References
- National comprehensive cancer network, NCCN clinical practice guidelines in oncology, cervical cancer, version 1. 2022.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. Cancer J Clin. 2022; 72:7-33.
[Crossref] [Google Scholar] [PubMed]
- Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928.
[Crossref] [Google Scholar] [PubMed]
- Cao T, Feng Y, Huang Q, Wan T, Liu J. Prognostic and safety roles in laparoscopic versus abdominal radical hysterectomy in cervical cancer: A meta-analysis. J Laparoendosc Adv Surg Tech A. 2015;25:990–998.
[Crossref] [Google Scholar] [PubMed]
- Frumovitz M, dos Reis R, Sun CC, Milam RM, Bevers MW, Brown J, et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet Gynecol. 2007;110:96–102.
- Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-assisted versus open radical hysterectomy: A multi-institutional experience for earlystage cervical cancer. Eur J Surg Oncol. 2016;42:513–522.
[Crossref] [Google Scholar] [PubMed]
- Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomoy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:82.
[Google Scholar] [PubMed]
- Sert B, Abeler V. Robotic radical hysterectomy in early-stage cervical carcinoma patients, comparing results with total laparoscopic radical hysterectomy cases. The future is now? Int J Med Robot. 2007;3:224–228.
[Google Scholar] [PubMed]
- Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–194.
[Google Scholar] [PubMed]
- Cantrell LA, Mendivil A, Gehrig PA, Boggess JF. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: A 3-year experience. Gynecol Oncol. 2010; 117: 260-265.
[Google Scholar] [PubMed]
- Tinelli R, Malzoni M, Cosentino F, Perone C, Fusco A, Cicinelli E, et al. Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: A multicenter study. Ann Surg Oncol. 2011; 18: 2622-2628.
[Google Scholar] [PubMed]
- Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018; 379: 1895-1904.
[Google Scholar] [PubMed]
- Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-assisted versus open radical hysterectomy: A multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol. 2016;42(4):513-522.
[Google Scholar] [PubMed]
- Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28(6): 82.
[Google Scholar] [PubMed]
- Matsuo K, Chen L, Mandelbaum RS, Melamed A, Roman LD, Wright JD. Trachelectomy for reproductive-aged women with early-stage cervical cancer: Minimally invasive surgery versus laparotomy. Am J Obstet Gynecol. 2019;220:469.
[Google Scholar] [PubMed]
Citation: Li Y, Na J, Wang X, Han S, Wang J (2024) Surgical Skills and Techniques in Robot-Assisted QM-C2 Radical Hysterectomy. J Blood Disord Transfus. 15:587.
Copyright: © 2024 Li Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

