Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Study to Determine Bioequivalence of Three Ceftiofur Crystalline Free Acid in Pigs
Luis Ocampo-Camberos1, Minerva Monroy-Barreto2, Agustín Nieto-Carmona1, Juan Angel Jaime3 and Lilia Gutierrez1*2Department of Analytical Chemistry, Universidad Nacional Autónoma de México and Avenida Universidad, Coyoacan, Mexico
3Department of Pharmaceuticals, National Autonomous University of Mexico, Mexico City, Mexico
Received: 02-Sep-2021 Published: 23-Sep-2021, DOI: 10.35248/0975-0851.21.s4.001
Abstract
Background: Antimicrobial resistance has the potential to affect sustainable development goals in food-producing livestock. Poor quality antibacterial pharmaceutical preparations significantly contribute to heighten this problem. Bioequivalence (BE) studies are very important for the development of dependable pharmaceutical preparations.
Methods: In this trial 3 Ceftiofur Crystalline Free Acid (CCFA) pharmaceutical preparations (1 reference and 2 experimental), intended for swine medicine and freely sold in Mexico, were tested to assess as to whether or not they can be regarded as generic ones.
Results: Three commercially available products of CCFA containing 200 mg of ceftiofur crystalline free acid were compared taking Excede® brand as reference preparation and preparations A and B as experimental ones. Thirty-six Landrace/Duroc pigs randomly divided into three groups received a single injection in phase 1 and after a washout period the same procedure was repeated in a crossover phase. Based on PK data obtained through HPLC analytical recollection of serum concentrations of ceftiofur, it is possible to conclude that preparations A and B cannot be regarded as bioequivalent to Excede® in pigs given that AUC0-168, MRT and K½el values obtained from preparations A and B are statistically different beyond a 20% limit from the corresponding ones obtained for the reference preparation, with confidence intervals >0.05.
Conclusion: Based on the area under the concentration vs time curve from zero to 168 h, mean residence time, and elimination constant values obtained from preparations A and B it is possible to conclude that they cannot be regarded as bioequivalent to Excede® in pigs (CI>0.05).
Keywords
Ceftiofur Crystalline Free-Acid; Bioequivalence; Pigs; PK/PD ratios
Introduction
The determination of the Bioequivalence (BE) of veterinary drug formulations has been set in the Ley General de Salud Animal in Mexico. Yet, its implementation awaits completion in many cases. Two drug preparations are considered to be bioequivalent when they are equally bioavailable having administered them through the same route and at the same dose [1,2]. The degree of similarity between two serum concentration profiles to be considered BE is when the confidence interval for untransformed data should be 80%–120% (the confidence interval should lie within ± 20% of the mean of the reference product). For logarithmically transformed data, the confidence interval is generally 80%–125% [3]. Usually the key pharmacokinetic parameters to evaluate BE are area under the concentration vs time curve (AUC), the elimination half-life (T½β), and the value of maximum serum concentration (CMAX), but other data can also be considered, such as K½el [4].
Ceftiofur Crystalline-Free Acid (CCFA) is an extended-release injectable formulation of ceftiofur. It is approved for treating Actinobacillus pleuropneumniae, Pasteurella multocida, Haemophilus parasuis, and Streptoccus suis in swine respiratory disease [5]. From the clinical viewpoint, optimal Pharmacokinetic/ Pharmacodynamics (PK/PD) ratio for ceftiofur indicates that antibacterial drug concentrations overtime should be slightly above the Minimum Inhibitory Concentration (MIC) values, for the longest possible time between dosing intervals i.e., T>MIC [6]. The pioneer preparation of Excede® from Zoetis has already presented its Pharmacokinetics (PK) [7], claiming that it allows a Dosage Interval (ID) as long as a week. In the treatment of pigs, this feature represents not only a notable therapeutic advantage, but also a reduction in the handling of the animals and even minimizes deaths due to stress during the capture and handling of animals with advanced pneumonia [8]. Surely motivated by the therapeutic success of the reference preparation of Ceftiofur Crystalline Free Acid (CCFA) in the treatment of diseases in pigs, two preparations with the same active ingredient, and in theory bioequivalent to the pioneering preparation, have been made available in the Mexican market. Their PK profiles have not been disclosed. Hence, this trial intended to study whether or not the long-acting preparations named A and B suffice the PK requirements to be regarded as true bioequivalent alternatives to Excede® in Mexico and how PK/PD ratios result.
Methods
Chemicals and reagents
Crystalline free acid of high purity (>95%) was purchased from Sigma Aldrich Chemicals and High-Performance Liquid Chromatography (HPLC) grade solvents were obtained from J.T. Baker®.
Drug-preparations
Three commercially available products of CCFA, containing 200 mg of ceftiofur crystalline free acid were compared. Excede® from Zoetis, Mexico was taken as the reference preparation and two other preparations, here described as A and B, were studied as manufacturers did not allow their names disclosed. The three CCFA preparations were purchased from a veterinary pharmacy near a pig production geographic area. All three concentrations of CCFA were previously analyzed.
Animals and housing
All study procedures and animal care activities were carried out following the Institutional Committee for Research, Care, and Use of Experimental Animals of the National Autonomous University of Mexico (UNAM), per Official Mexican Regulation NOM-062- ZOO-1999 [9]. In all, 36 Landrace/Duroc pigs with an average initial weight of 9.2 ± 1.6 kg, from the National Autonomous University of Mexico (UNAM)-Experimental Ranch (CEIEPP, in Jilotepec, State of Mexico), were included in this trial. The absence of any other type of medication was ensured and pigs did not receive any drug 20 days before the start of this study. Pigs were housed in previously assigned and identified lots in groups of 12 animals in elevated weaning cages equipped with simple 5-mouth feeders in stainless steel, Dura-Tuff plastic floor made of high impact polypropylene and a water system of variable height with 1 nipple drinker.
Experimental design
Phase 1. Animals were randomly divided into three groups of 12 pigs each according to VICH guidelines [VICH GL52-Bioequivalence, 2015) [10], as follows: reference preparation receiving a single injection of ceftiofur crystalline free acid as in Excede® (Zoetis, Mexico) (group EXC) at a dose of 5 mg/kg, injected IM in the lateral view of the neck, utilizing 18 gauge needles 2.5 cm long and an approximate volume of 0.5 mL. Similarly, pigs from groups A and B were injected as for EXC group. All three CCFA preparations tested were prepared as 10% suspensions.
Assisted by technicians, blood samples were taken by jugular puncture, collecting 5-7 mL in Vacutainer tubes without additive, at the following times: before injection, and at 2, 4, 8 hours the first day and then once a day at noon until day 7 as suggested by Tantituvanont, et al. [11]. Serum was recovered from blood samples after centrifuging them (4°C; 3500 rpm/15 min) and immediately stored and fully identified by freezing at -20°C until analyzed.
Phase 2, crossover. After a 21 day washout period, pigs were interchanged relocating 6 animals from A and B groups to EXC group and vice versa. Then the remaining 6 pigs from group A were interchanged with 6 animals from group B.
Chromatographic method
A High-Performance Liquid Chromatographic method (HPLC) was implemented as described by Jacobson, et al. [12]. Determination of ceftiofur in bovine plasma by HPLC-DAD, (Journal of Pharmaceutical and Biomedical Analysis 40 1249-1252), using a Jasco LC model UV-VIS. The analytical HPLC system consisted of a PU-2089 solvent delivery system, UV-2075 UV-DAD, and X-LC autosampler with a Shiseido Capcell Pak C18 MG III column (150 × 4.6 mm). Detection and quantitation were performed at a wavelength of 265 nm. A recovery percentage of 88.5% and a reproducibility coefficient with a variation of less than 6% were achieved. The ceftiofur calibration curve (0.1-10 μg/mL) and had a mean correlation coefficient of 0.999. A stock or 1 mg/mL stock solution was prepared in phosphate buffer (pH=2.0) and stored at -70°C (considering 1 month of activity under these conditions).
Sample treatment
To 500 μL of serum, add 50 μL of 20% (w/v) trichloroacetic acid and stir in Vortex for 20 seconds and centrifuge at 4500 rpm for 10 minutes. The supernatant was collected and then ready for Solid-Phase Extraction (SPE) procedure. The SPE cartridges used for sample clean up were preconditioned with 2 mL of ACN and 2 mL of water. The supernatant was then loaded onto a cartridge and passed through under gravity. The cartridge was washed with 3 mL 5 % methanol in water. A full vacuum was applied and the compounds were eluted with 3 mL methanol and the eluent dried under nitrogen. The residue was reconstituted in 0.5 mL of the mobile phase. The liquid was transferred to chromatography vial for HPLC analysis. The following relationship (Table 1 and Figure 1) was obtained for pig serum.
| Concentration (µg/ml) | AUC in water* | AUC in pig serum* | % recovery |
|---|---|---|---|
| 0.1 | 4529 | 5213 | 85.6 |
| 0.2 | 6523 | 6653 | 88.9 |
| 0.4 | 10025 | 11250 | 92.5 |
| 0.8 | 17956 | 18532 | 87.3 |
| 1.6 | 31025 | 32564 | 83.5 |
| 3.2 | 59856 | 60125 | 85.4 |
Note: *ɳ=4
Table 1: Concentrations of ceftiofur vs. mean values of Area Under the Curve (AUC) obtained from the chromatograms, either when diluting the standard in water or in pig serum. No standard deviations are presented due to its Small Dispersion (SD).
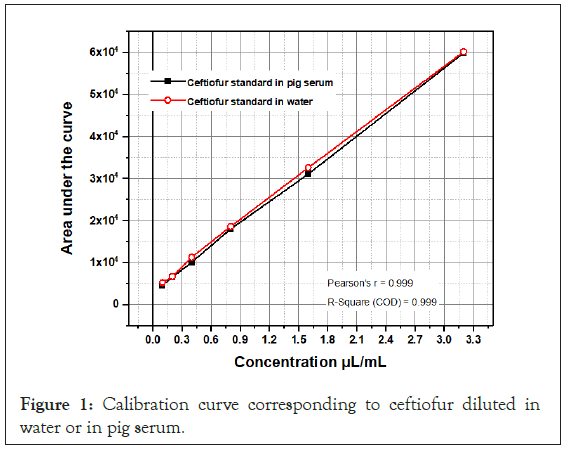
Figure 1: Calibration curve corresponding to ceftiofur diluted in water or in pig serum.
Pharmacokinetics and statistical analysis
Data was run through compartmental and non-compartmental models using the programs: PKAnalyst, WinNonlin, and using ORIGIN for graphing. Data obtained through both models were indistinguishable. The essential pharmacokinetic parameters obtained were: AUC0-168 (μg/mL/h)=area under the curve by integral; AUMC (μg/mL/h)=area under the moment curve; AUCT (μg/mL/h)=area under the curve by the trapezoidal method; K½el (h)=elimination constant; CMAX (μg/mL)=maximum plasma concentration; TMAX (h)=time to achieve CMAX; MRT (h)=mean residence time; Fr (%)=relative bioavailability=AUC0-168 of preparation A/AUC0-168 of Excede® × 100. Pharmacokinetic parameters were evaluated utilizing ANOVA and Bonferroni t-test using JMP software. Preparations were considered generic if AUC0-168, K½el, and CMAX did not vary more than 20% as compared with the reference preparation (Excede®, Zoetis México).
Results
The implemented method presented an average recovery rate of 92% (range 87%-102%), with a linearity between 1 and 7 μg/mL limit of 1 μg/mL and an intra-assay and inter-assay error of 3% and 5% respectively. Figure 2 shows test serum samples from pigs medicated with CCFA of the three preparations studied at 84 h post-injection. (r2>0.988), with a detection limit of 0.5 μg/mL, a quantification
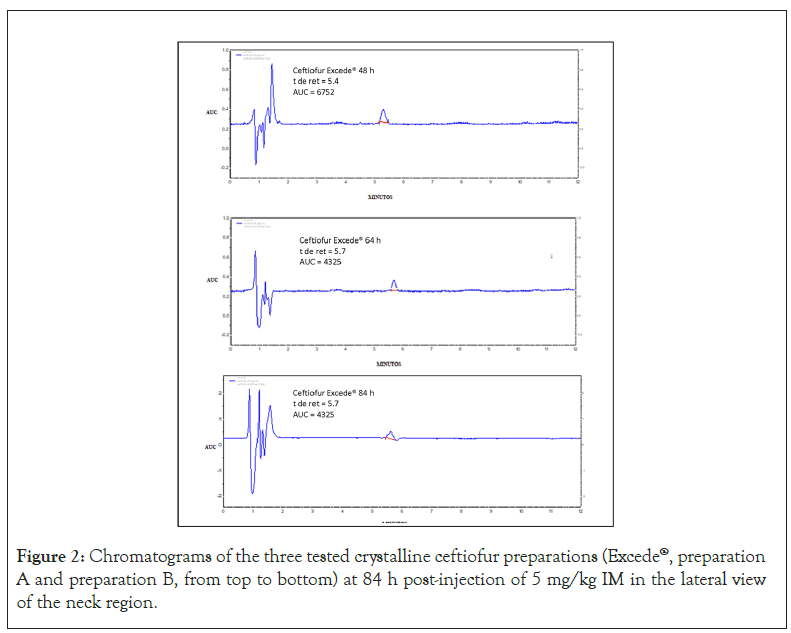
Figure 2: Chromatograms of the three tested crystalline ceftiofur preparations (Excede®, preparation A and preparation B, from top to bottom) at 84 h post-injection of 5 mg/kg IM in the lateral view of the neck region.
Pharmacokinetic data from phase 1 and phase 2 (crossover) is summarized in Table 2, and Figures 3 and 4 present mean ± 1 SD serum concentrations of ceftiofur both during phase 1 and at phase 2 (crossover). Table 3 shows the statistical comparison between the pharmacokinetic parameters obtained for Excede® and preparations A and B by ANOVA Test with log-transformed and untransformed data. Based on PK data obtained it is possible to conclude that preparations A and B cannot be regarded as bioequivalent to Excede® in pigs given that AUC0-168, MRT and K½el values obtained from preparations A and B are statistically different beyond a 20% limit from the corresponding ones obtained for the reference preparation, with confidence intervals >0.05.
| Parameter | Exceed® | Preparation A | Preparation B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 Crossover | Phase 1 Crossover | Phase 1 Crossover | ||||||||||
| X | ± DE | X | ± DE | X | ± DE | X | ± DE | X | ± DE | X | ± DE | |
| K½el (h) | 7.91 | 2.01 | 7.97 | 2.11 | 3.04 | 0.53 | 2.86 | 0.61 | 2.13 | 0.25 | 2.17 | 0.17 |
| TMAX (h) | 11.42 | 2.9 | 11.5 | 3.04 | 4.39 | 0.77 | 4.13 | 0.88 | 3.08 | 0.36 | 3.13 | 0.24 |
| CMAX (µg/mL) | 1.41 | 0.32 | 1.44 | 0.23 | 1.26 | 0.57 | 1.29 | 0.38 | 1.47 | 0.38 | 1.6 | 0.51 |
| AUC0-168 (µg/mL/h) | 45.24 | 18.93 | 46.05 | 15.88 | 15.02 | 7.17 | 15.22 | 6.97 | 12.18 | 3.17 | 13.81 | 4.97 |
| AUMC (µg/mL/h2) | 1121 | 702 | 1139 | 575 | 133 | 66 | 136 | 85 | 75 | 22 | 88 | 36 |
| MRT (h) | 22.83 | 5.81 | 23.01 | 6.08 | 8.78 | 1.54 | 8.26 | 1.76 | 6.15 | 0.72 | 6.27 | 0.48 |
| AUCT (µg/mL/h) | 61.91 | 26.68 | 63.95 | 21.73 | 41.71 | 24.13 | 41.55 | 19.41 | 44.99 | 16.22 | 46.63 | 14.15 |
| T >MIC* (h) | 132 | 109 | 70 | 60 | 60 | 64 | ||||||
| AUC0-168/MIC* | 226 | 230 | 75 | 76 | 61 | 69 | ||||||
| Fr (%) | - | 33.2 | 33.1 | 26.9 | 29.98 | |||||||
Note: a,b,c,dhighlight statistically significant differences within a row *An arbitrary value obtained from literature was set at 0.2 µg/mL. AUC0-168 (µg/mL/h)=area under the curve by integral; AUMC (µg/mL/h): Area Under the Moment Curve; AUCT (µg/mL/h): Area under the curve by the trapezoidal method; K½el (h)=elimination constant; CMAX (µg/mL)=maximum plasma concentration; TMAX (h)=time to achieve CMAX; MRT (h)=Mean Residence Time; Fr (%)=relative bioavailability=AUC0-168 of preparation A/AUC0-168 of Excede® × 100; T >MIC=time at which the concentration of ceftiofur is equal or higher than the minimum inhibitory concentration set at 0.2 µg/mL in this case (taken from graphs); AUC0-168/MIC=ratio of area under the curve/minimum inhibitory concentration set at 0.2 µg/mL in this case.
Table 2: Pharmacokinetic parameters and PK/PD ratios obtained for Exceed® and for the pharmaceutical preparations of free acid crystalline ceftiofur "A" and "B" in pigs, derived from the phase 1 and the crossover (phase 2), of this bioequivalence study.
| Parameter | Exceed® (Reference) |
Preparation A | Preparation B | ANOVA | ANOVA (log) |
|---|---|---|---|---|---|
| Phase 1 | |||||
| AUC0-168 | 45.24 ± 19 | 15.02 ± 7 | 12.18 ± 17 | >0.05; >0.01 | >0.05; >0.01 |
| K½el | 7.91 ± 2 | 3.04 ± 0.5 | 2.13 ± 0.2 | >0.05; >0.01 | >0.05; >0.01 |
| CMAX | 1.41 ± 0.3 | 1.26 ± 0.6 | 1.47 ± 0.4 | > 0.5 | > 0.5 |
| MRT | 22.83 ± 6 | 8.78 ± 2 | 6.15 ± 0.7 | >0.05; >0.01 | >0.05; >0.01 |
| Phase 1, crossover | |||||
| AUC0-168 | 46.05 ± 16 | 15.22 ± 7 | 13.81 ± 5 | >0.05; >0.01 | >0.05; >0.01 |
| K½el | 7.97 ± 2 | 2.86 ± 0.6 | 2.17 ± 0.2 | >0.05; >0.01 | >0.05; >0.01 |
| CMAX | 1.44 ± 0.2 | 1.29 ± 0.4 | 1.60 ± 0.5 | > 0.5 | > 0.5 |
| MRT | 23.01 ± 6 | 8.26 ± 2 | 6.27 ± 0.5 | >0.05; >0.01 | >0.05; >0.01 |
Note: Bioequivalence evaluation by Steinijans and Diletti’s non-parametric test.
Arithmetic mean of individual differences=-1.66667E-01.
90% confidence interval (preparation A or B vs. Excede®)=Prep A vs. Excede® 86.0%-114.0%; Prep B vs. Excede® 92.0%-110%
Table 3: Statistical comparison between the pharmacokinetic parameters obtained for Excede® and preparations A and B by ANOVA Test with log transformed and untransformed data.
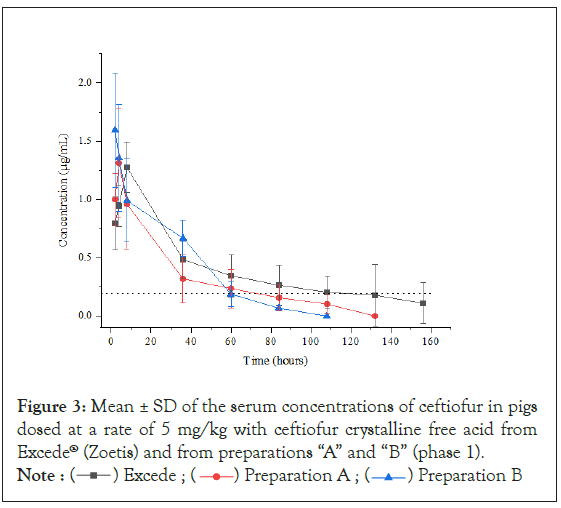
Figure 3: ± SD of the serum concentrations of ceftiofur in pigs
dosed at a rate of 5 mg/kg with ceftiofur crystalline free acid from
Excede® (Zoetis) and from preparations “A” and “B” (phase 1).
Note : Excede ;
Excede ; Preparation A ;
Preparation A ; Preparation B
Preparation B
Figure 4: Mean ± SD of the serum concentrations of ceftiofur in pigs
dosed at a rate of 5 mg/kg with ceftiofur crystalline free acid from
Excede® (Zoetis) and from preparations “A” and “B” (phase 1).
Note :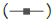 Excede ;
Excede ; Preparation A ;
Preparation A ; Preparation B
Preparation B
Discussion
It has been pointed out that antimicrobial resistance has the potential to affect almost all sustainable development goals and that poor quality antibacterial pharmaceutical preparations may contribute in a significant manner to contribute to heighten this problem [13]. Bioequivalence studies are very important for the development of sound pharmaceutical preparations. Their justification can be viewed as a form of maintaining an economic balance in the national market for veterinary drugs while improving animal health. But above this, the importance of BE studies is to regulate the quality of supposedly generic products and as such, demonstration of BE must be part of the registration file submitted to a given regulatory agency. In this study, concentration profiles of ceftiofur and PK data derived indicate that all three preparations of CCFA are absorbed to a greater or lesser extent and generate CMAX values of 1.7 μg/mL and 1.8 μg/mL and 1.9 μg/mL for Excede® and preparations A and B, respectively and no statistically significant differences were detected. Considering that ceftiofur is a time-dependent drug, the absence of differences in the CMAX value is also of minor importance. TMAX values were also similar, a fact that was somehow predictable given the sampling times chosen. In contrast, K½el was particularly longer in Excede®, with a value of 7.91 h, and 3.04 h or 2.13 for preparations A and B, respectively (P<0.05 in all instances). As stated above, this parameter complies well with the PK/PD of T ≥ CMI, which is of great importance for the clinical efficacy of ceftiofur [6,14]. Consequently, relative bioavailability and MRT values for Excede® vs preparations A and B show statistically significant differences (P<0.05 in all cases). AUC0-168 represents the exposure to ceftiofur in base to the fraction of the dose reaching the systemic circulation considering the systemic clearance of the drug. Hence, given the K½el, AUC0-168, and MRT differences observed in this trial between the reference preparation and products A and B, and considering than these differences are greater than 20%, it is feasible to conclude that preparations A and B cannot be considered generic brands of CCFA. Furthermore, because BE can be seen as the absence of a significant difference in the rate and extent to which the active ingredient reaches either the bloodstream or tissues in a given pharmaceutical equivalent(s) [15], lack of BE would fail to accomplish such concentrations and AUC0-168/MIC ratio would be considerably smaller in preparations A and B. Consequently, clinical efficacy may be compromised [6]. This latter is only an assumption and clinical work is needed to characterize if indeed there is a quantifiable difference. Also, it is important to highlight a methodological peculiarity of this study and that is that the samples were obtained from a veterinary pharmacy and not directly from the manufacturer as it should be in an official study from the country's authorities (SADER in the case of Mexico). This study was driven by an academic interest.
It has been pointed out that the breakpoint of ceftiofur vs many pathogens and consequently the limit minimum serum therapeutic concentration is also 0.2 μg/mL [16]. Then, it can be inferred that the three products present useful activities for up to 3 days (72 h) for preparation A, up to 4.6 days (110 h) for preparation B and 6-7 days (158 h) for the reference preparation. Again, this is very important given the arguments above, and surely this may be seen in the daily clinical work in swine medicine [6, 17].
Conclusion
The absence of differences in CMAX and TMAX values could indicate the existence of BE among all three CCFA preparations tested. Yet, ceftiofur is a time-dependent drug and these two parameters are not as important as K½el and AUC0-168, which were particularly larger in Excede® (P<0.05 in all instances). Consequently, PK/PD most important ratios for the clinical efficacy of ceftiofur are T ≥ CMI and to some extent, AUC0-168/MIC and these ratios would be certainly higher than the ones that could have been obtained for preparations A and B. Hence, from this particular viewpoint, preparations A and B cannot be regarded as BE to Excede®.
Acknowledgments
The authors are grateful to the research site staff members involved in the development of the Study
Author Contributions
Serán colocadas una vez hayan sido definidos los autores.
Disclosure of Funding Support
This study was full sponsored by Ultra Laboratorios, S.A. de C.V. Jalisco, Mexico.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest with regard to the content of this article.
REFERENCES
- Food and Drug Administration. U.S. Department of Health and Human Services, Center for Veterinary Medicine (CVM). Bioequivalence Guidance. Silver Spring. 2006.
- Food and Drug Administration. Bioavailability and Bioequivalence Requirements. Silver Spring. 2015; 21: 5.
- European Medicines Agency. Conduct of Bioequivalence Studies for Veterinary Medicinal Products. European Medicines Agency. 2011.
- Toutain PL, Koritz GD. Veterinary drug bioequivalence determination. J Vet Pharmacol Ther. 1997; 20: 79-90.
- Food and Drug Administration. Freedom of information summary. Original new drug application Excede for swine. 2004; 141-235.
- Papich MG. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet Microbiol. 2014; 171: 480–486.
- ZOETIS. Excede for swine (ceftiofur crystalline-free acid). 2013.
- Lawhorn B. Swine pneumonia. Texas A&M systems. Agrilife Extension. 2010.
- Mexican Official Standard. Technical Specifications for the Production, Care and Use of Laboratory Animals. United Mexican States Department of Agriculture, Livestock. Rural Development Fisheries and Food.1999.
- VICH. Bioequivalence: Blood Level Bioequivalence Study. Brussels: International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. 2015.
- Tantituvanont A, Yimprasert W, Werawatganone P, Nilubol D. Pharmacokinetics of ceftiofur hydrochloride in pigs infected with porcine reproductive and respiratory syndrome virus. J Antimicrob Chemother. 2009; 63: 369-373.
- Jacobson GA, Martinod S, Cunningham CP. Determination of ceftiofur in bovine plasma by HPLC-DAD. J Pharm Biomed Anal. 2006; 40 (5): 1249-1252.
- Clifford K, Desai D, Prazeres da Costa C, Meyer H, Klohe K, Winkler A, et al. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bulletin of the World Health Organization. 2018; 96: 662-664.
- Mawby DI, Whittemore JC, Genger S, Papich MG. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med. 2014; 28: 72–77.
- Huixiao H, George P, Fritz S, Srinubabu G, Sudhakar A. J. Bioequivalences & Bioavalability. J Bioequiv Availab 2014; 1: 1-2.
- Deshpande L, Pfaller M.A, Jones RN. In vitro activity of ceftiofur tested against clinical isolates of Escherichia coli and Klebsiella pneumoniae including extended spectrum beta-lactamase producing strains. Int J Antimicrob Agents. 2000; 15 (4): 271-5.
- Toutain PL, Lees P. Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine. J Vet Pharmacol Ther. 2004; 27 (6): 467–77.
Citation: Ocampo-Camberos L, Monroy-Barreto M, Nieto-Carmona A, Jaime JA, Gutierrez L (2021) Study to Determine Bioequivalence of Three Ceftiofur Crystalline Free Acid in Pigs. J Bioequiv Availab. S4: 001.
Copyright: © 2021 Ocampo-Camberos L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was full sponsored by Ultra Laboratorios, S.A. de C.V. Jalisco, Mexico.

