PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2018) Volume 9, Issue 2
Study of the Immune Response to Hepatitis B Virus Vaccine among Healthy Vaccinated Students in Khartoum, Sudan
Abstract
The aim of this study was to describe the immune response to HBV vaccine among healthy, vaccinated Academy of Health Science students in Khartoum state. A total of eighty one samples (n=81) were obtained from healthy vaccinated students, the samples were involving 12 (14.2%) males and 69 (85.8%) female. The mean of Age (years), Weight (kg), TWBCs (cell/μl) and differential lymphocytes count (%) were 22.22 ± 1.1 year, 58.42 ± 12.5 kg, 5.8 ± 1.7 cell/μl and 38.6 ± 9% respectively. 2.5 ml blood sample was collected from each student in EDTA blood container. TWBCs and differential lymphocytes count were measured from whole blood by using Sysmex Haematological analyzer, and then the plasma was separated from whole blood by centrifugation at 3000 RPM for 5 min. All plasma samples were examined for the presence of anti-HBsAg using Enzyme Linked Immune Sorbent Assay (ELISA). The results showed that out of 81 blood samples investigated, 80 (98.77%) were positive for anti HBsAg while only one (1.23%) was negative. The mean of ELISA reading in the male was higher than female 2.7005 and 2.668 respectively, and there is insignificant effect of gender, weight, TWBCs and differential lymphocytes count in the immune response to HBV vaccine with p value 0.675, 0.070, 0.092 and 0.604 respectively. The study concluded that all most all students produced antibody immune response to HBV vaccine and there are variations in ELISA readings. Further studies with more sample size and by using more advanced technique (quantitative ELISA) should be done to clarify the results.
Keywords: HBV; HBV vaccine; Antibody immune response; Healthy students
Introduction
Hepatitis is inflammation of the liver which can be caused by several insulting agents including viruses, Hepatitis B virus infection is a major public health problem worldwide, roughly 30% of the world's population show serological evidence of current or past infection. Most HBV infections are acquired via horizontal transmission among adolescents and young adults, the virus is spread predominantly by percutaneous or mucosal exposure to infected blood and various body fluids including saliva, menstrual, vaginal and seminal fluids, which have all been implicated as vehicles of human transmission [1-3].
Chronic hepatitis B virus infection is a serious public health problem leading to cirrhosis, hepatocellular carcinoma and death. Over 750,000 people die of hepatitis B each year, and about 300,000 of these are due to liver cancer. Sudan is classified among countries with high hepatitis B surface Ag (HBsAg) endemicity of more than (8%). Exposure to HBV infection ranges of (47%) to (78%) with a hepatitis B surface antigen (HBsAg) seroprevalence ranging from as low as (6.8%) in central Sudan to as high as (26%) in southern Sudan [4-6].
Hepatitis B infection can be prevented by HBV vaccine, vaccination against hepatitis B has been available since the beginning of the 1980s, the recombinant hepatitis B vaccine was introduced in 1986 and has gradually replaced the plasma-derived vaccine. The active substance in recombinant hepatitis B vaccine is HBsAg that has been produced in yeast or mammalian cells into which the HBsAg gene has been inserted by using plasmid [7-9].
Vaccination is recommended to all newborns and high-risk groups of hepatitis B infection including Homosexually active men, persons at high risk of sexually transmitted HBV infection, injection drug user, household members living with HBV carrier patients, sexual partner of HBV carriers, patients of hemodialysis units, patients who receive clotting factor concentrates and persons at occupational risk of HBV infection [10-12]. Vaccine includes 20 μg of surface antigen for adults and 10 μg for children. The vaccine should be given by intramuscular injection in anterolateral aspect of thighs of infants and children less than 2 years of age and in the deltoid muscle of older children, adolescents and adult, it is generally administered in three doses with the second dose given one month after the first dose and the third dose given sex months after the first dose [10,13]. Post-vaccination testing should be performed 1-2 months after completion of the vaccine series. Anti-HBs response less than 10 IU/mL after completion of the first vaccine series is called 'non-responders'. Re-vaccination is recommended for non-responders. So administration of a single dose of vaccine followed by measurement of anti-HBs antibody response 1-3 months later [10,14]. Antibody tests should be considered in health care workers at high risk of infection or in the cases of hemodialysis patients [15].
This study was sought to describe the immune response to HBV vaccine among healthy, vaccinated students.
Materials and Methods
This study a cross-sectional ®laboratory based conducted in Khartoum state, during the period of January to April 2017. A total of eighty one samples (n=81) were obtained from healthy, vaccinated students three month post vaccination. All samples were examined for TWBCs and differential lymphocytes count from whole blood by using Sysmex Haematological analyzer. In addition, plasma was separated from whole blood by centrifugation and all plasma samples were examined for the presence of anti-HBsAg by a commercially available enzyme - linked immunosorbent assay "HBsAg ELISA" kit (Fortress Diagnostics Limited, unit 2 C Antrim technology parks, Antrim, BT4I IQS the United Kingdom). The assays were performed following the instructions in the manufacturer manual. Positive and negative controls were included in each assay.
Procedure of ELISA test
All reagents and specimens were settled to reach room temperature. 50 μl of positive control, negative control and specimen were added to their respective wells in the micro-titer plate followed by adding 50 μl of enzyme conjugate to each well except the blank, then the plate was covered with plate cover and incubated for 30 min at 37°C. By the end of incubation period each well was washed 5 times with diluted wash buffer and 50 μl of chromogen A and chromogen B solutions were added to each well including blank, then the plate was incubated at 37°C for 15 min and stop solution was added by the end of the later incubation. Each well Absorbance was read against blank well by using ELISA reader as final step of ELISA procedure.
Calculation and Interpretation of results
The results were calculated by relating each specimen absorbance (A) to the cut off (c.o.) of the plate. Cut off value (C.O.) was calculated by using the following equation Cut off value (C.O.)=NC × 2.1 (NC is mean of the three negative controls reading) (=.05*2.1=0.105). Only samples shows Absorbance more than cut of value were considered as positive, while those shows Absorbance less than cut of value were considered as negative.
Quality control and of the results
Reagent, standard and control were checked for storage, stability and preparation before starting work. Each micro-plate was considered separately when the results was calculated and interrelated.
Method used for data collection
Data was collected by using administrated questionnaire include the following information Gender, Weight and Age.
Data analysis
The data that collected from questionnaire and laboratory results were analyzed by SPSS version 15 computerized programs.
Results
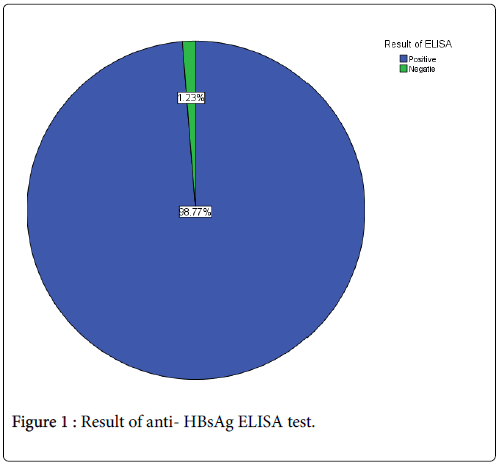
A total of eighty one samples (n=81) were obtained from healthy, vaccinated academy of health science students, the samples were involving 12 (14.2%) males and 69 (85.8%) female as that showed in (Table 1). The mean of Age (years), weight (kg), TWBCs (cell/μl), differential lymphocyte count (%) and ELISA reading were 22.22 ± 1.1, 58.42 ± 12.5, 5.8 ± 1.7, 38.6 ± 9 and 2.67 ± 0.8 respectively (Table 2). The result showed that out of 81 blood samples investigated, 80 (98.77%) were positive for anti HBsAg while only one (1.23%) was negative (Figure 1).
| Gender | Frequency | Percent % |
|---|---|---|
| Male | 12 | 14.8 |
| Female | 69 | 85.2 |
| Total | 81 | 100 |
Table 1: Distribution of participants according to the gender.
| Statistics | Variables | ||||
|---|---|---|---|---|---|
| Age | Weight | TWBCs | Differential lymphocyte count | ELISA reading | |
| Mean | 22.22 ± 1.1 | 58.42 ± 12.5 | 5.8 ± 1.7 | 38.6 ± 9 | 2.67 ± 0.8 |
| Median | 22 | 56 | 5.7 | 38.7 | 3.06 |
| Mode | 22 | 57 | 5 | 28.9 | 3.04 |
| Maximum | 28 | 117 | 10.2 | 57.4 | 3.116 |
| Minimum | 20 | 38 | 2.7 | 16.8 | 0.021 |
Table 2: Mean, Median, Mode, Maximum and Minimum of Age, Weight, TWBCs, Differential Lymphocytes count and ELISA reading.
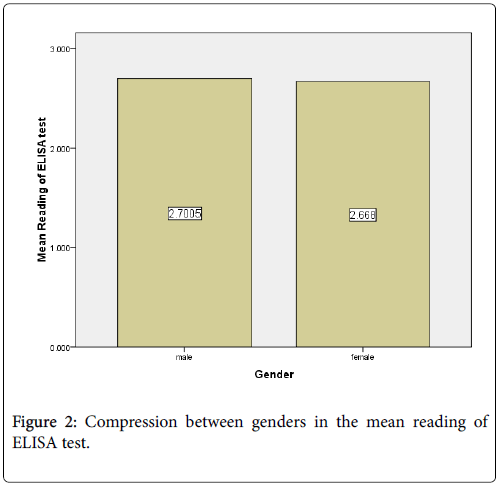
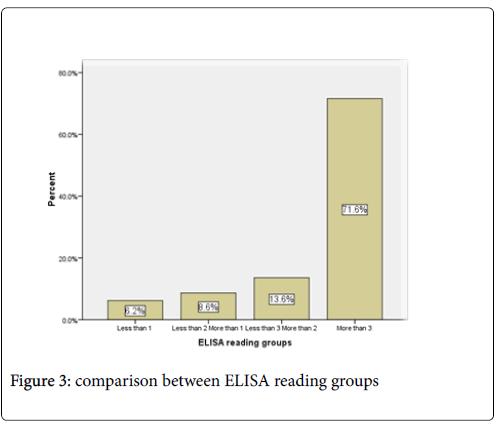
The mean of ELISA reading in the males (2.7005) was higher than female (2.668) (Figure 2). Out of 80 positive samples there were 76.1%, 13.6 %, 8.6% and 6.2% show ELISA reading above 3, less than 3 more than 2, less than 2 more than 1 and less than 1 respectively. (Figure 3).
There is insignificant effect of gender, weight, TWBCs and differential lymphocytes count in the immune response to HBV vaccine, with p value 0.675, 0.070, 0.092 and 0.604 respectively Tables 3 and 4.
| Variables | ELISA test result | Mean | P. value | Interpretation |
|---|---|---|---|---|
| Weight | Positive | 58.14 | 0.07 | Insignificant Difference in weight |
| Negative | 81 | |||
| TWBCs | Positive | 5.79 | 0.092 | Insignificant Difference in TWBCs |
| Negative | 8.7 | |||
| Differential lymphocytes count | Positive | 38.67 | 0.604 | Insignificant Difference in Differential lymphocytes count |
| Negative | 33.9 |
Table 3: There is in significant difference in the means of weight, TWBCs and Differential lymphocytes count depending on ELISA test results.
| Gender | Result of ELISA | p. value | Interpretation | |
|---|---|---|---|---|
| Positive | Negative | |||
| Male | 12 | 0 | 0.675 | Insignificant difference in the result of ELISA test according to the Gender |
Table 4: Chi-squire test: There is insignificant difference in the results of ELISA test according to the Gender
Discussion
Hepatitis B virus infection is a major public health problem worldwide. Chronic HBV can lead to cirrhosis, hepatocellular carcinoma and eventually death. Recombinant HBV vaccine was introduced to protect against the infection [16,17]. The present study was aimed to detect the immune response to recombinant HBV vaccine among healthy, vaccinated students in Khartoum state.
This study demonstrates that there is high efficacy rate of recombinant HBV vaccine with (98.77%) of participants produces anti-HBsAg, this high efficacy rat is agree with the results of several studies obtained in Canada, America and by WHO whom reported (95-100%) and more than (95%) efficacy rate respectively [18,19]. This closeness of findings from different studies which carried out in various country may be explained by excellent effectiveness and immunogenic activity of recombinant vaccine regardless the age and socioeconomic status. In contrast, our result disagreed with that reported in Gambia by Maimuna et al., [20] whom describe that the vaccine efficacy against infection with HBV was (95.1%), This difference in mounting antibody responses may be due to a constellation of factors for example environment, exposure to other diseases and the difference in genetic makeup. In addition to that, they may use anther technique to measure the antibody levels. Several studies suggested that the weight and gender may effect on the function of the immune system, in recent study we find that the impact of weight, gender, TWBCs and differential lymphocyte count in the immune response to HBV vaccine was insignificant, and this may be due to high purity and immunogenic activity of the recombinant vaccine. Besides, qualitative technique was used in this study to detect the antibody to vaccine instead, using of quantitative technique to measure the antibodies level may be more helpful.
It is worth mentioning that the only participant that failed to produce antibody after successful three doses of vaccination was screened for the presence of HBsAg, and the result shows that she has chronic HBV infection and this may explain why she was not responded to vaccine. In addition, this finding may conflict the published reports that vaccination of patients with HBV may lead to severe adverse reaction which is not noted with this case.
Conclusion
All most all healthy vaccinated Academy of Health Science students were produced antibody to HBV vaccine. Gender, weight, TWBCs and differential lymphocytes count have insignificant effect in immune response against HBV vaccine and there were variations between samples in ELISA readings. Therefore, Screening of all vaccinated individual under high risk groups and measuring their antibodies levels to determine their protective capability against HBV infection is recommended. Further, studies with more sample size and using more advanced techniques (e.g. quantitative ELISA) should be done to clarify the results.
Acknowledgements
Special thanks to all students whom participated in this study, with our best wishes to them to success in their life. Also, we would like to acknowledge Mr. Moamen Ezz-aldin Mohammed and Mr. Ahmed Mahjob. For their support in laboratory analysis of the samples.
References
- Dény P, Zoulim F (2010) Hepatitis B virus, from diagnosis to treatment. J Pathol Biol 58: 245-253.
- Trepo C, Chan H LY, Lok A (2014) Hepatitis B virus infection. Lancet 384: 2053-2063.
- Uo Z, Li L, Ruan B (2012) Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis 16: 82-88.
- Mudawi H M (2008) Epidemiology of viral hepatitis in Sudan. J Clin Exp Gastroenterol 1: 9-13.
- Schädler S, Hildt E (2009) HBV life cycle: Entry and morphogenesis. Viruses 1: 185-209.
- Adrian M (2009) Hepatitis B and Hepatocellular Cracinoma. Hepatology 49: 56-60.
- Chen DS (2009) Hepatitis B vaccination, The key towards elimination and eradication of hepatitis B. J Hepatol 50: 805-816.
- Kristina C (2009) Studies on Hepatitis B Vaccination and Factors Associated with Vaccine Response. Div Infect Dis 67: 0345-0082.
- CDC (2016) Centers for disease, control and prevention, epidemiology and prevention of vaccine. Preventable diseases, Hepatitis B.
- Kwon SY, Lee CH (2011) Epidemiology and prevention of hepatitis B virus infection. Korean J Hepatol 17: 87-95.
- Fisman DN, Agrawal D, Leder K (2002) The effect of age on immunologic response to recombinant hepatitis B vaccine, a meta-analysis. J Clin Infect Dis 35: 1368-1375.
- Bertoletti A, Gehring A (2006) The immune response during hepatitis B virus infection. J Gen Virol 87: 1439-1449.
- WHO (2015) Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Guidel Prev care Treat Pers with chronic Hepatitis B Infection.
- Nikhil K, Jennifer T, Maria S, Simon C (2013) Autoimmune hepatitis: clinical review with insights into the puringeric mechanism of disease. J Clin Transl Hepatol 1: 79-86.
- Chisari FV, Isogawa M, Wieland SF (2010) Pathogenesis of hepatitis B virus infection. J Pathol Biol 58: 258-266
- Chisari FV, Ferrari C, Mondelli MU (1989) Hepatitis B virus structure and biology. Microb Pathog 6: 311-325.
- Gish RG, Given BD, Lai CL, Locarnini SA, Lau JN, et al. (2015) Chronic hepatitis B, virology, natural history, current management and a glimpse at future opportunities. Antiviral Res 121: 47-158.
- P Public Health Agency of Canada ramoolsinsup C (2002) "Management of viral hepatitis B". J Gastroenterol Hepatol 17: 125-145
- Elke L, Pierre V (2016) Hepatitis B and the need for a booser dose. Clin Infect Dis 53: 68-75.
- Maimouna M, Ingrid P (2013) Observational Study of Vaccine Effecacy 24 Years, after Start of Hepatitis B vaccination in Two Gambian Villages: No Need for a booster Dose 8: 3.
Copyright: © 2018 Gorish BMT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.