Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 2
Stingless Bee (Meliponulla baccaerii) Honey Antibacterial Activities Against Salmonella typhi, Escherichia coli, Staphylococcus aureus and Enterococcus faecalis
Kasim Roba Jilo*Received: 26-Jan-2024, Manuscript No. JBP-24-24703; Editor assigned: 29-Feb-2024, Pre QC No. JBP-24-24703 (PQ); Reviewed: 14-Mar-2024, QC No. JBP-24-24703; Revised: 21-Mar-2024, Manuscript No. JBP-24-24703 (R); Published: 26-Mar-2024, DOI: 10.35248/2155-9597.24.15.506
Abstract
Background: This study aims to explore the antimicrobial activity of Ethiopian stingless bee honey against human pathogenic microbes, which are increasingly developing drug resistance. Reports indicate that stingless bee honey possesses numerous medicinal properties, including potential therapeutic benefits. It serves as an antiseptic, antimicrobial, anticancer, anti-inflammatory agent, and promotes wound healing.
Methods: Four concentrations 25%, 50%, 75% and 100% were used to examine the antibacterial effects on human pathogens using a Completely Randomized Design (CRD). The inhibitory effects of these concentrations were observed over exposure periods of 48 hours and 72 hours, compared with both a negative control (sterilized water) and a positive control (Chloramphenicol) against two Gram-negative and two Gram-positive bacteria. A P-value of <0.005 was considered significant.
Results: In the disk diffusion assay, antibacterial activity was observed for stingless bee honey against the tested strains. The significance of the time depended on the working concentration prepared and the species of bacteria used. The standard drug inhibited more Gram-negative bacteria than Gram-positive. The 100% concentration inhibited Salmonella typhi and Escherichia coli more than it did Staphylococcus aureus and Enterococcus faecalis.
Conclusion: This research provides a novel perspective on the antibacterial effects of stingless bee honey against pathogenic Gram-negative bacteria such as Escherichia coli ATCC 25922, Salmonella typhi ATCC 8759, and Gram- positive bacteria like Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212. The significance of time and concentrations was found to depend on the bacterial species. The bacterial species-dependent nature of the significance of time and concentrations was observed. These bacteria were identified to have a Minimum Inhibitory Concentration (MIC) of 25%. The honey samples tested in this work demonstrated antibacterial activity against both Gram-positive and Gram-negative bacteria. The results reported here highlight the potential of using stingless bee honey to control bacterial growth. It was revealed by this study that honeydew honey produced by stingless bees has promising antibacterial activity against pathogenic bacteria, including antibiotic-resistant strains.
Keywords
Antibacterial effects; Enterococcus faecalis; Escherichia coli; Meliponulla baccaeri; Salmonella typhi; Staphylococcus aureus; Stingless bees; Stingless bee honey
Abbreviations
AMR: Antimicrobial Resistance; ATCC: American Type Culture Collection; MHA: Mueller-Hinton Agar; MIC: Minimal Inhibitory Concentration
Background
Humans have used honey as a medicinal source for millions of years [1]. Pot-honey, also known as stingless bee honey, Meliponine honey, Pot-honey, and Keller-honey in Malaysia, is a ‘wonder fluid’ that astounds with its countless healing properties [2]. Stingless bee honey is widely used as a functional food and is reported to possess numerous medicinal properties, such as antiseptic, antimicrobial, anticancer, anti-inflammatory, and wound-healing properties [3]. It targets a variety of ailments, such as gastroenteritis, cataracts, and even aids in wound-healing [4]. However, knowledge about it remains relatively limited and its theoretical medicinal value lacks evidence linked to specific bioactive components [5].
Stingless bee honey promotes the growth of apoptotic and necrotic cells in testicular tissue and has been found to have various therapeutic effects including anti-diabetic effects through α-amylase and α-glycosidase inhibition [6]. The potent inhibitory activity of stingless bee honey has recently sparked increased interest in using honey to eliminate antibiotic-resistant bacteria [7]. Stingless bee honeys from Costa Rica are primarily valued for their ethno pharmacological use as a wound dressing while in Western Maharashtra, India, the propolis of the stingless bee (Trigona sp.) is a popular folk medicine remedy for various ailments [8,9]. Stingless bee honey holds significant therapeutic potential, particularly as an antimicrobial agent [10]. Stingless bee honey promotes the growth of apoptotic and necrotic cells in testicular tissue [2].
Antimicrobial activity of stingless bee honey has been tested against six wound pathogens in Malaysia using agar well diffusion [11]. Modern science recognizes that the traditional use of stingless bee honey has significant potential as a valuable addition to modern medicine, and it is considered to have higher medicinal value than other bee species [12]. Stingless bee honey, particularly from Malaysia, offers numerous benefits, especially in the medical field, because of a vast array of active phytochemical compounds [13].
The native people of Northern Australia highly value stingless bee honey as a food source, and it holds cultural significance, playing a part in the social traditions and rituals of these people [14]. Similarly, in Western Maharashtra, India, the propolis of the stingless bee (Trigona sp.) is a common remedy in folk medicine for various ailments [9]. Stingless honey bees, which belong to the Meliponinae species, are native to tropical and subtropical regions. They have undertaken evolutionary worsening and are unlikely to cause harm or injury to humans [15]. Stingless bees can be classified into two genera: Melipona and Trigona [16]. These bees, common in tropical countries, are known for their honey production [17].
Stingless bees, also known as meliponines, makes eusocial bees large group and they are unique in that their stingers are immature and incapable of stinging a person [18]. Classified under family Apidae and sub-family Meliponinae, stingless bees are the smallest species of bees that produce honey [19]. This study is designed to explore the antibacterial properties of stingless bee honey against specific pathogenic microbes mentioned above.
Staphylococcus aureus is a bacterium known to cause a range of diseases through both suppurative and non-suppurative methods [20]. Stingless bees, specifically those of the Meliponini tribe, are highly social creatures native to tropical and sub-tropical ecosystems [21,22].
Stingless bee honey is a valuable food product known for its therapeutic benefits [23]. The Meliponinae, large group of bees that lack a sting, are found in various tropical and subtropical regions around the world [24]. Stingless bee or pot-honey honey is a natural product produced by a diverse group of highly eusocial bees [25]. These stingless honey bees, which are part of the Meliponinae species, are indigenous to various tropical and subtropical regions and are unique in their lack of a sting [15]. Stingless bees represent a vast monophyletic class of highly eusocial bees that are commonly found in abundance in warm, humid forests around the world [26].
With the rapid increase in microbial resistance against prescribed antibiotics becoming an issue, it’s essential to screen for effective, safe, affordable, and readily available therapeutics from various medicinal plants like herbs for their potential antimicrobial effects [27]. One of the significant challenges the world faces today is the antimicrobial resistance exhibited by most bacterial pathogens to available antibiotics and the escalating cost of discovering effective antimicrobial agents [28]. In recent years, the demand for an effective cure for infectious diseases has boosted efforts to find new drugs, especially from natural sources. The rise in microbial resistance towards modern antimicrobial drugs has become a topic of interest among scientists who are developing novel drugs with less or no microbial resistance and broad-spectrum inhibition activity [2]. Therefore, studying antimicrobial activities is essential to investigate plant resources for medicinal values [29].
Honey, particularly from the Apis mellifera species, has been observed to have various biological effects, including antimicrobial, antifungal, antioxidant, and immune properties. Its topical application as an antibacterial and healing agent has been proven effective and is a cost-effective alternative [30]. The primary goal of antimicrobial or antibacterial applications is to prevent or combat infections, especially during the healing period. Pot-honey, due to its antimicrobial and antiseptic properties, can be used as an antibacterial ingredient in pharmaceutical formulations [16]. In modern times, honey is globally traded due to its exceptional medicinal value. Like other food supplements, the healing properties of honey are advancing in pharmacological science [2].
The creation of new drugs has been the primary solution chosen to address thisproblem [31]. The antimicrobial activity of honey depends on several factors, including its botanical origin, and geographical and entomological sources [32]. Currently, antimicrobial agents are the world’s only factor for eradicating infectious diseases [32]. One of the significant challenges today is the antimicrobial resistance exhibited by most bacterial pathogens to available antibiotics and the escalating cost of discovering effective antimicrobial agents [28]. Several studies have tested the antimicrobial activity of combinations of honey and various substances [33]. Therefore, this study aims to test the antimicrobial activities of stingless bee honey against the aforementioned human pathogenic microbes to find alternative solutions to these problems. Stingless bee species called Melliponulla baccaeri has been identified in Ethiopia and based on this background information this research is highlighting the potential of stingless bee honey as an alternative to antibiotics, according to the growing concern over antibiotic-resistant bacteria. The study is investigating the antibacterial properties of this honey against specific pathogenic microbes under various conditions. The aim is to deepen our understanding of the significance of stingless bee honey and stimulate further research into alternatives for drug- resistant microbes. This could potentially pave the way for the integration of stingless bee honey into pharmaceutical products.
Materials and Methods
Setting
The study was conducted in the Microbiology Laboratory at the Ethiopian National Biotechnology Research Institute in Holeta, Oromia, Ethiopia in 2023. The study was organized as a factorial experiment in (CRD) [28]. The factors included four species of bacteria and four concentrations, with both negative and positive controls.
Sample collection
During the collection process, the sealed portion of the pot was carefully broken with a sterile needle. The tip of a sterile disposable syringe was then dipped into the opening of the honey pot [34]. Collected honey was tested against Enterococcus faecalis ATCC 29212, Salmonella typhi ATCC 8759, Eshcheria coli ATCC 25922, and Staphylococcus aureus ATCC 25923 [33].
Sample preparation
The sample selected for this study was stingless bee honey. These samples were collected from the West Shoa zone, specifically from Gedo, and were stored at 4°C until the experiment was conducted [5]. A fresh culture of test organisms (50 μl) was swabbed over the surface of Mueller Hinton agar (Oxoid, UK) plates using a sterile cotton swab in eight different angles. This ensured that the organism was uniformly distributed on the agar surface [11].
Concentration preparation
Four concentrations of stingless bee honey were used in the study: 100%, 75%, 50%, and 25%. The preparation of these concentrations was described as follows: 10 grams of stingless bee honey was considered as 100% concentration, 7.5 grams of stingless bee honey mixed with 2.5 ml of sterilized distilled water was considered as 75% concentration, 5 grams of stingless bee honey mixed with 5 ml of sterilized distilled water was considered as 50% concentration, and 2.5 grams of stingless bee honey mixed with 7.5 ml of sterilized distilled water was considered as 25% concentration, which was the minimum inhibitory concentration. The bacteria were incubated for 24 hours, 48 hours, and 72 hours, and data were collected only for 48 hours and 72 hours since no inhibition was seen at 24 hours [29].
Antimicrobial disks selection
Antibiotic disks, specifically Chloramphenicol (30 μg, C-30), were stored at 4°C and used as a positive control. A negative control was also added, along with all treatments, to the plate [35]. The dried discs were then placed onto the surface of the inoculated Nutrient agar plates. Agar plates were incubated at 37°C for 24 hours. The diameter of the inhibition zones was measured in millimeters (mm) using calipers [36]. Antimicrobial tests were performed to determine the inhibitory properties of spot-honey against foodborne pathogens using an agar well diffusion assay [37].
Antimicrobial activity
The evaluation of the antimicrobial activity of the samples was conducted using a 96-well microtiter plate-based Minimum Inhibitory Concentration (MIC) assay. The tests were performed against American Type Culture Collection (ATCC) strains, which served as a reference for antimicrobial activity. The strains tested included Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Salmonella typhi, and Enterococcus faecalis [8]. The bacterial suspension was then introduced into each of the petri dishes containing Mueller Hinton Agar (MAH) media [38].
Application
With the use of sterile forceps or a disk dispenser, an antibiotic disk was placed on the plate after dried and slightly pressed down to ensure contact with the surface of the plate [35]. The antimicrobial properties were analysed using the well diffusion method (mm) against foodborne pathogens [39]. For the inoculation of plates, a fresh culture suspension of the test microorganisms (100 μL) was spread on Mueller Hinton agar.
Inoculation of plates
The plates were inoculated with a fresh culture suspension of the test microorganisms (100 μL), which was spread on Mueller Hinton agar [40]. After quarter minutes adjusting turbidity, sterile cotton swap was dipped into the standardized bacterial suspensions; then, 100 microliters of bacteria in fluid form were added to the plate [35].
Minimum Inhibitory Concentration (MIC) determination
The honey sample was diluted with sterilized distilled water to a lower concentration to determine its minimum inhibitory concentration. The minimum inhibitory concentration of the sample from this specific geographical location was 25%. The sample was prepared by adding 2.5 grams of stingless bee honey to 7.5 ml of sterilized distilled water, resulting in a 25% concentration, which is the minimum inhibitory concentration for these bacteria.
Incubation
For incubation, the plates were inverted and incubated at 37°C or at an optimum growth temperature for the tested microorganism. After 48 hours and 72 hours, the zones of inhibition were measured. The results obtained were then compared for all treatments and time exposures [35].
Collection and maintenance of test organisms
The antibacterial effects of stingless bee honey on the growth of four different species of bacteria were examined. The bacteria selected for this study were Escherichia coli, Staphylococcus aureus , Salmonella typhi, and Enterococcus faecalis. Universal bottles, each containing nutrient broth 10 ml, individually and inoculated with bacteria using an inoculum loop. They were then incubated at 37°C for up to 48 hours. These bacterial cultures were then stored at 4°C until they were used (Table 1).
| Bacterial species | Gram +/- |
|---|---|
| Salmonella typhi | -ve |
| Escherichia coli | -ve |
| Staphylococcus aureus | +ve |
| Enterococcus faecalis | +ve |
Table 1: Gram positive and Gram negative of different bacteria species.
Disk diffusion assay (Evaluation zone of inhibition)
In evaluation of inhibition zones made by these, 25%, 50%, 75%, and 100% concentrations of stingless bee honey filled into sterilized petri plates (90 mm). Bacteria were then inoculated onto the petri plates, allowed to solidify, and individual plates were marked for the inoculated organism. Disks were used to test these solutions. Chloramphenicol and solvents (distilled water) were used.
Statistical analysis
In the statistical analysis, the data obtained from the study were prepared in triplicate and analysed using Analysis of Variance (ANOVA). The differences between mean values were considered significant at p-values<0.05. For pair comparisons, Duncan’s test was utilized in R software.
Results
The standard drug exhibited more inhibition of S. typhi, E. coli, and S. aureus at 72 hrs and 48 hrs (Figure 1). However, it did not significantly inhibit E. faecalis (Table 2). Time didn’t show significant effect on S. typhi and E. faecalis at a 100% concentration, but it was significant for E. coli and S. aureus. Time also had significant effects on S. typhi and S. aureus, but not on E. coli and E. faecalis at 75% concentrations. At a 50% concentration, time had significant effects on E. coli and S. typhi, but not on E. faecalis and S. aureus. At the lowest inhibitory concentration of 25%, time did show a significant effect on S. typhi, but it was significant for E. coli, S. aureus, and E. faecalis.
| Tested products | S. typhi at 48 hrs | S. typhi at 72 hrs | E. coli at 48 hrs | E. coli at 72 hrs | S. aurues at 48 hrs | S. aurues at 72 hrs | E. faecalis at 48 hrs | E. faecalis at 72 hrs | Mean | CV |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug (positive control) | 12.33d | 37.33a | 15.00d | 29.60b | 21.00c | 30.33b | 21.00c | 22.00c | 23.58 | 13.69 |
| S100% | 20.33ab | 22.67ab | 20.33ab | 16.67b | 20.00ab | 27.33a | 21.33ab | 23.67ab | 21.54 | 19.3 |
| S75% | 13.67b | 18.33a | 11.33bc | 11.00bc | 8.33c | 12.00bc | 13.33b | 13.67b | 12.71 | 16.69 |
| S50% | 11.33a | 6.67ab | 9.67ab | 5.00b | 6.00ab | 8.33ab | 7.00ab | 8.67ab | 7.83 | 35.15 |
| S25%` | 4.00ab | 1.33bc | 6.00a | 1.67bc | 0.00c | 6.33a | 0.00c | 5.00a | 3.04 | 53.27 |
| S. water (negative control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: a,b,c,d-Values of similar letters that are not significantly different; Whereas: S100%=100 percent of stingless bee honey concentration; S75%=75 percent of
Stingless bee honey concentrations; S50%=50 percent of stingless bee honey concentrations; S25%=25 percent of stock solution prepared, S. water=Sterilized water, Drug=Chloramphenicol.
Table 2: Antibacterial properties of stingless bee honey at different concentrations (%) and exposure periods of 48 and 72 hrs.
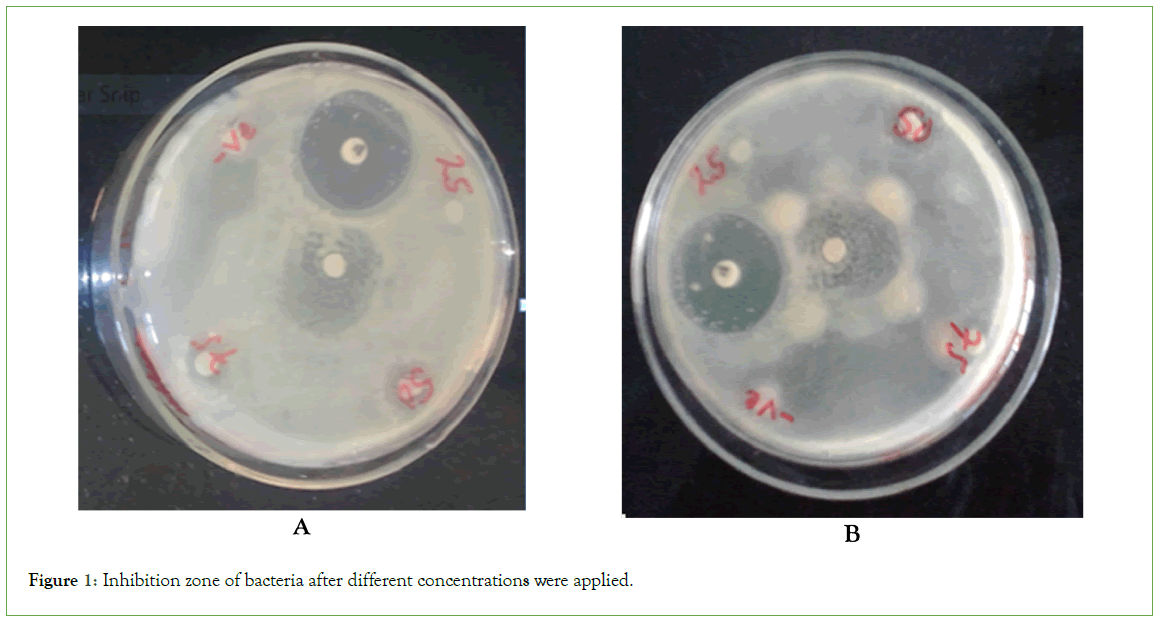
Figure 1: Inhibition zone of bacteria after different concentrations were applied.
Discussion
In the discussion, it was found that the standard drug had no significant effect on S. typhi and E. coli. This could be attributed to the fact that they are Gram-negative bacteria, which may be due to the external cover found on their outer layer. Likewise, Gram- positive bacteria, both S. aureus and E. faecalis, were significantly inhibited by standard drugs, which align with the reports of [29]. The inhibition zone of bacteria varied based on bacterial species, working concentration prepared, and exposure periods, which is similar to the reports of [29]. Stingless bee honey had significant effects against Gram-positive bacteria (S. aureus, Enterococcus faecalis) and Gram-negative bacteria (E. coli), either significantly stronger or equivalent to standard antibiotics, which is parallel to the reports of [17]. The positive control exhibited higher bactericidal activity for S. aureus than for E. coli, which aligns with this finding [28]. Furthermore, in agreement with, our results also showed that Gram-negative bacteria like E. coli exhibited the lowest inhibition zone by positive control [39].
The research reported that a concentration of 30% (w/v) did not inhibit bacterial growth in this test, which supports our finding that bacteria were not inhibited below a 25% concentration, indicating it as the minimum inhibitory concentration [31]. Stingless bee honey inhibited S. aureus at 100% in Cape Coast=11.46 ± 0.2 10, which is lower than Ethiopian Stingless bee honey according to our findings [34]. This might be due to differences in botanical origin.
In line with our findings, a study conducted showed that Stingless Bee Honey (SBH) had a more inhibitory effect on the test microbes than commonly used antibiotics, although the activity against the Gram-negative bacterium (Pseudomonas aeruginosa) was limited [34]. Another research also stated that stingless bee honey exhibited the highest mean inhibition (2.2 ± 0.4 cm), which agrees with our findings [7]. Agrees with those who reported that E. coli was not inhibited at 50%, 25%, and 12.5% concentrations of stingless bee honey. It was inhibited below a 25% concentration, and a 25% concentration is the MIC for this experiment [1]. Previous studies reported that, at a 100% honey concentration, among the fifteen honey samples, stingless bee honey showed a maximum zone of inhibition against E. coli (13 mm), which is lower than our finding [19]. Therefore, we can conclude that stingless bee honey recorded a maximum inhibition of 23 mm at a 100% concentration.
This finding contradicts the report by, which stated that Salmonella typhi was not inhibited at all concentrations, even by 100% [19]. S. aureus was inhibited at 31.6 mm, whereas E. coli was inhibited at 12.00 mm. This suggests that Gram-positive bacteria were inhibited more than Gram-negative bacteria, which aligns with our findings [30]. S. aureus was inhibited at 32 mm, while E. coli was inhibited at 23 mm, indicating that S. aureus is more inhibited than E. coli. This is similar to the results reported by Boanerges R, et al. (2013) [41]. Our findings also show that S. aureus and E. faecalis were more inhibited than E. coli, with inhibition zones of 32 mm and 25 mm respectively for S. aureus and E. faecalis, compared to 23 mm for E. coli [14]. A previous study reported that the maximum inhibition for S. aureus was 32.6 mm, whereas for E. coli it was 27.3 mm, suggesting that Gram-negative bacteria were less inhibited than Gram-positive bacteria [30].
Our results are also in agreement with a study that found S. aureus to be inhibited the most at a 100% concentration (32 mm), whereas E. coli was inhibited at 23 mm, suggesting that Gram-negative bacteria were less inhibited than Gram-positive bacteria. Another study reported that the average inhibition zones of Gram-positive strains treated with stingless bee honey were 18.30 mm (Standard Error (SE) ± 1.07), whereas Gram-negative strains exhibited zones of 10.28 mm (SE ± 0.56), indicating that Gram-positive bacteria were more sensitive (p<0.05) [33]. Reports showed that among the Gram-positive strains used in the assay, S. aureus ATCC 25923 was sensitive to all four honeys evaluated. In contrast, among the Gram-negative bacteria, the E. coli strain was less sensitive to all four honeys tested, which aligns with our findings [10]. In line with our findings, Gram-positive bacteria were found to be more sensitive than Gram-negative bacteria at all concentrations and exposure periods [10]. A previous study reported that commercial honey samples were not active against the Gram-negative bacterium, E. coli which aligns with our findings [40].
Another study reported that the zones of inhibition increased with the increasing concentration of Stingless Bee Honey (SBH), with the highest inhibitory effect observed on Gram-positive bacteria [34]. Similarly, a 100% concentration inhibited more significantly than 75%, 50%, and 25%. Chloramphenicol inhibited E. coli at 32 ± 0.0 and S. aureus at 23 ± 0.0, which is consistent with our findings where E. coli was inhibited at 24 mm and S. aureus was inhibited at 15 mm [37]. Research has reported that the positive control inhibited E. coli at 38.33 ± 0.58 and S. aureus at 33.33 ± 1.53, which aligns with our findings [13]. This suggests that stingless bee honey inhibited the bacteria more than the positive control, indicating its potential as a promising natural product for combating bacteria that are developing resistance against medically prescribed drugs.
It was reported that E. coli and S. aureus were inhibited by stingless bee honey at 8.67 ± 0.58a and 16.33 ± 1.15a respectively [39]. This is similar to our findings, which showed inhibition zones of 16.67b and 27.33a for E. coli and S. aureus respectively. However, a report stating that S. aureus was inhibited at 3.89 ± 3.74a and E. coli was inhibited at 5.37 ± 4.30a by stingless bee honey contradicts our findings [32].
Limitations
This study was primarily focused on investigating the antibacterial activities of stingless-bee-honey. However, it was limited in scope due to several factors. Firstly, the specific mechanisms underlying these antibacterial effects were not examined. Secondly, the particular components of the bacteria that were affected by the honey were not identified. These limitations were largely due to constraints in our research facilities. Understanding these aspects is essential for approving stingless-bee-honey as a medicinal food, especially considering the growing issue of microbial resistance. Therefore, we recommend further research to delve into these areas. Moreover, this study did not explore the potential use of stingless-bee-honey in pharmaceutical products on a global scale. Given the potential results of our research, we believe that future studies should investigate this potential, which could have significant implications for the healthcare industry worldwide. In conclusion, while our study provides valuable insights into the antibacterial properties of stingless-bee-honey, it also highlights the need for more comprehensive research in this field.
Recommendations
Further research on stingless bee honey: Given its significant antibacterial activity, particularly against Gram-positive bacteria, more research should be conducted on stingless bee honey. This could include exploring its potential uses in medical and health applications.
Investigate botanical origins: The study found that the antibacterial activity of stingless bee honey varied depending on its botanical origin. Therefore, further research should be conducted to understand how different botanical origins affect the antibacterial properties of the honey.
Explore concentration effects: The study found that a concentration below 25% did not inhibit bacterial growth. Future studies could explore this further, perhaps investigating the effects of different concentrations on a wider range of bacteria.
Gram-negative bacteria: The study found that Gram-negative bacteria were less inhibited than Gram-positive bacteria. Further research could explore why this is the case and whether there are ways to increase the honey’s effectiveness against Gram-negative bacteria.
Conclusion
In conclusion, our study confirmed that the inhibition zone of both Gram-negative and Gram-positive bacteria varies based on the type of treatment, the working concentration prepared from the stock solution of the experiment, and the exposure period of the experiment. The positive control exhibited higher bactericidal activity for S. aureus than for E. coli. The research reported that a concentration of 30% (w/v) did not inhibit bacterial growth in this test, which supports our finding that bacteria were not inhibited below a 25% concentration, indicating it as the minimum inhibitory concentration. Stingless bee honey inhibited S. aureus at 100% in Cape Coast=11.46 ± 0.210, which is lower than Ethiopian Stingless bee honey according to our findings. This might be due to differences in botanical origin.
Our findings also show that S. aureus and E. faecalis were more inhibited than E. coli, with inhibition zones of 32 mm and 25 mm respectively for S. aureus and E. faecalis, compared to 23 mm for E. coli. A previous study reported that the maximum inhibition for S. aureus was 32.6 mm, whereas for E. coli it was 27.3 mm, suggesting that Gram-negative bacteria were less inhibited than Gram-positive bacteria. Our results are also in agreement with a study that found S. aureus to be inhibited the most at a 100% concentration (32 mm), whereas E. coli was inhibited at 23 mm, suggesting that Gram-negative bacteria were less inhibited than Gram-positive bacteria. Another study reported that the average inhibition zones of Gram-positive strains treated with stingless bee honey were 18.30 mm (Standard Error (SE) ± 1.07), whereas Gram-negative strains exhibited zones of 10.28 mm (SE ± 0.56), indicating that Gram- positive bacteria were more sensitive (p<0.05). These findings suggest that stingless bee honey has significant potential as an antibacterial agent, particularly against Gram-positive bacteria.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
Data generated during the course of this research are available upon reasonable request, and can be obtained by contacting the corresponding author.
Acknowledgements
First and foremost, I would like to express my profound gratitude to the Holeta National Biotechnology Research Institute in Ethiopia. Lastly, I would like to acknowledge the Oromia Agricultural Research Institute for their generous financial support, which was instrumental in all aspects of this work.
Competing Interests
The authors declare no competing interests.
Funding
Not applicable: This is funded as part our normal daily job under Oromia agricultural research institute.
References
- Brown E, O’Brien M, Georges K, Suepaul S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement Med Ther. 2020;20:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zulkhairi-Amin FA, Sabri S, Mohammad SM, Ismail M, Chan KW, et al. Therapeutic properties of stingless bee honey in comparison with european bee honey. Adv Pharmacol Sci. 2018;2018.
[Crossref] [Google Scholar] [PubMed]
- Shahira I, Sujanto R, Ramly NS, Ghani AA. The Composition and functional properties of stingless bee honey: A review. Malaysian J Appl Sci. 2021;6(1):111-127.
- Rosli FN, Hazemi MH, Akbar MA, Basir S, Kassim H, Bunawan H. Stingless bee honey: Evaluating its antibacterial activity and bacterial diversity. Insects. 2020;11(8):500.
[Crossref] [Google Scholar] [PubMed]
- Fletcher MT, Hungerford NL, Webber D, Carpinelli de Jesus M, Zhang J, Stone IS, et al. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci Rep. 2020;10(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Ali H, Abu Bakar MF, Majid M, Muhammad N, Lim SY. In vitro anti-diabetic activity of stingless bee honey from different botanical origins. Food Res. 2020;4(5):1421-1426.
- Ng WJ, Sit NW, Ooi PA, Ee KY, Lim TM. The antibacterial potential of honeydew honey produced by stingless bee (Heterotrigona itama) against antibiotic resistant bacteria. Antibiotics. 2020;9(12):871.
[Crossref] [Google Scholar] [PubMed]
- Zamora G, Beukelman K, van den Berg B, Arias ML, Umaña E, Aguilar I, et al. The antimicrobial activity and microbiological safety of stingless bee honeys from Costa Rica. J Apic Res. 2014;5(5):503-513.
- Choudhari MK, Punekar SA, Ranade RV, Paknikar KM. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J Ethnopharmacol. 2012;141(1):363-367.
[Crossref] [Google Scholar] [PubMed]
- Domingos SC, Clebis VH, Nakazato G, de Oliveira Jr AG, Takayama Kobayashi RK, Peruquetti RC, et al. Antibacterial activity of honeys from Amazonian stingless bees of Melipona spp. and its effects on bacterial cell morphology. J Sci Food Agric. 2021;101(5):2072-2077.
[Crossref] [Google Scholar] [PubMed]
- Omar S, Mat-Kamir NF, Sanny M. Antibacterial activity of Malaysian produced stingless-bee honey on wound pathogens. J Sustain Sci Manag. 2019;14(3):67-79.
- Rajab NF. Stingless bee honey and its potential value: A systematic review. Food Res. 2018;2(2):124-133.
- Zakaria NN, Jaafar NM, Mohamad AZ. Antioxidant, antibacterial and anti-diabetic activities of stingless bee honey from selected areas in Peninsular Malaysia. In IOP Conference Series: Earth and Environmental Science. 2020;596(1):012093.
- Boorn KL, Khor YY, Sweetman E, Tan F, Heard TA, Hammer KA. Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time‐kill methodology. J Appl Microbiol. 2010;108(5):1534-1543.
[Crossref] [Google Scholar] [PubMed]
- Tuksitha L, Chen YL, Chen YL, Wong KY, Peng CC. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J Asia Pac Entomol. 2018;21(2):563-570.
- Abd Jalil MA, Kasmuri AR, Hadi H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol Physiol. 2017;30(2):66-75.
[Crossref] [Google Scholar] [PubMed]
- Al-Hatamleh MA, Boer JC, Wilson KL, Plebanski M, Mohamud R, Mustafa MZ. Antioxidant-based medicinal properties of stingless bee products: Recent progress and future directions. Biomolecules. 2020;10(6):923.
[Crossref] [Google Scholar] [PubMed]
- Pimentel TC, Rosset M, de Sousa JM, de Oliveira LI, Mafaldo IM, Pintado MM, et al. Stingless bee honey: An overview of health benefits and main market challenges. J Food Biochem. 2022;46(3):e13883.
[Crossref] [Google Scholar] [PubMed]
- Anil GB, Pattabhiramaiah M, Reddy MS. Evaluation of antibacterial activity of stingless bee. Life Sci Informatics Publ. 2020;6(4):24-34.
- Ondusko DS, Nolt D. Staphylococcus aureus. Pediatr Rev. 2018;39(6):287-298.
[Crossref] [Google Scholar] [PubMed]
- Menegatti C, Lourenzon VB, Rodriguez-Hernandez D, da Paixao Melo WG, Ferreira LL, Andricopulo AD, et al. Meliponamycins: Antimicrobials from stingless bee-associated Streptomyces sp. J Nat Prod. 2020;83(3):610-616.
[Crossref] [Google Scholar] [PubMed]
- Shanahan M, Spivak M. Resin use by stingless bees: A review. Insects. 2021;12(8):719.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Han B, Chen X, Gao J, Zhao S, Sun L, et al. Quantification of bioactive components and evaluation of microbial community and antibacterial activity from Heterotrigona itama and Tetrigona binghami honeys. Int J Food Sci Technol. 2023;58(5):2247-2257.
- Biluca FC, Braghini F, de Ferreira GC, Costa dos Santos A, Ribeiro DHB, Gonzaga LV, et al. Physicochemical parameters, bioactive compounds, and antibacterial potential of stingless bee honey. J Food Process Preserv. 2021;45(2):e15127.
- Ilechie AA, Kwapong PK, Mate-kole E, Kyei S, Darko-takyi C, Azuka A, et al. The efficacy of stingless bee honey for the treatment of bacteria-induced conjunctivitis in guinea pigs. J Exp Pharmacol. 2012;63-68.
- Akhir RA, Bakar MF, Sanusi SB. Antioxidant and antimicrobial activity of stingless bee bread and propolis extracts. In AIP conference proceedings 2017;1891(1).020090.
- Atef NM, Shanab SM, Negm SI, Abbas YA. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull Natl Res Cent. 2019;4(1):9.
- Wavinya F, Omolo M, Malebo H, Sifuna A, Nyongesa P, Nonoh J. Antibacterial activity of honey from wild species of stingless bees; Plebenia hylderbrandii and Meliponula bocandei. J Biosci Med. 2021;9(7):67-84.
- Roba K, Bedewi Z. Evaluating Croton macrostachyus: Honey nectar, and pollen antimicrobial activities against Escherichia coli, Shigella boydii, Staphylococcus aureus, and Bacillus subtilis. Clin Microbiol. 2022;11(1000270):1-7.
- Tenório EG, de Jesus NR, Nascimento AR, Teles AM. Antimicrobial activity of honey of stingless bees, Tiúba (Melipona fasciculata) and Jandaira (Melipona subnitida) compared to the strains of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. In AIP Conference Proceedings. 2015;1702(1):120006.
- Chan-rodríguez D, Ramón-sierra J, Lope-ayora J, Sauri-duch E, Cuevas-glory L, Ortiz-vázquez E. Antibacterial properties of honey produced by Melipona beecheii and Apis mellifera against foodborn microorganisms. Food Sci Biotechnol. 2012;21(3):905-909.
- Nweze JA, Okafor JI, Nweze EI, Nweze JE. Comparison of antimicrobial potential of honey samples from Apis mellifera and two stingless bees from Nsukka, Nigeria. J Pharmacogn Nat Prod. 2016;2(4):1-7.
- Nishio EK, Ribeiro JM, Oliveira AG, Andrade CG, Proni EA, Kobayashi RK, et al. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci Rep. 2016;6(1):21641.
[Crossref] [Google Scholar] [PubMed]
- Kwapong PK, Ilechie AA, Kusi R. Comparative antibacterial activity of stingless bee honey and standard antibiotics against common eye pathogens. J Microbiol Biotechnol Res. 2013;3(1):162-168.
- LaPierre L, Cornejo J, Asun A. Laboratory guide: Methodologies for antimicrobial susceptibility testing. Asia-Pacific Economic Cooperation. 2020.
- Akhir RA, Bakar MF, Sanusi SB. Antioxidant and antimicrobial potential of stingless bee (Heterotrigona itama) by-products. J Adv Res Fluid Mech Therm Sci. 2018;42(1):72-79.
- Hasali NH, Zamri AI, Lani MN, Mubarak A, Ahmad F, Chilek TZ. Physico-chemical analysis and antibacterial activity of raw honey of stingless bee farmed in coastal areas in Kelantan and Terengganu. Malays Appl Biol. 2018;47(4):145-151.
- Kustiawan PM, Zulfa AF, Batistuta MA, Hanifa DN, Setiawan IM. Comparative analysis of phytochemical, total phenolic content, antioxidant and antibacterial activity of two species stingless bee propolis from East Kalimantan. Malays J Med Heal Sci. 2022;18(7):50-55.
- Mahmood AL, Lani MN, Hassan Z, Nilai BB, Sembilan N, Bahri S, et al. Antioxidant and antimicrobial properties of Indo-Malayan stingless bee (Heterotrigona itama) honey from different seasons and distribution of flowers. Food Res. 2021;5(2):498-507.
- Julika WN, Ajit A, Sulaiman AZ, Naila A. Physicochemical and microbiological analysis of stingless bees honey collected from local market in Malaysia. Indonesian J Chem. 2019;19(2):522-530.
- Pimentel RB, da Costa CA, Albuquerque PM, Junior SD. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey. BMC Complement Altern Med. 2013;13:1-4.
[Crossref] [Google Scholar] [PubMed]
Citation: Jilo KR (2024) Stingless Bee (Meliponulla baccaerii) Honey Antibacterial Activities against Salmonella typhi, Escherichia coli, Staphylococcus aureus and Enterococcus faecalis. J Bacteriol Parasitol. 15:506.
Copyright: © 2024 Jilo KR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

