PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 0, Issue 0
Spatiotemporal Distribution and Associated Risk Factors for Middle East Respiratory Syndrome-Coronavirus In Dromedary Camels: Review
Abdallahi Abdurehman* and Jafer KedirEthiopia
Received: 29-Sep-2020 Published: 23-Nov-2020, DOI: 10.35248/1948-5948.20.12.446
Abstract
The Middle East respiratory syndrome (MERS) is caused by Coronaviruses(CoV). Dromedary camels are likely to be a natural host of MERS, and transmission between camels is clearly documented. The first evidence of dromedaries being the reservoir of MERS-CoV came from serological studies. MERS-CoV was found circulating in dromedary camels during the last 20 years and neutralizing antibodies were detected in camels.High levels of MERS-CoV antibodies have been observed in dromedaries in the Middle East and Africa.Serological follow-up of dromedary dams and their calves has shown a typical pattern of juvenile infection.Differences in virus susceptibility and pathogenicity between animals of different species could be explained by a distinct tissue distribution of dipeptidyl phosphatase 4, the MERS-CoV receptor. Detection of MERS-CoV in dromedaries is performed to understand the epidemiology and evolutionary dynamics of the virus and to reduce the risk of human transmission. Sero-prevalence reports spatiotemporal distribution of MERS-CoV in dromedary camels among countries on the world where it was null in North America in 2005 and Australia in 2014. But it ranges from 29-100% in other studied countries. It was 100% in Saudi arabia, United Arab Emirates, Oman, Jordan. Protective experimental immunizations in dromedaries have already started using a modified vaccinia virus Ankara (MVA) vaccine expressing the MERS-CoV spike protein.
Keywords
Middle east respiratory syndrome; Coronavirus; Mers-cov; Mers coronavirus; Dromedary camels; Epidemiology
Introduction
The MERS is among viral diseases that affect camels. It affects mainly the respiratory system, and caused by Middle East respiratory syndrome Coronavirus (MERS-CoV). The virus belongs to the family Coronaviridae, single-stranded RNA of positive polarity. MERS-CoV, a member of the Betacoronavirus genus lineage C, was first identified in Saudi Arabia in 2012.
The virus specific antibodies have been detectedin the serum of dromedary camels across Northern Africa, including Tunisia, Egypt, Sudan, Ethiopia, Nigeria, Kenya and Somalia, andacross the Arabian Peninsula, including Jordan, Saudi Arabia, Qatar,Oman and United Arab Emirates [1-4].Genomic and epidemiologic studies comparing MERS-CoV sequences from household clustersand camels, and of dromedary farms and human contacts in UAE [5,6], and of patients with corresponding MERS-CoV positive camels in Saudi Arabia demonstrate camels as potential source of human infection [7].
A vaccine expressing the MERS-CoV spike protein confer mucosal immunity in dromedary camels with serum neutralizing antibodies and reduction of excreted infectious virus and viral RNA transcripts in vaccinated animals [8]. Surveillance and epidemiological studies reveals that infected dromedary camels serve as a reservoir with spill-over human infections via close contacts [9-14].
Dromedary camels assumed as the only reservoir for MERSCoV until 2015where studies in Qatar on 15 healthy alpacas (Vicugnapacos) in 20 herd that shared a barn with dromedaries were 100%seropositive to viruses [15]. An otherstudyshowed similar to dromedary camels, infected alpacas didn’t develop fever, but unlike dromedary camels, none of the alpacas had any observable nasal discharge over the course of infection. All infected animals could mount neutralizing antibodies to MERS-CoV[16]. That viruse could infect bat cell lines derived from six species as well as pig, camel, sheep, nonhuman primates, and human cell lines. The transmission and infection nature of MERS-CoV is via the respiratory secretions; coughing or droplet nuclei of an infected person [17]. Its cellular receptor, later identified as the DPP4 receptor, conserved across many mammalian species and various tissues like lung and kidney epithelium [18].
The exact source of MERS-CoV and how it is transmitted to humans is not known. Since, the role of camels and other animals in the epidemiology of MERS-CoV and the route of transmission to humans remains unclear. Thus, further investigation of MERSCoV transmission within and among species is necessitates a better understanding of the role of potential reservoirs during an outbreak. However, it is still unclear whether camels are the natural reservoir of the virus and the only source of human infection. Clearly, transmission from camels to humans does take place, and camel exposure is a risk factor for humaninfection but such transmission is not efficient and infectionis not directly proportional to exposure.The aims of this review is to summarize the spatiotemporal distribution and associated risk factors for MERS-CoV occurrence in camels globally.
Etiology of Middle East Respiratory Syndrome Corona Virus
The MERS is caused by Coronaviruses. CoVs are enveloped, single-stranded positive-sense RNA viruses displaying a large genome of 26 kb to 32 kb. The viruse is a putative member of a new species [19] within the order Nidovirales, family Coronaviridae, subfamily Coronavirinae, genus Betacoronavirus, subgroup2c [20].
The coronaviruse has two phylogenetic clades, clade A (earliest case) and clade B (new case) [21]. Basedon genotype and serological characteristicsthe viruses are classified within 4 groups: Alphacoronaviruses (αCoVs), Betacoronaviruses (βCoVs), Gammacoronaviruses, and Deltacoronaviruses [22,23]. MERS-CoV had frequently been referred to as a SARS-like virus,"SaudiSARS"or the novel coronavirusuntil 2013 [24]. or Human Coronavirus Erasmus Medical Center/2012(HCoV-EMC/2012) is the name of a novel strain of coronavirus isolated from the sputum of the first person to become infected with what was later named Middle East respiratory syndrome coronavirus, or MERS-CoV [19,25,26].
Occurences of Middle East Respiratory Syndrome–Coronaviruses in Camel
The global population of camels is estimated to be about 30 million, 95% of these being dromedary camels [27]. Dromedary camels inhabit the Middle East region, North and East Africa and North-western parts of Asia. Of the total of 28.5 million dromedary camels worldwide, 77% are in Africa, the largest camel populations found in Somalia (6.2 million), Sudan (4.8million), Kenya (3 million) and Ethiopia (2.3 million). Only 4% are in the Arabian Peninsula. The highest density of camels by land area in the Arabian Peninsula is found in Qatar and UAE [27].
The first evidence of dromedaries as reservoir of MERS-CoV came from serological studies. High levels of MERS-CoV antibodies have been observed in dromedaries in the Middle East and Africa [1-4,28,29]. Serological follow-up of dromedary dams and their calves has shown a typical pattern of juvenile infection. Maternal antibodies against MERS-CoV in dromedary calves generally disappear between 4 and 8 months of age, permitting infection to occur during the sero negative period;young infected dromedaries then develop antibodies that persist for a long time [30,31]carried out study in different parts of Africa, have encompassed diverse geographical and ecological variables, study findings may well be relevant in regions such as Saudi Arabia where zoonotic MERS remains a recurrent threat. Furthermore, it is not clear that transmission of MERS-CoV to humans is absent in Africa. Another study has reported evidence of humans with MERS-CoV seropositivity in Kenya [32]. Further studies are needed to assess whether or not zoonotic MERS-CoV transmission occurs in Africa and epidemiological data provide identification of situations of highest risk. Better understanding of the risk factors and virus transmission dynamics of MERS-CoV within camels is important in responding to the global health threat posed by MERS-CoV [33]. However, in a few cases, MERS-CoV has been isolated at the age of 20 days or even at younger, indicating that maternal antibodies are not necessarily protective. A plausible hypothesis could indeed be that young camels that lack antibodies have a high probability of being infected and in turn, expose the mothers to infection or re-infection [34]. The longitudinal study conducted by Meyer et al. [34] on natural MERS-CoV infections in camels confirms assumptions from preliminary cross-sectional studies in camels [3,30,35]. MERS-CoV infection appears to predominantly affect young, immunologically naive animals. Serum antibodies might not have been sufficient to mediate protective immunity in the respiratory tract because dams and calves were sporadically infected even as maternal antibodies peaked at day 7 postparturition [34]. Findings of virus isolation from calves but not dams are in line with earlier observations of reduced viral load in seropositive camels on reinfection [36,37] indicating that neutralizing antibodies might not provide sterile immunity but could still reduce the viral replication level. The predominance of infection in young animals is better explained by the absence of immunity than by other factors, such as social group density, because the number of newborn camels in our study was negligible compared with the overall size of the herd at the farm. Moreover, young camels were not kept in a contiguous group but in small compartments, where they had more contact with their mothers than with other young animals. Calves are likely to have been infected through fomites or through adult animals shedding low quantities of virus.
Camel breeding, even if involving a small number of newborn animals, should be classified as a risk for human acquisition of MERS-CoV. The greatest risk should be assumed for the time after the fourth month of life until the first wave of natural infections, which should occur during the first year of life in camels raised in MERS-CoV–endemic regions. Measures for the prevention of infection, such as personal protective equipment, hand hygiene, and environmental sanitation, as applied on the farm in our study, should be sufficient for protection, given that no human MERSCoV illnesses occurred among staff and only 2 of 300 workers with regular contact with camels had detectable MERS-CoV–specific IgG antibodies. Because persons with underlying disease and the elderly show the most severe outcomes of MERS-CoV infection, these groups should generally avoid farms where camel calves are being raised.
So far, the specific source of infection for young dromedaries is not known, although it is likely to be from other dromedaries. Extensive investigations in other animal species, including rodents, ticks, horses and small ruminants, have not demonstrated other reservoirs of infection to date. Their host range is very wide and includes both mammalian and avian species. Coronaviruses can cause acute and chronic respiratory, enteric, neurological and hepatic diseases in their hosts [38].
MERS-CoV has been found in dromedary camels in several countries in the Middle East and Africa. Highpercentage of seropositive dromedaries were foundin Arabian Peninsula and Africa. Serological tests showed a higher incidenceof virus infections in adult than young camels [39]. Also, young dromedaries (≤ 2 years) had lower viral load than theadult ones, which indicates a higher risk of infection for humans during the reproductiveseason (spring) when a number of immunocompetent camels increased. The diseases acquired in dromedaries at age of less than one year and becomes a source of infection to humans, but not known how the virus is transmitted from camels to humans (FAO, 2017).
In an attempt to investigate the time frame of MERS-CoV introduction to dromedary camel population, multiple studies screened stored serum samples. All 151 dromedary camel serum samples obtained in 2003 from UAE (100%) were seropositivity. Archived serum samples, obtained from dromedary camels in Saudi Arabia from1992 to 2010 had high seropositivity ranging from 72 % to 100 % [30]. From Egypt, 189 stored dromedary camel serum samples collected in 1997, and from Sudan and Somalia, collected between 1983 and 1984, were tested, and 81% have neutralizing antibodies to virus [4].
Serum samples collected from 105 dromedary camels living in the Canary Islands, a Spanish archipelago located just off the southern coast of Morocco, between 2012 and 2013, 14 % have antibodies against MERS-CoV [40]. In an attempt to screen feral camels in Australia, 307 dromedary camels’ sera from two different locations were sampled between December 2013 and June 2014. All tested negative for specific viruseantibodies [41].
MERS-CoV is a zoonotic virus transmitted from animals to humans. The origins of the virus are not fully understood but, it is believed that originated in bats and transmitted to camels sometime in the distant past [17]. MERS-CoV is a zoonotic virus, and dromedary camels are a reservoir host [5,42,43]. Bats are a likely original reservoir; coronaviruses similar to MERS-CoV have been identified in bats [44], but epidemiologic evidence of their role in transmission is lacking. Infection of other livestock species with MERS-CoV is possible [45]; however, attempts to inoculate goats, sheep, and horses with live MERS-CoV did not produce viral shedding [16], and no epidemiologic evidence has implicated any species other than dromedaries in transmission. Sporadic zoonotic transmission from dromedaries has resulted in limited human-tohuman transmission chains, usually in healthcare or household settings [46-49].
Transmission following exposure to camel feces may be biologically plausible, although no epidemiologic evidence indicates the likelihood of such transmission. Similarly, although transmission following consumption of raw camel milk may be biologically plausible, epidemiologic studies have not consistently identified milk consumption as a unique risk factor for MERS-CoV infection or illness, independent of other direct or indirect camel exposures [11,50]. No epidemiologic evidence supports transmission associated with camel urine or meat.Thus, further investigation of MERS-CoV transmission within and among species is necessitates a better understanding of the role of potential reservoirs during an outbreak [51]. However, it is still unclear whether camels are the natural reservoir of the virus and the only source of human infection. Clearly, transmission from camels to humans does take place, and camel exposure is a risk factor for human infection but such transmission is not efficient and infectionis not directly proportional to exposure. but camels are a major reservoir host for the viruse and an animal source of infection in humans [51]. Strains of viruse identical to human strains have been isolated from camels in several countries, including Egypt, Oman, Qatar, and Saudi Arabia [46,52,53].
The strongest evidence of camel-to-human transmission of MERSCoV comes from a study in Saudi Arabia whereviruse isolated from a man with fatal infection and his camelswere identical; showing that viruse can infect dromedary camels and transmitted to humans by close contact.
Spatio Temporal Distributions and Spread of the Diseases
At the end of January 2020, a total of 2519 laboratory-confirmed cases of MERS, including 866 associated deaths (case-fatality rate: 34.3%) were reported globally. The majority of these cases were reported from Saudi Arabia (2121 cases), including 788 related deaths with a case-fatality rate of 37.1% (WHO, 2020).
Since September 2012 and as of 19 February 2020, 2,527 cases confirmed; including 904 case fatalities 2494 cases of MERS-CoV, including 912 deaths, have been reported by health authorities worldwide. Up to July18 2018, a total of 2229 laboratory-confirmed cases of MERS, including 827 case fatalities deaths (case–fatality rate: 37.1%) in 27 countries were reported to WHO worldwide, with most being reported in Saudi Arabia (1854 cases with 717 deaths) were reported globally; (WHO, 2018).Epidemiologic studies have provided evidence of endemic MERS-CoV infection among dromedaries in the Greater Horn of Africa as far back as 1983 [54] and in Saudi Arabia as far back as 1992-1993 [30].
Multiple surveillance studies explored the extent of MERSCoV infection in dromedaries. Presence of specific MERS-CoV antibodies in dromedary camels’ sera was used as an indicator of previous exposure to the virus, while the presence of MERS-CoV RNA material in nasal secretions, usually identified through RTPCR, indicated current infection and active viral shedding. Serum samples from 303 dromedary camels from Saudi Arabia were screened in 2013 and found to have high seropositivity of 72 % to MERS-CoV [30].
All serum samples from 50 dromedary camels in Oman were positive for MERS-CoV specific antibodies [55]. Similar results were reached from a larger study conducted in the United Arab Emirates (UAE), where 500 dromedary camels’ sera screened in 2013 revealed 96% seropositivity [3].
In Africa, a study assessed the geographic distribution of MERSCoV among dromedaries by investigating serum samples from Nigeria, Tunisia, and Ethiopia [56]. In Nigeria, serum samples collected between 2010 and 2011 from 358 adult dromedaries distributed over 4 provinces were tested, and 94 % were positive for MERS-CoV antibodies. In Tunisia, 48.5 % of 204 serum samples of dromedaries collected from three provinces tested positive for MERS-CoV [56]. In Ethiopia, 96.3 % of the serum samples collected between 2011 and 2013 from 188 dromedaries from three regionswere positive for MERS-CoV antibodies.
An increase in seropositivity rate with age was observed which confirms the trend observed inEthiopia in a previous study [9]. Thus a higher virus RNA detection rate in young animals compared with older animals which could be related to a lack of prior immunity as published in previous studies in Saudi Arabia [57,58]. Young animals were naïve and more susceptible to virus infection [34,58].
Sero-prevalence reports spatiotemporal distribution of MERS-CoV in dromedary camels among countries on the world (Table 1). It was null in North America [2,59]. But it ranges from 29-100% in other studied countries. It was 100% in Saudi Arabia, United Arab Emirate, Oman and Jordan (Table 1).
| Year | Countries | Number examined | Prevalence (%) | References |
|---|---|---|---|---|
| 1983-1997 | Sudan and Somalia | 189 | 81 | Muller et al. (84) |
| 1992-2010 | Saudi Arabia | 264 | 87 | Alagaili et al. (17) |
| 1992-2013 | Kenya | 774 | 30 | Corman et al. (1) |
| 1993 | Saudi Arabia | 131 | 90 | Hemida et al. (2) |
| 2003 | UAE | 151 | 100 | Meyer et al (3) |
| 2005 | UAE | 33 | 91 | Alexandersen et al. (59) |
| 2005 | North America | 6 | 0 | Alexandersen et al. (59) |
| 2009, 2013 | Tunisia | 204 | 54 | Reusken et al. (14) |
| 2010-11 | Nigeria | 358 | 94 | Reusken et al. (14) |
| 2011-13 | Ethiopia | 188 | 97 | Reusken et al. (14) |
| 2012-13 | Canary-Islands | 105 | 14 | Reusken et al. (28) |
| 2012-13 | Oman | 50 | 100 | Reusken et al. (28) |
| 2012-13 | Saudi Arabia | 310 | 90 | Hemida et al. (35) |
| 2012-2015 | Pakistan | 565 | 55.8 | Saqib et al. (125) |
| 2013 | Egypt | 110 | 94 | Perera et al. (62) |
| 2013 | Jordan | 11 | 100 | Reusken et al. (28) |
| 2013 | Qatar | 14 | 79 | Haagmans et al. (10) |
| 2013 | Saudi Arabia | 206 | 95 | Alagailiet al. (30) |
| 2013 | UAE | 182 | 96 | Meyer et al. (3) |
| 2013 | Oman | 76 | 7% | Nowotny andKolodziejek (124) |
| 2013 | Egypt | 52 | 92 | Chu et al. (113) |
| 2013 | Saudi Arabia | 5 | 100% | Memish et al. (63) Azhar et al. (12) |
| 2013 | Kenya | 335 | 46.9% | Deem et al. (115) |
| 2013-14 | Saudi Arabia | 21 | 100% | Hemida et al. (2) |
| 2014 | Australia | 2 | 0% | Hemida et al. (2) |
| 2014-2015 | Iraqi | 18 | 85% | Thwiny et al. (126) |
| 2014-2016 | Saudi Arabia | 584 | 70.9% | Kasem et al. (7) |
| 2014-2016 | Egypt | 2541 | 71.2% | Ali et al. (65) |
| 2015 | UAE | 376 | 29% | Yusof et al. (128) |
| 2015 | Burkina Faso | 525 | 84.6% | Miguel et al. (31) |
| 2015 | Ethiopia | 632 | 99.4% | Miguel et al. (31) |
| 2015 | Morocco | 343 | 100% | Miguel et al. (33) |
| 2015-2017 | Saudi Arabia | 689 | 56.4 | Kasem et al. (7) |
| 2016 | Saudi Arabia | 171 | 84.21% | Harrathand Abu Duhier (118) |
| 2018 | Israel | 71 | 71.8% | Harcourt et al. (117) |
| 2019 | UAE | 11 | 82% | Lau`et al. (120) |
Table 1: Spatiotemporal distribution of MERS-CoV in dromedary camels among countries on the world (Sero-prevalence reports).
Risk Factors for the Occurrence and Spread of Middle East Respiratory Syndrome-Coronavirus
Host risk factors
Middle East Respiratory Syndrome-Coronavirus pinpointed a zoonotic introduction of a novel coronavirus probably originating from bats into human populations [32]. The viruse was found circulating in dromedary camels from last 20 year [60], and neutralizing antibodies were detected in camels [61]. Dromedary camels are the primary animal host for MERS-CoV and the only species from which antibodies specific to the virus detected serologically among livestock [35,62].
The first study on domestic animal host shows IgG antibodies specific to MERS-CoV in dromedary camel herds in Oman and the Canary Islands. One hundred percent of the camels tested in Oman (n=50) and 14 percent (n=105) of Spanish camels were positive for the viruse antibodies. Several species of domestic animals in various countries including Oman, Egypt, Jordan, and Saudi Arabia were screened for antibodies against viruse, including sheep, goats, cattle, and buffalo, but all were negative [9,20,35,62].
Coronavirus RNA sequences found in bat fecal samples are closely related to MERS-CoVsequences [63]. The viruse grows readily in bat-derived cell lines and unlikely to be immediate source for most human cases because human contact with bats is uncommon. Theviruse excreted in the nose of dromedaries seems to be much higher than that of other animal species described so far, suggesting a more prominent role of dromedaries in the transmission of MERS-CoV to humans [64].
Sex: male camels showed a higher positivity (83.5%) to viruse antibodies than the female camels (66.5%). For MERS-CoV RNA the male camels showed 20% positivity while female camels exhibited 4.9% [7]. A previous study stated that there was no difference in the seroprevalence rates between female camels (82.7%) and males (85.1%), while viruse RNA level was higher in females (7.1%) than in males (2.6%) [65]. This variation is due to the difference in sample numbers and the age of animals included in both studies
Another point highlighted by the study on camel function as a risk factor is the function of camels which is also related to sex. Camels raised for milking show the highest sero-prevalence followed by camels raised for their meat and also camels used for transport activities have the lowest seroprevalence [58]. The higher seropositivity rate in females bred for milking could be related to the high viral RNA detection rates in younger animals, e.g. calves [34].
A plausible hypothesis could indeed be that young camels that lack antibodies have a high probability of being infected and in turn, expose the mothers to infection or re-infection. The lower seropositivity rate in camels bred for their meat or for transport activities, which are mostly males, could also be linked with the fact that males are often separated from the herd (the two sexes are only mixed during the reproduction activities) and have thus fewer contacts with other camels (i.e. females and calves) [31].There is convincing evidence that dromedary camels are host animals for the strain of MERS-CoV that infects humans. Whether camels are indeed the reservoir for MERS-CoV or whether they function as a vehicle for the virus from a yet unidentified animal reservoir to humans remains to be established.
Age: adult camels had a higher seroprevalence of MERS-CoV antibodies (86.6%) compared to young camels less than 2 years (57.7%) in Saudi Arabia [7]. While young animals less than 2 years showed a high positivity (15.4%) to MERS-CoV RNA compared to adult animals, previous studies have shown high seropositivity in adults compared to juvenile camels that exhibited a high infection rate [14,57].
Two hundred and three samples from live dromedary camels in Saudi Arabia were collected in 2013 and found to have high seropositivity (72 %) to MERS-CoV [30]. Seropositivity was higher among adults dromedary camels (two years and older) compared to juvenile dromedary camels (less than two years of age), 95 % vs. 55 % respectively [59]. In the same study, 202 dromedary camels’ nasal swabs were tested for the presence of MERS-CoV RNA material using RT-PCR; 25% were positive. In other words, onefourth of the tested dromedary camel population was shedding the virus and was potentially infectious. Of those shedding the virus, 71% were juvenile and 29% were adult dromedary camels older than two years.
Biology of the pathogen as a risk factor
Middle East Respiratory Syndrome-Coronavirus viruses can be recovered from the full-length Complementary DNA (cDNA) clone, using susceptible Vero A66 and Huh-7 cells, with titers of around 106 plaque-forming unit/ml (p.f.u./ml) at 72 h posttransfection (h.p.t.). The recovered viruses can be cloned by three rounds of plaque purification, and their phenotypic and genotypic properties can be determined. MERS-CoV rescued from both cell lines induce a Clear Cytopathic Effect (CPE), characterized by the induction of cell fusion, which was more apparent in Huh-7 cells [66].
In both cell lines, viral mRNAs could be readily detected at 7 h Post-Infection (PI) and reached maximum levels around 13 h p.i. Viral RNA levels remained more or less constant until 24 h p.i. in Vero cells, whereas the amount isolated from Huh7 cells declined due to the more rapid development of cytopathology in this cell line between 13 and 24 h p.i. After the peak of viral RNA, accumulation had been reached, the titer of virus released from MERS-CoV infected Vero cells increased steadily from ~5×105 to ~5×107p.f.u./ml. The bulk of the viral progeny is released significantly earlier from Huh7 cells, although the final titers at 24h p.i. are comparable to that obtained from Vero cells [67].
Differences in virus susceptibility and pathogenicity between animals of different species could be explained by a distinct tissue distribution of DPP4, the MERS-CoV receptor. DPP4 distribution in the respiratory tract was similar among llamas and pigs but differed from that of dromedary camels [64]. In contrast, DPP4 was barely detected in the respiratory tract of sheep, probably accounting for the lack of infection reported here. These results are in concordance with those reported that MERS-CoV experimentally inoculated sheep showed no clinical disease and that only small amounts of virus were detected in nasal swab samples.DPP4 (also named CD26) has been identified as the receptor for MERSCoV [68]. All HCoV receptors identified to date are exopeptidases, although their proteolytic activity is not necessary for the virus to bind to the receptors, nor for them to enter the host cell [69,-71].
A comparative analysis of HCoV receptor expression across the respiratory tract of humans may provide clues regarding differences in pathobiology between HCoVs. In cell lines and ex vivo lung cultures, DPP4has expressed in type I and II alveolar cells, ciliated and non-ciliated bronchial epithelium, bronchial submucosal glands, endothelium, alveolar macrophages and leukocytes [72]. This largely corresponds with viral tropism in ex vivo human lung cultures, which show infection of non-ciliated cells in bronchi, bronchioles, endothelial cells and type I and II pneumocytes, but rarely in alveolar macrophages [26,73,74]. Remarkably, the binding site of DPP4 is different in different species, explaining why not all animals can be infected with MERS-CoV.
Environmental risk factors
Lifestyle: Surprisingly, there was no observed difference between nomadic and sedentary herds in the seropositivity rate or virus RNA positive rate [31]. Two hypotheses may explain this pattern. Firstly, the sedentary lifestyle is found in animal production systems where animals live at high density in ‘commercial’ farms. In such situations, the virus may be introduced more easily to the herd with animals being bought from other sources and the virus once introduced will amplify to infect most of the susceptible animals, since they are in close contact with each other. The virus appears to have a density-dependent transmission pattern.
In contrast to this, nomads are long-distance travelers who connect to different regions. Consequently, they have multiple opportunities to come into contact with other camel populations during their travels, or through indirect contacts with water points and thus increasing the probability of encountering animals shedding MERS-CoV. In supportof these interpretations, the lowest seroprevalence was found for the mixed lifestyle which is associatedwith medium herd sizes and relatively small range movements [31].
Herd size: The role of camel density in shaping the large spatial scale (i.e. national) variation pattern in seropositivity and virus RNA detection rates is supported by the identification of a herd size effect on serological prevalence. Higher seropositivity rate was found in large or medium size herds as compared with small herds, suggesting that the transmission of the virus is density dependent. More studies are now necessary to better describe the virus transmission dynamics within herds and between herds, with mechanistic models accounting for a disease transmitted through close contact and the possibility of re-infections.Such a model would allow determining the minimum size of a camel herd required for the MERS-CoV to persist in that herd without ‘fadeouts’: i.e. critical community size [2].
Seasonal difference: MERS-CoV spread among dromedary camels shows that the virus produces acute epidemics in calves, often born in Spring [17,75]. Such outbreaks may cause an increase in the number of primary cases and increased opportunities for subsequent transmission, multiplying the number of admissions of MERS-CoV cases to hospitals with the possibility of further triggering hospital outbreaks as previously reported [27,49,76-78] but not find evidence to explain a seasonal pattern on human-tohuman transmission [79].
Transmission
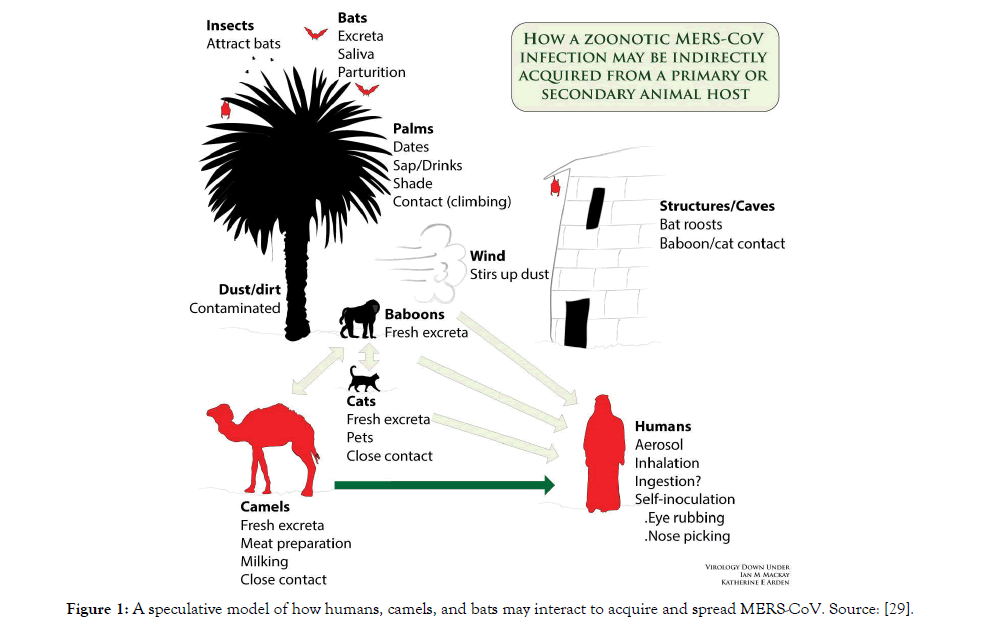
The exact source of MERS-CoV and how it is transmitted to humans is unknown. Initial investigations have indicated that MERS-CoV originated from bats; sequences related to MERS CoV have been found in several bat species [48]. Limited, non-sustained human-to-human transmission in health-care settings continue to occur, primarily in the Kingdom of Saudi Arabia, due to the nonspecificity of MERS symptoms resulting in late diagnosis of MERS. Even though, it is unclear how the virus is transmitted from camels to humans, the WHO advises avoiding contact with camels and to eat only fully cooked camel meat, pasteurized camel milk, and to avoid drinking camel urine (WHO, 2016). A speculative model of how humans, camels, and bats may interact to acquire and spread MERS-CoV is given by [29].
The world today is watching the evolution of the situation in China with concern and fear, where at the end of 2019 an increase was registered in patients with a respiratory infection infected by a new coronavirus. This has now been identified with the acronym COVID-19, pinpointed in the city of Wuhan. The appearance of a new infectious disease is always a complex situation, especially if it is an epidemic of significant extension or severity. The cases increased rapidly in Wuhan and Hubei Province, and they extended in smaller numbers and with limited transmission chains throughout China. Imported cases and secondary cases have been reported in more than 24 countries. On January 30, 2020, WHO declared this epidemic as a Public Health Emergency of International Concern. The COVID-19 virus has been identified and sequenced genetically [80,81]. It is related to other coronaviruses that circulate in bats (including the SARS coronavirus), leading to the belief that its natural reservoir is probably these flying mammals. The intermediate host, which is probably another mammal, has not yet been identified. The point of contact with humans could be a live animal market in Wuhan, which today is shut down [82,83]. Itis possible that this virus went unnoticed for several weeks in a city of 11 million inhabitants and at the beginning of the flu season, until the alert was given due to the increase in severe cases (pneumonia) and it was possible to isolate and identify the coronavirus COVID-19 in several patients. The jump of a virus from animals to humans (spillover) is common among coronaviruses. This happened with SARS in 2002–2003 and with MERS since 2012. It has been shown that the 2019-nCoV virus is transmitted easily from person to person, as groups of intrafamily cases and transmission to health personnel have been identified. The transmission capacity, which is usually estimated using the so-called basic reproduction number or R0, is a controversial variable of this new disease. An R0 value less than 1 indicates a low extension capacity of an infectious disease, while R0 values greater than 1 indicate the need to use control measures to limit estension. Reliable estimates place the R0 value of the COVID-19 in 1.4–2.5, similar to theR0 ofthe coronavirus SARS at the beginning of the epidemic (2.2–3.7). This value was reduced to anR0 of 0.67–1.23 at the end of the epidemic. By contrast, the coronavirus MERS has always remained at lower R0 values (0.29–0.80)[84]. It seems that the COVID-19 could be more easily transmitted than SARS. However, there is a need to exercise caution. The R0 value indicates the transmission potential of an infectious disease. A higher R0 does not mean a more extensive disease. The flu, for example, whose R0 value ranges around 1.3 each year, infects millions of people worldwide. Neither does the R0 indicate the transmission rate either. R0 is also an average value: there are people who, although infected, will not transmit the disease to anyone, while others may transmit it to many more people. These individuals, called «super-spreaders», were protagonists oftwo extraordinary events during the SARS epidemic in Toronto (Canada) and MERS in Seoul (South Korea) when, from one patient who was a «super-spreader», dozens of patients, visitors and health personnel from two hospitals were infected. Control measures, such as those used in China, can significantly reduce the R0 of a disease. In this initial phase of the COVID-19 epidemic, its R0 value is being estimated from multiple assumptions and using complex mathematical models.As epidemiologists, some of us approach these mathematical models with circumspect: a popular saying states «All the models are wrong, but some are useful». This saying also applies to another controversial parameter appearing at the start of all epidemics: the number of real cases. Current statistics, without entering into discussions about the Chinese authorities’ communication policy or transparency, probably reflect a bias towards the most severe cases which are the most likely to have reached out to the health system. Numbers for mild cases and asymptomatic cases are likely to be lower than reality. In recent weeks the detection capacity (RTPCR test) of infected patients in the epidemic zone has increased, and this fact could partly explain the increase in case numbers, although many patients may still be undiagnosed. This possibility leads to the discussion about the estimation of the fatality rate of this disease, which currently stands at around 2.0%, with more than 40,000 cases and 1000 deaths ( WHO, 2020). The mortality rate for SARS was around 10%, so the disease caused by COVID-19 seems, for now, to be less severe.
Recent research shows the human to human transmission route of SARS-CoV-2, these studies data shows a person who has been visited Wuhan city market their family members also find infected by this virus [85]. According to WHO guideline person to person transmission occurred via direct contact or droplet spreading by the infected individual via coughing and sneezing. Likewise [86], has suggested the presence of this dangerous virus in fecal swab and blood that indicates the multiple transmission routes of infection. It's has been already shown that the transmission of SARS-CoV and MERS-CoV occurred through nosocomial transmission and considered airborne pathogens.
Risk for camel-to-camel or camel-to-human transmission may be influenced by crowding, mixing of camels from multiple sources, transportation, and characteristics of live animal markets [28]. Phylogenetic modeling has provided supportive evidence that longterm MERS-CoV evolution has occurred exclusively in camels, with humans acting as a transient and usually terminal host [87].
Pathogenesis
A very important stage after transmission is the binding of coronavirus to host cell receptors. It is noteworthy that SARSCoV-2 share the same cellular receptor with SARS-CoV genera. The spike protein of coronavirus from all four families, guides to coronavirus entry into the host cell[88]. Corona viruses entre into the host cell by a two-step process: first host cell receptor recognized for viral attachment and fuse viral and host cell membrane. The spike protein is present in two very different forms pre-fused (before fusion to host) and post-fused (after fusion to host cell).
The pre-fused spike protein displays a homo-trimer structure with three receptor binding S1 receptor binding side and resting at the top of trimeric S2 [89-91]. The post-fusion structure is a coiled-coil structure with contained only S2 [92,93]. The virus invasion may have two pathways (a) the ACE-2 receptor (b) using the integrin receptor.Angiotensin-Converting Enzyme-2 (ACE-2) receptor presents the cell membrane of the cells of the lungs, heart, and kidney. ACE-2 is expressed by type I and type II alveolar epithelial cells. Among them, type II is shown more than 80% ACE-2 receptor.
Men had a higher level of ACE-2 receptor rather than women. This enzyme considers as the main entry point for coronavirus [92,94]. SARS-CoV-2 can also fuse directly to the cell surface in the detection Beta-CoV receptor reveals that human cells expressing ACE-2 receptor have a crucial role to play in binding SARS-CoV-2, Spike (S) glycoprotein, and ACE-2 host receptor [95].
A 30 % difference in the S1 unit of S protein sequence between SARS-CoV and SARS-CoV-2. The RGD-motif of S protein, which is different in sequence from SARS-CoV and MERS-CoV, shows tightly binding to lung cells. It has long been known that SARSCoV is primarily a respiratory disease, so it also needed protease from the respiratory tract such as trans-membrane protein serine-2 (TMPRSS-2) and HAT [96-98] (Figure 1). The TMPRSS-2 and HAT both activate the binding affinity of the S-protein cleavage trimer.
Figure 1: A speculative model of how humans, camels, and bats may interact to acquire and spread MERS-CoV. Source: [29].
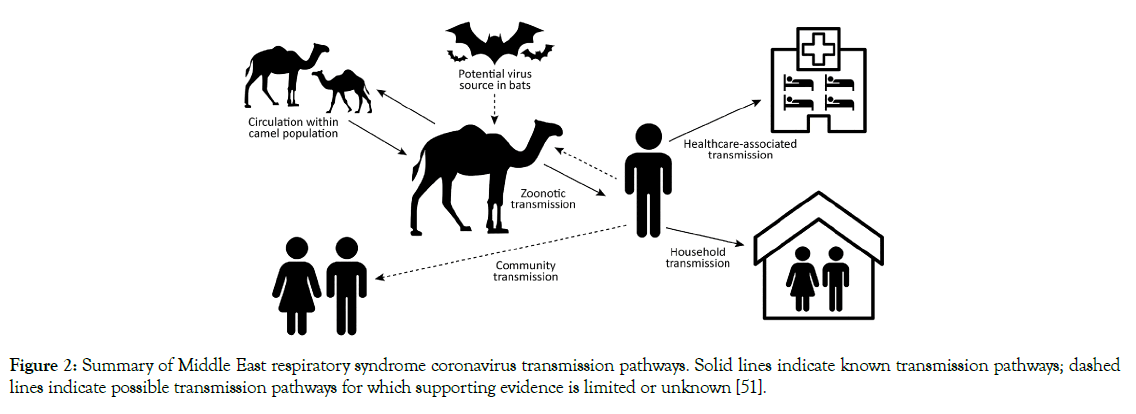
Some studies support that the S-protein and ACE-2 increase the affinity 10 to 20-fold for SARS-CoV-2 [99,100]. After binding SARSCoV-2 to host cell receptor, it required a serial activation of kinase and protease like activities for the internalization of the virus. The phagocytosis mechanism is complex, where the interconnected, and cross-activation of proteins take participates inside the cells. TMPRSS-2 and HAT cleaves pattern for S-fragments differ from each other; HAT cleaves S protein mainly at R667, where TMPRSS-2 cleaves at multiple sites, both cleavages enhance the cell-virus fusion [97]. The infection of the target cell by SARSCoV-2 occurred due to S-pseudotypedvirions, which is less sensitive to cathepsin inhibitor when the target cell expresses TMPRSS-2 [97,98]. Pseudo virions are still producing by SARS-CoV-2; still, TMPRSS-2, rely on endosomal cathepsin for the entry. Meanwhile, other accessory proteins may be involved in viral binding and invasion, such as cathepsin [101] 2005) and clathrin (Figure 2), while potential molecules facilitated an uncertain membrane invasion of SARS-CoV-2 [102-104]. SARSCoV- 2 cell entry Depends on ACE-2 and TMPRSS2, and it is Blocked by a Clinically Proven Protease Inhibitor. The lack of a complete understanding of the phagocytosis mechanism that is critical for SARS-CoV-2 to the host's which pathway involvement.
Figure 2: Summary of Middle East respiratory syndrome coronavirus transmission pathways. Solid lines indicate known transmission pathways; dashed lines indicate possible transmission pathways for which supporting evidence is limited or unknown [51].
A recent study shows that Ca+2 ions increase infectivity and entry into MERS-CoV and Rubella virus cells [68,105] because of the presence of negatively charged peptide on fusion protein (S-protein). Studies show that the spike protein of coronavirus has evolutionary changes and obtain some features for its adaptations in human host cells [106,107]. In some research article it has been showing the involvement of Ca+2 ions play a significant role in which is several receptorbased events and initiates internalization of pathogenicity of the virus by altering the actin filaments and cytoskeleton arrangements through affecting the actions of several proteins [108]. When a virus binds an integrin receptor association (α5β1), a serial activation of kinase activates that contributes to the internalization is needed. The binding of virus or virus particle induce Ca+2 response inside the host cell that lead cellular response. The integrin α5-subunit and β1-subunit provide a docking site for various kinases, such as β-subunit Focal Adhesion Kinase (FAK)and α-subunit Talin Adapter Proteins [109]. The tyrosine kinase FAK plays a vital role as a key mediator of the integrin signaling event controller. RGD motif of spike protein (S-Protein) interaction to integrin stimulates FAK tyrosine phosphorylation result's in FAK signaling activate, meanwhile at time stimulated FAK promotes phospholipase C-γ (PLC-γ) activities that directly participate in the generation and catalyzed of inositol triphosphate 3 (IP3) and Diacylglycerol (DAG).Thus, the PLC-γ activates coronin, found as an actinbinding protein within cytosol and thus further stimulation of PLC-γ these proteins proceed IP3 and this diffuse towards Endoplasmic Reticulum (E.R.) and binds to Inositol Triphosphate-3 Receptor (IP3R), present at E.R. this results in immobilization of Ca+2 into the cytosol [110,111].
Phagocytosis is a complex mechanism by interconnected and crossactivation of intracellular proteins. Moreover, in this complex mechanism, cytosolic proteins of the host also play a role in virus engulf, one of the best proteins talin, which a ubiquitous cytosolic protein that docks the α5-subunit of integrin and acts as a substrate for the Ca+2 activated protease, called calpain. Thus, an increase in Ca+2 concentration in host cells leads to re-armament or deform the actin filaments by binding on α-actin that provide an intact binding between actin filaments and help into the invasion of the virus into lung cells (Figure 2). So, this fact can be possible; the concentration of Ca+2 into lung cells also increases the binding and entry of SARS-CoV-2 inside the cell, and that plays a vital role in the pathogenicity of the virus. Nonetheless, if the concentration of Ca+2 ions in lung cells reduces, this may be a step towards reducing the degree of coronavirus infection.
Prevention and Control of the Disease
Protective experimental immunizations in dromedaries have already started using a modified vaccinia virus Ankara (MVA) vaccine expressing the MERS-CoV spike protein [112]. Preliminary data showed a significant reduction in excretion of infectious virus and viral RNA in small numbers of vaccinated and challenged dromedaries compared to controls. Protection is correlated with the presence of serum neutralizing antibodies againstMERS-CoV.
In spite of frequent reports of nosocomial infection of MERSCoV, human-to-human transmission is not sustainable and MERS is considered to be a zoonotic disease. To date, WHO has declared that the overall transmission patterns of MERS remain unchanged, i.e. multiple introductions from animals to humans and secondary transmission in healthcare settings (WHO, 2017). Therefore, identification of the zoonotic sources of MERS-CoV might guide control strategies at the human–animal interface to stop future human infection. If the spillover process of MERS-CoV from animals to humans could be stopped, we may be able to put an end to further nosocomial outbreaks in the Middle East and beyond. Available serological studies have indicated that the seropositivity of MERS-CoV neutralizing antibodies is much lower in juvenile than in adult camels, suggesting that MERS-CoV infection in camels may target young animals [113-116]. In agreement with the serological findings, the detection rate of MERS-CoV RNA in the nasal and/or rectal swabs of juvenile camels was higher than in those of adult camels [117]. In addition, a recent study found that MERS-CoV mainly targeted camels of less than 4 years of age, particularly calves, and the infection in juvenile camels manifested as an acute, epidemic and time-limited infection. Thus, delaying the social separation of calves or avoiding contact with camels aged less than 4 years might be a simple but effective measure to reduce spillover of MERS-CoV from camels to humans [118]. Although there is no evidence of sustained human-tohuman transmission of MERS-CoV, nosocomial infection may sometimes lead to MERS outbreaks. The MERS outbreak in the Republic of Korea, the largest MERS outbreak ever recorded outside of Saudi Arabia, was a result of nosocomial transmission: a single exported case with a travel history in the Middle East resulted in 185 laboratoryconfirmed human infections in Korea and one in China, with 36 deaths (WHO, 2015). The outbreak pattern in Korea was similar to the hospital outbreaks that occurred in the Middle East, which were attributed to failures of infection prevention and control in healthcare settings [119].
Enhancing infection prevention and control awareness and implementation measures is critical to preventing the possible spread of MERS-CoV in health-care facilities. It is not always possible to identify patients with MERS-CoV early because some have mild or non-specific symptoms. For this reason, it is important that all health-care facilities establish and implement clear triage policies for rapid screening and assessment of potential MERSCoV cases and all cases with acute respiratory symptoms. It is also important for health-care workers to apply standard precautions consistently with all patients, regardless of their diagnosis, in all work practices all of the time. Droplet precautions should be added to the standard precautions when providing care to any patient with symptoms of acute respiratory infection (WHO, 2017) [118- 120].
Health-care facilities that provide care for patients suspected of or confirmed to be infected with MERS-CoV should take appropriate measures to decrease the risk of transmission of the virus from an infected patient to other patients, health-care facility workers (medical and service personnel) and visitors [121-125]. These measures involve interventions at the patient-carer interface and other general measures such as linen management, cleaning and disinfection and waste management. Contact precautions and eye protection should be added when caring for probable or confirmed cases of MERS-CoV infection and airborne precautions should be applied when performing aerosol-generating procedures. Hospital cleaning staff should also be informed of and trained to take proper precautions when cleaning rooms of MERS-CoV patients (WHO, 2017).
MVA-specific antibodies that cross-neutralize camelpox virus are another very important advantage of this vaccine since outbreaks of camelpox still occur in dromedaries. Another approach would be to add a MERS-CoV component to the already existing attenuated camelpox vaccine Ducapox. Since Ducapox has been used in the Middle East for many years, the acceptance of such a vaccine can be anticipated [126-128]. However, it is important for the success of a vaccine to adhere strictly to the exact time of vaccination since the window of the disappearance of maternal antibodies and appearance of antibodies as a result of infection is narrow. Therefore, early diagnosis, prompt isolation of suspected cases and timely contact tracing of case contacts are key strategies to prevent nosocomial transmission.
Conclusion
Collaboration between human and animal health sectors in affected countries is essential to understanding the risk of transmission of MERS-CoV between animals and humans, whether there is any seasonal variation in the circulation of the virus in animals and the natural reservoir(s) of MERS-CoV. Given limited knowledge of mechanisms of MERS-CoV transmission, current precautions to prevent zoonotic transmission, such as recommendations to avoid consumption of raw camel milk and meat, are prudent despite the lack of epidemiologic evidence linking these exposures to MERSCoVinfec- tion. Such precautionary recommendations, while appropriate in the context of limited knowledge, should not be interpreted as evidence of an epidemiologic association with MERSCoV transmission. It is important to work towards limiting the spread of infection in animal populations (through development of vaccines and better management of infected animals/herds) so as to reduce the opportunity for further human exposure. In addition, a better understanding of transmission in health-care settings, especially the exposures that result in human-to-human transmission, the potential role of asymptomatic infected healthcare workers and the possible role of environmental contamination, is urgently needed.
Continuous epidemiologic and virologic monitoring is required to determine other exposures resulting in transmission and to assess for the possibility of improved virus fitness and adaptation. The disease is widely distributed in high camel population rearing areas in the world indicating the need for action via planned vaccination. Until additional evidence is available to further refine recommendations to prevent MERS-CoV transmission, continued use of existing precautionary recommendations is necessary.
REFERENCES
- Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, Drosten C, et al. Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. Journal of Virology. 2014;88:11297-11303.
- Hemida MG, Chu DK, Poon LL, Perera RA, Alhammadi MA, Ng HY, et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20:1231-1234.
- Meyer B, Müller MA, Corman VM, Reusken C, Ritz D, Godeke G, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552-559.
- Muller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, et al. Middle East respiratory syndrome coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg Infect Dis. 2014;20:1983-1997.
- Muller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, et al. Middle East respiratory syndrome coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg Infect Dis. 2014;20:1983-1997.
- Muhairi SA, Hosani FA, Eltahir YM, Mulla MA, Yusof MF, Serhan WS, et al. Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2016;52:848-854.
- Kasem S, Qasim I, Al-Hufoï¬ A, Hashim O, Alkarar A, Abu-Obeida A, et al. A cross-sectional study of MERS-CoV-speciï¬c RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J Infect Public Health. 2018;11:331-338.
- Haagmans BL, Brand JMA, Raj VS, Volz A, Wohlsein P, Smits SL, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77-81.
- Reusken CB, Ababneh M, Raj VS, Meyer B, Eljarah A, Abutarbush S, et al. The middle east respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveillance. 2013;18:20662.
- Haagmans BL, Al Dhahiry SHS, Reusken CB, Raj VS, Galiano M, Myers R, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infectious Diseases. 2013;14:140-145.
- Alraddadi BM, Watson JT, Almarashi A, Abedi GR, Turkistani A, Sadran M, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49-55.
- Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of middle east respiratory syndrome coronavirus. N Eng J Med. 2014;370:2499-2505.
- Farag EA, Reusken CB, Haagmans BL, Mohran KA, Raj VS, Pas SD, et al. A high proportion of MERS-CoV shedding dromedaries at the slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 2015;5.
- Reusken, CB, Farag EA, Jonges M, Godeke GJ, El-Sayed AM, Pas SD, et al. The middle east respiratory syndrome coronavirus (MERS-CoV) RNA and neutralizing antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveillance. 2014;19:23.
- Reusken CB, Raj VS, Koopmans MP, Haagmans BL. Cross-host transmission in the emergence of MERS coronavirus. Current Opinions Virology. 2016;16:55-62.
- Adney DR, Bielefeldt-Ohmann H, Hartwig AE, Bowen RA. Infection, replication, and transmission of Middle East respiratory syndrome coronavirus in alpacas. Emerging Infectious Diseases. 2016.
- Alagaili AN, Briese T, Omar NMS, Mohammed OB, Ian Lipkin W. Waterpipe smoking as a public health risk: potential risk for transmission of MERS-CoV, Saudi J Biol Sci. 2018;26:938-941.
- Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-erasmus medical center. Nature. 2013;495:251-254.
- Van Boheemen S, De Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. Microbiology. 2012;3:00473-512.
- Raj VS, Osterhaus AD, Fouchier RAM, Haagmans BL. Middle East respiratory syndrome emergence of a novel human coronavirus. Current Opinion in Virology. 2014;5:58-62.
- Daniel KC, Poon LM, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, et al. middle east respiratory syndrome coronaviruses in dromedary camels, egypt. Emerg Infect Dis. 2014;20:1049-1053.
- Daniel KC, Poon LM, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, et al. middle east respiratory syndrome coronaviruses in dromedary camels, egypt. Emerg Infect Dis. 2014;20:1049-1053.
- Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, et al. Discovery of seven novel mammalian and avian coronaviruses in the genus delta-coronavirus supports bat coronaviruses as the gene source of alpha-coronavirus and betacoronavirus and avian coronaviruses as the gene source of gamma-coronavirus and delta-coronavirus. J Virol. 2012;86:3995-4008.
- Tina HS. Scientists race to understand deadly new virus: sars-like infection causes severe illness, but may not spread quickly among people. Science News. 2013;183:5-6.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in saudi arabia. N Eng J Med. 2012;367:1814-1820.
- Chan JF, Chan KH, Choi GK. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis. 2013;207:1743-1752.
- Gossner C, Danielson N, Gervelmeyer A, Berthe F, Faye B, KaasikAaslav K, et al. Human-Dromedary Camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses and Public Health. 2014.
- Reusken CB, Haagmans BL, Muller MA, Gutierrez C, Godeke G, Meyer B, et al. Middle east respiratory syndrome coronavirus neutralizing serum antibodies in dromedary camels: a comparative serological study. Lancet Infectious Diseases. 2013;13:859-866.
- Mackay MI, Arden EK. Middle East Respiratory Syndrome: An emerging coronavirus infection tracked by the crowd. Journal of Virus Research. 2015;202:60-88.
- Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, De Wit E, et al. Middle east respiratory syndrome coronavirus infection in dromedary camels in saudi arabia. Microbiology. 2014;5:e01002-14.
- Miguel E, Chevalier V, Ayelet G, Ben Bencheikh MN, Boussini H, Chu DK, et al. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Eurosurveillance. 2017;22:30498.
- van Doremalen NT, Bushmaker WB, Karesh V, Munster J. Stability of middle east respiratory syndrome coronavirus in milk. Emerg Infect Dis. 2014;20:1263-1264.
- Miguel E, Véronique Ch, Ayelet G, Bencheikh M, Boussini H, Chu D, et al. Risk factors for MERS coronavirus infection in dromedary camels in Ethiopia, Morocco and Burkina Faso, 2015. Eurosurveillance. 2017;22.
- Meyer B, Juhasz J, Barua R, Das Gupta A, Hakimuddin F, Corman VM, et al. Time course of MERS-CoV infection and immunity in dromedary camels. Emerg Infect Dis. 2016;22:2171-2173.
- Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, et al. middle east respiratory syndrome coronavirus seroprevalence in domestic livestock in saudi arabia, 2010 to 2013. Euro Surveillance. 2013;18:20659.
- Hemida MG, Al-Naeem A, Perera RA, Chin AW, Poon LL, Peiris M, et al. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerging Infectious Diseases. 2015;21.
- Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. J Infect Dis. 2014;14:140-145.
- Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Revision. 2005;69:635-664.
- Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist. 2014;7:281-287.
- Patteril NA, Woo PC, Drosten C. Acute Middle East respiratory syndrome coronavirus infection in livestock Dromedaries, Dubai, 2014. Emerg Infect Dis. 2015;21:1019-1022.
- Crameri G, Durr P, Barr J, Yu M, Graham K, Williams O, et al. Absence of MERS-CoV antibodies in feral camels in Australia: implications for the pathogen’s origin and spread. One Health. 2015;1:76-82.
- Reusken CB, Schilp C, Raj VS, De Bruin E, Kohl RHG, Farag EA, et al. The middle east respiratory syndrome coronavirus infection of alpaca in a region where MERS-CoV is endemic. Emerg Infect Dis. 2016.
- Wernery U, Lau SK, Woo PC. Middle East respiratory syndrome (MERS) coronavirus and dromedaries. Vet J. 2017;220:75-79.
- Corman VM, Jores J, Meyer B, Younan M, Liljander A, Said MY, et al. Antibodies against MERS coronavirus in dromedary camels, kenya, 1992-2013. Emerg Infect Dis. 2014;20:1319-1322.
- Kandeil A, Gomaa M, Shehata M, El-Taweel A, Kayed AE, Abiadh A, et al. Middle east respiratory syndrome coronavirus infection in non-camelid domestic mammals. Emerg Microbes Infect. 2019;8:103-108.
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N Eng J Med. 2013;369:407-416.
- Drosten C, Meyer B, Müller MA, Corman VM, Al-Masri M, Hossain R, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828-35.
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819-1823.
- Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, et al. middle east respiratory syndrome coronavirus outbreak in jeddah a link to health care facilities. N Engl J Med. 2015;372:846-854.
- Khudhair A, Killerby ME, Al Mulla M, Abou Elkheir K, Ternanni W, Bandar Z, et al. Risk factors for MERS-CoV seropositivity among animal market and slaughterhouse workers, Abu Dhabi, United Arab Emirates, 2014–2017. Emerg Infect Dis. 2019;25:927-935.
- Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT. Middle East respiratory syndrome coronavirus transmission. Emerg Infect Dis. 2020;26: 191-198.
- Guery B, Poissy J, Mansouf L, Sejourne C, Ettahar N, Lemaire X, et al. Clinical Features and Viral Diagnosis of Two Cases of Infection with Middle East Respiratory Syndrome Coronavirus: A Report of Nosocomial Transmission. Lancet. 2013;381:2265-2272.
- Raj SV, Elmoubasher AB, Farag A, Chantal BE, Reusken CB, Mart M, et al. Isolation of MERS coronavirus from dromedary camel, Qatar. Emerg Infect Dis. 2014;20:1339-1342.
- Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance. 2012;17:20285.
- Reusken CB, Messadi L, Feyisa A, Ularamu H, Godeke GJ, Danmarwa A, et al. Geographic distribution of middle east respiratory syndrome-coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014;20:1370-1374.
- Reusken CB, Farag EA, Haagmans BL, Mohran KA, Godeke GJ, Raj VS, et al. Occupational exposure to dromedaries and risk for middle East respiratory syndrome-coronavirus infection, Qatar, 2013-2014. Emerg Infect Dis. 2015;21:1422-1425.
- Hemida MG, Elmoslemany A, Al-Hizab F, Alnaeem A, Almathen F, Faye B, et al. Dromedary camels and the transmission of Middle East respiratory syndrome coronavirus. Transboundary Emerging Diseases. 2017;64:344-353.
- Miguel E, Perera RA, Baubekova A, Chevalier V, Faye B, Akhmetsadykov N, et al. Absence of Middle East respiratory syndrome coronavirus in camelids, Kazakhstan, 2015. Emerg Infect Dis. 2016;22:555-557.
- Alexandersen S, Kobinger GP, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, the United Arab Emirates in 2005. Transboundary Emerging Diseases. 2014;61:105-108.
- Sharif-Yakan A, Kanj SS. The emergence of middle east respiratory syndrome coronavirus in the middle east: origins, transmission, treatment and perspectives. Pathology of Pathogens. 2014;10:1004457.
- Milne-Price S, Miazgowicz KL, Munster VJ. The emergence of the Middle East respiratory syndrome coronavirus (MERS-CoV). Pathogenic Diseases. 2014;71:121-136.
- Perera RA, Wang P, Gomaa MR, El-Shesheny R, Kandeil A, Bagato O, et al. Seroepidemiology for MERS coronavirus using microneutralization and pseudoparticle virus neutralization assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveillance. 2013;18:20574.
- Memish ZA, Cotten M, Meyer B, Watson SSJ, Al Sahafi AJ, Al Rabeeah AA, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1015.
- Júlia VA, Judith MA, Brand V, Widagdo W, Muñoz M, Raj VS, et al. Livestock susceptibility to infection with middle east respiratory syndrome coronavirus. Emerg Infect Dis. 2017;23:232-240.
- Ali M, El-Shesheny R, Kandeil A, Shehata M, Elsokary B, Gomaa M, et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill. 2017;22:30487.
- Almazan F, Marta L, Diego D, Sola I, Zuñiga SA, Jose L, et al. Engineering a replication-competent, propagation-defective middle east respiratory syndrome coronavirus as a vaccine candidate. Journal of Microbiology.2013;4:e00650-713.
- De Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, Van Nieuwkoop S, Limpens RW, et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporine a or interferon-alpha treatment. J Gen Virol. 2013;94:1749-1760.
- Raj VS, Smits SL, Provacia LB, van den Brand JM, Wiersma L, Ouwendijk WJ, et al. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4 mediated entry of the middle east respiratory syndrome coronavirus. J Virol. 2014;88:1834-1838.
- Hocke AC, Becher A, Knepper J. emerging human middle east respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs. Am J Resp Crit Care Med. 2013;188:882-886.
- Li W, Moore MJ, Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454.
- Delmas B, Gelf J, L’Haridon R. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417-420.
- Chan RW, Hemida MG, Kayali G. Tropism and replication of Middle East respiratory syndrome coronavirus from dromedary camels in the human respiratory tract: an in vitro and ex vivo study. Lancet Respir Med. 2014;2:813-822.
- Dijkman R, Jebbink MF, Koekkoek SM. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J Virol. 2013;87:6081-6090.
- Kindler E, Jónsdóttir HR, Muth D, Hamming OJ, Hartmann R, Rodriguez R, et al. Efficient replication of the novel human betacoronavirus emc on primary human epithelium highlights its zoonotic potential. Microbiology. 2013;4:00611-612.
- Wernery U, Corman VM, Wong EY, Tsang AK, Muth M, Lau SK, et al. Acute middle east respiratory syndrome coronavirus infection in livestock dromedaries, Dubai. Emerg Infect Dis. 2015;21.
- Drosten C, Muth D, Corman V, Hussain R, Al Masri M, HajOmar W, et al. An observational, laboratory-based study of outbreaks of MERS-Coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60:369-377.
- Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with the middle east respiratory syndrome-coronavirus infection: a single-center experience in saudi arabia. International Journal of Infectious Diseases. 2014;29:301-306.
- WHO (World Health Organization). Middle east respiratory syndrome-coronavirus (MERS-CoV). update on the middle east respiratory syndrome coronavirus (MERS-CoV) transmission from animals to humans, and interim recommendations for at risk groups. 2017.
- WHO (World Health Organization). Middle east respiratory syndrome coronavirus (MERS-CoV). 2018.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus:implications for virus origins and receptor binding. Lancet. 2020;6736:30251-30258.
- WHO (World Health Organization;MERS situation update). Middle east respiratory syndrome coronavirus (MERS-CoV) Epidemic and pandemic diseases. 2020.
- Callaway E, Cyranoski D. What scientists want to know about the coronavirus outbreak. Nature. 2020;577:605-607.
- Cohen J. Wuhan seafood market may not be the source of novel virus spreading globally. Science. 2020.
- Wu P, Hao X, Lau EH, Wong JY, Leung KS, Wu JT, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China. Eur Surveill. 2020;25:2000044.
- Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S. Novel wuhan (2019-nCoV) coronavirus. Am J Resp Crit Care Med. 2020;201:7-8.
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. E Microb Infect. 2020;9:386-389.
- Dudas G, Carvalho LM, Rambaut A, Bedford T. MERS-CoV spillover at the camel-human interface. eLife. 2018;7:e31257.
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237-261.
- Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118-121.
- Walls AC, Tortorici MA, Bosch BJ, Frenz B, Rottier PJ, DiMaio F, et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114-117.
- Yuan Y, Cao D, Zhang Y, Ma J, Qi J, Wang Q, et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;10:15092.
- Li F, Berardi M, Li WH, Farzan M, Dormitzer PR, Harrison SC, et al. Conformational states of the severe acute respiratory syndrome coronavirus spike protein ectodomain. J Virol. 2006;80:6794-6800.
- Walls AC, Tortorici MA, Snijder J, Xiong X, Bosch BJ, Rey FA, et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci USA. 2017;114:11157-11162.
- Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011-1033.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020,94:e00127-120.
- Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T, et al. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873-882.
- Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122-4134.
- Bertram S, Glowacka I, Müller MA, Lavender H, Gnirss K, Nehlmeier I, et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol. 2011;85:13363-13372.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. National Microbiology. 2020;5:562-569.
- Song W, Gui M, Wang X. Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2011;14:e1007236.
- Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond, SL, Bates P, et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proceedings of National Academic Science. 2005;102:11876-11881.
- Millet JK, Whittaker GR. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Research. 2015;202:120-134.
- Mathieu D, Felix AR, Margaret K. Rubella virus: First calciumrequiring viral fusion protein. PLoS Pathog. 2014;10:004530.
- Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, et al. Ca2+ ions promote fusion of middle east respiratory syndrome coronavirus with host cells and increase infectivity. J Virol. 2020;94:e00426-520.
- Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. 2020, Antiviral Res. 2020;177:104759.
- Yan S, Sun H, Bu X, Wan G. An evolutionary RGD motif in the spike protein of SARS-CoV-2 may serve as a potential high-risk factor for virus infection?. Preprints. 2020.
- Fox JE, Goll DE, Reynolds CC, Phillips DR. Identification of two proteins (actin-binding protein and P235) that are hydrolyzed by endogenous Ca2+-dependent protease during platelet aggregation. J Biol Chem. 1985;260:1060-1066.
- Lawson C, Schlaepfer D. Integrin adhesions: Who’s on first? What's on second?. Cell Adh Migr. 2012;6:302-306.
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D. Identification of severe acute respiratory syndrome in Canada. N Eng J Med. 2003;348:1995-2005.
- Trimble WS, Grinstein S. T.B. or not T.B.: Calcium regulation in mycobacterial survival. Cell. 2007;130:12-14.
- Chen SMS, Damdinjav B, Perera RA, Chu DKW, Khishgee B, Enkhbold B, et al. Absence of middle east respiratory syndrome-coronavirus in bactrian camels, southern mongolia. Emerg Infect Dis. 2016;21:1269-71.
- Chen Y, Chan K, Kang Y, Chen H, Luk HK, Poon RS, et al. A Sensitive and specific antigen detection assay for middle east respiratory syndrome coronavirus. Emerg Microbes and Infect. 2015;4:e26.
- Chu DK, Poon LL, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, et al. Middle East respiratory syndrome coronaviruses (MERS-CoV) in dromedary camels, Egypt. Emerg Infect Dis. 2014;20:1049-1053.
- Corman VM, Muller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV/EMC) infections. Eurosurveillance. 2012;17:20334.
- Deem SL, Fèvre EM, Kinnaird M, Browne AS, Muloi D, Godeke GJ, et al. Serological evidence of MERS-CoV antibodies in dromedary camels (camelus dromedaries) in laikipia county, kenya. PLoS One. 2015;10:e0140125.
- FAO (Food and Agricultural Organization: FAO MERS Situation update). Middle East Respiratory Syndrome Coronavirus-FAO Emergency Prevention System for Animal Health. 2017.
- Harcourt JL, Rudoler N, Tamin A, Leshem E, Rasis M, Giladi M, et al. The prevalence of middle east respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in israel. Zoonoses and Public Health. 2018;65:749-754.
- Harrath R, Abu Duhier FM. Seroprevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J Med Virol. 2018;90:1285-1289.
- Kam YW, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R, et al. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One. 2009;4:e7870.
- Lau SKP, Li KSM, Luk HKH, He Z, Teng JLL, Yuen KY, et al. Middle East respiratory syndrome coronavirus antibodies in Bactrian and hybrid camels from Dubai. mSphere. 2020;5:e00898-919.
- Liu R, Wen Z, Wang J, Ge J, Chen H, Bu Z, et al. Absence of middle east respiratory syndrome coronavirus in bactrian camels in the west inner mongolia autonomous region of china: surveillance study results from july 2015. Emerg Microbes Infect. 2015;4:73.
- Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, et al. Molecular Basis of Binding between Novel Human Coronavirus MERS-CoV and Its Receptor CD26. 2013;500:227-231.
- Müller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D, et al. Presence of Middle-East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infectious Diseases. 2015;15:559-564.
- Nowotny, N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveillance. 2014;19:20781.
- Saqib M, Sieberg A, Hussain MH, Mansoor MK, Zohaib A, Lattwein E, et al. Serologic evidence for the middle east respiratory syndrome coronavirus infection in dromedary camels, punjab, pakistan, 2012-2015. Emerg Infect Dis. 2017;23:33.
- Thwiny HT, Al Hamed TA, Nazzal AR. Seroepidemiological study of middle east respiratory syndrome (MERS) virus infection in Iraqi dromedary camels. 2018;88:191-200.
- WHO. Novel Coronavirus (2019-nCoV) Situation Report 15 [accessed 4 Feb 2020]. 2020.
- Yusof MF, Eltahir YM, Serhan WS, Hashem FM, Elsayed EA, Marzoug BA, et al. Prevalence of middle east respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50:509-513.
Citation: AbdurehmanA(2020) Spatiotemporal Distribution and Associated Risk Factors for Middle East Respiratory Syndrome-Coronavirus In Dromedary Camels: Review. J MicrobBiochem Technol. 12:446 Doi: 10.35248/1948-5948.20.12.446
Copyright: © 2020 Abdurehman A, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited