Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 11
Spatial Distribution of Tomato Early Blight and Reaction of Some Tomato Varieties to the Disease in Southern Tigray, Ethiopia
Hailu Negesa1* and Getachew Ayana22Ethiopian Institute of Agricultural Research, Melkassa Agricultural Research Center, P.O. BOX 436, Adama, Ethiopia
Received: 29-Sep-2021 Published: 27-Nov-2021
Abstract
Early blight disease caused by Alternaria species is the most destructive fungal disease of tomato in Ethiopia and in southern Tigray in particular. However, the importance and distribution status of the disease has not been studied in this area. Besides, the reaction of released tomato varieties to the disease was not well documented in Ethiopia. Therefore, the present study was conducted to identify the distribution of early blight in southern Tigray and to evaluate the reaction of some released tomato varieties to the disease under glasshouse conditions. Results revealed that tomato early blight was prevalent up to 89.3% and significantly (p<0.01) varied in disease intensity among the districts and peasant associations of the study areas. Severity of the disease was higher in the Raya Azebo than in the Raya Alamata district with a mean of 42.1% and 25.6%, respectively. Similarly, under peasant association level Wergaba and Gerjele were highly severed relatively, with 50% and 44.4% mean values, respectively. On the other hand, Limhat and Selam Bekalsi peasant associations had the lowest disease severity with the mean of 11.4% and 12.3%, respectively. Tested tomato varieties have been shown significant differences in their reaction to the disease. Two of the tested varieties were shown a resistant reaction to the disease; whereas, four varieties have been indicated a moderately resistant to the disease. Overall, the study identified the importance of tomato early blight in southern Tigray and the existence of promising varieties to resist the risk of early blight disease. Meanwhile, Future works should focus on the evaluation of promising varieties and integration of management options.
Keywords
Early blight; Incidence; Resistance; Severity; Variety
Introduction
Tomato (Solanum lycopersicum L.) is one of the most popular vegetable crops widely grown throughout the world. Adaptability, potential yield and its flexibility for food are the main reasons for popularity of the crop. It is originated from the Andean region of Colombia, Chile, Peru, and Bolivia [1].
From the world, Ethiopia ranks 98, 161 and 58 in terms of production quantity, area harvested and yield per hectare, respectively. From the African continent Egypt, Nigeria and Tunisia are the leading producers of the crop [2]. Tomato is one of the most important and widely grown vegetables in Ethiopia [3,4]. In 2018/19 and 2019/20 meher cropping season tomato production was estimated to cover 4322.31 and 6012.28 hectares in Ethiopia, with an estimated production of 25996.6 and 38523.1 tons, respectively [5]. From 182,361,395 tons of estimated world tomato production, Ethiopia shares 45561 tons of estimated contribution [2]. From 6,520,863 hectares of land covered by vegetables tomato shares only 2.4% hectares of land next to Ethiopian cabbage, red pepper, green pepper, and head cabbage. Similarly, tomato ranks 5th in terms of production capacity from vegetables produced in the country. Nationally, the average national yield of tomato was estimated to be 6.4 tons per hectare [5].
Tomato is one of the most important crops in the industrialized world. It is a key food and cash crop for farmers wherever it is produced. The crop is grown for its fruits, consumed in fresh and processed forms (salads, tomato paste, sauce, ketchup, and juice forms). Tomato foods are rich in nutrients, minerals and vitamins [6]. It is a high-value commodity crop, which has been given top priority in the vegetable research system of Ethiopia.
Despite its importance, the average national yield was significantly low due to different biotic and abiotic constraints [7]. Among the biotic constraints, tomato early blight is one of the most important and frequently occurring diseases across the world and in Ethiopia particularly [8,9]. It is the worst damaging disease and causes a reduction in the quantity and quality of tomato yield. Every one percent increase in the intensity of the disease was estimated to reduce up to 1.36% of the yield and complete crop failure could occur under the severe disease condition [7]. Reports indicated, 50% to 86% yield losses can be caused by early blight [10,11]. In the case of Ethiopia, 14.2% to 52.9% yield loss was reported due to this disease [9]. In recent years, the disease has been causing serious problems on tomato production in Ethiopia [12,7]. Correspondingly, southern Tigray is failed under the hanging of this disease [9]. Obviously, the intensity and distribution of this disease are various from place to place [13-15]. However, there is little profiled information available on the status of distribution and intensity of the disease in southern Tigray.
In addition, a limited number of studies conducted in the country paid attention to evaluate bio-agents and integration of fungicide with host resistance to manage the disease [9,12,16]. Less effort was given to study the resistance reaction of available tomato genotypes. In most cases, application of fungicides has been recommended to fight this disease properly. However, indiscriminate use of fungicides considerably increases hazards to the human being and the environment [17]. In another way, the easiest, economical, harmless and effective management approach is using resistant crops [18]. Therefore, knowing the reaction of tomato varieties is very important for the management of the disease. Keeping this in mind, the present study has been needed to develop a clear picture of the current status of the distribution and intensity of tomato early blight in southern Tigray. Besides, to know the reaction of some released tomato varieties to aggressive isolate of the pathogen from the study area.
Materials and Methods
Description of the study area
Assessment of the disease and sample collection have been conducted in the Southern zone of Tigray region during January 2020 in the winter (Bega) season under an irrigation cropping system. The survey was carried out in the major tomato growing districts of the zone namely Raya Alamata and Raya Azebo districts of the Southern Tigray zone, Ethiopia. The study area is found at a distance of 665 km from Addis Ababa to the northern part of the country. The area is found between an elevation range of 930- 3171 meters above sea level with an annual rainfall of 600-750 mm. (Table 1) (Figure 1).
| Districts | Altitude Ranges | Prevalence (%) | Incidence (%) | Severity (%) | |||
|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | ||||
| Raya Azebo | 1504 - 1662 | 100 | 45 - 94.0 | 72.7a | 28 - 62.1 | 42.1a | |
| Raya Alamata | 1404 - 1619 | 78.6 | 0 - 94.4 | 48.8b | 0 - 48.0 | 25.6b | |
| Overall | 1404-1662 | 89.3 | 0 - 94.4 | 60.5 | 0-62.1 | 34.05 | |
| LSD(0.05) | - | - | - | 8.5 | - | 4.2 | |
Table 1: Altitude ranges and disease intensity of tomato early blight by districts in Southern Tigray, 2020.
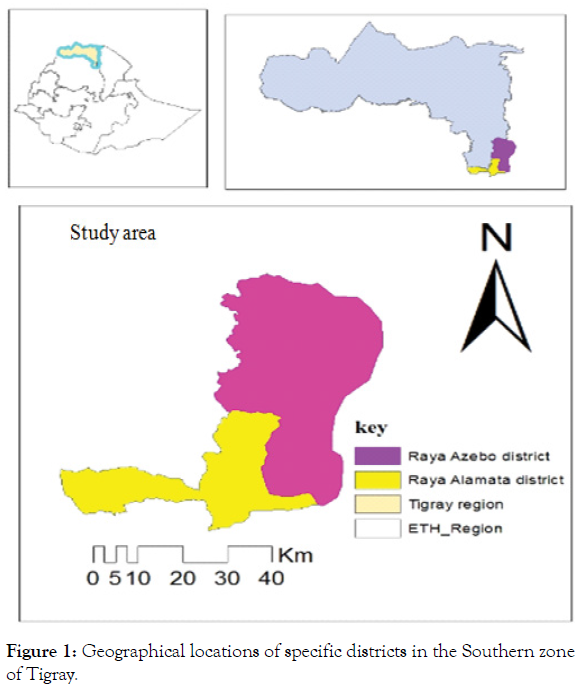
Figure 1: Geographical locations of specific districts in the Southern zone of Tigray.
Assessment of tomato early blight
A purposive sampling strategy was used to select the districts and peasant associations across the study areas by consulting Zonal and districts agricultural offices, respectively. Assessment of the fields has been done by the distance of 4 to 6 km intervals based on random sampling along the main and feeder roadsides on the preplanned routes. Early blight incidence and severity were assessed through direct visual observation of the disease symptoms in the fields. The assessment was carried out along the two diagonals (in an ‘’X’’ fashion) using 2m2 quadrants at least 5 to 10 m far apart from each other approximately. In each field, 5 quadrants were systematically assigned to the respective points and tomato plants within the quadrant were counted and recorded as an infected and healthy plant. Similarly, assessment of early blight severity was done from ten plants randomly from each quadrant using a 0-9 disease scoring scale according to Ghosh P, et al. [19] where 0=no visible infection, 1= 0-10% leaf area are infected, 2=10-20%, 3=20-30%, 4=30-40%, 5=40-50%, 6=50-60%, 7=60 -7 0%, 8=70- 80% and 9=80-90% of leaf damaged. Prevalence of the disease was calculated using a formula described by Pandey K, et al. [20]. Disease incidence and disease index were also calculated for each field based on the formula used by scholars previously. Samples were collected from each quadrant separately in paper bags, labeled and transported to Ambo Agricultural Research Center laboratory using an icebox and identification of associated Alternaria species has been done in the laboratory.
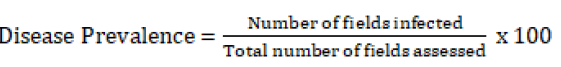
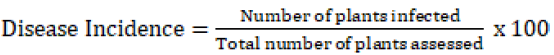
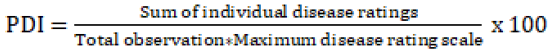
Evaluation of tomato varieties for their reaction to the disease
Evaluation of the varieties for their reaction to the disease was carried out at Ambo Agricultural Research Center from June to August 2020 under glasshouse conditions using 11 released tomato varieties. Two varieties ARP Tomato d2 and Melkashola [9,12] were used as tolerant and susceptible checks, respectively (Table 2). Before sowing, the seed was washed thoroughly by distilled water and disinfected in 1% sodium hypochlorite solution for 5 minutes. Sterilized seeds of 11 tomato varieties (Table 2) were sown on a plastic pot containing sterilized soil composed of topsoil, compost and sandy soil with a ratio of 2:1:1 in the glasshouse. The treatments were arranged in randomized completely block design (RCBD) with three replications. Two pots per replication with two plants per pot were used for each of the varieties for inoculation. Isolate for inoculation was selected based on a pathogenicity test conducted under glasshouse conditions to select virulent isolate from identified Alternara species. Inocula of the selected isolate was prepared by dislodging the conidia using a glass road after pouring 10 ml of distilled water from fungal colonies incubated for 12 days. Then, the suspension was filtered using cheesecloth to remove mycelial masses and the final concentration of the suspension was adjusted to 3 × 106 conidia/ml [21] using a hemocytometer.
| Districts | Peasant Association | Prevalence (%) |
Incidence (%) | Severity (%) | ||
|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | |||
| Raya Azebo | Wergaba | 100 | 78 - 94.4 | 80.1ab | 41 - 62.1 | 50.0a |
| K. Adisho | 100 | 45 - 90.0 | 66.9ab | 28 - 56 | 39.6bc | |
| Werebaye | 100 | 56 - 87.2 | 67.7ab | 31 - 48 | 41.1bc | |
| B. Delbo | 100 | 65 - 86.4 | 75.4ab | 32 - 42 | 37.8bc | |
| Raya Alamata | Gergele | 100 | 65 - 93.5 | 84.1a | 40 - 48 | 44.4ab |
| K. Lemlem | 100 | 46 - 72.0 | 63.0b | 30 - 36 | 34.3c | |
| Limhat | 57.1 | 0 - 50.5 | 24.1c | 0 - 20 | 11.4d | |
| S. Bekalsi | 57.1 | 0 - 50.0 | 24.0c | 0 - 26 | 12.3d | |
| LSD(0.05) | - | - | - | 17.2 | - | 8.4 |
Table 2: Prevalence and intensity of tomato early blight across peasant associations of the southern zone of Tigray, 2020 under irrigation cropping system.
Inoculation was done at 35 days old seedlings by spray inoculation method until runoff using hand sprayer. All inoculated plants were covered with transparent polyethylene paper for 48 hours immediately after inoculation to maintain humidity that facilitates the condition for a pathogen to infect [22]. Disease severity assessment was done by seven-day intervals at 7, 14, 21, 28, 35, 42 days after inoculation [20] using a 0-5 disease rating scale [13]. Percent severity Index (PDI) and area under the disease progress curve (AUDPC) were worked out using a formula described by Pandey K, et al. [20]. Finally, reaction class of the varieties was marked based on the value of percent severity index (PSI) according to Lohith MR, et al. [23] <1=immune; 1-10=highly resistant; 10.1-25=resistant 25.1-40=moderately resistant; 40.1-50=susceptible and >50=highly susceptible.
Data analysis
Data on the incidence and severity of early blight was analyzed using two stages nested design GLM procedure of SAS 9.4 statistical software. Mean separation was done using the LSD test at significance levels of 0.05. Analysis of variance for Percent Severity Index (PSI) and AUDPC data of tomato varieties reaction was done using the GLM procedure of SAS software. Treatment means separation was done using the Least Significant Difference (LSD) test at the alpha level of 5%. An infection rate of the disease across the inoculated tomato varieties was computed by Minitab 16 software.
Results
Occurrence and distribution of tomato early blight
Tomato early blight disease was prevalent in both districts by different levels of disease intensity. Magnitude of the disease was varied from slight to severe infection depending on the variability of the variety grown, irrigation type, seed source, previous history of the farm, seedling production type, growth stage, and irrigation frequency divergence farmers were used in their fields. The results have been shown that tomato early blight was more prevalent in Raya Azebo than Raya Alamata district. The disease was 100% prevalent in Raya Azebo district whenever it was 78.6% prevalent in Raya Alamata district (Table 1). Averagely around 89.3% prevalence of the disease was recorded during the field assessment. Statistically, there was a significant difference (p < 0.01) among the districts in the intensity of the disease (Table 1).
The overall mean incidence and severity of the disease were 60.5% and 34.05% from both assessed districts, respectively. However, the higher incidence of the disease was recorded in Raya Azebo district with a mean value of 72.7%, whereas the lowest was recorded from Raya Alamata district with a mean value of 48.8%. Similarly, the higher disease severity was recorded in Raya Azebo district whenever the lowest disease severity was recorded in Raya Alamata district with a mean of 42.1% and 25.6%, respectively. Present results indicated that disease severity was ranged from 0-62.1% whenever disease-free fields were observed in Raya Alamata district. In contrast, fields with the maximum disease severity were observed in Raya Azebo district (Table 1).
Distribution and intensity of tomato early blight across the peasant associations
Results indicated that the disease was 100% prevalent in all peasant associations of Raya Azebo district and two of Raya Alamata district. The extent of disease incidences and severities across the peasant associations were significantly different from each other. The highest incidence of the disease was recorded from Gerjele, Wergaba, Bagedelbo, Werebaye and Kara Adisho peasant associations with the mean of 84.1%, 80.1%, 75.4%, 67.7%, and 66.9%, respectively (Table 2). Statistically, there was a significant difference (p <0.01) among peasant associations in disease intensity (Table 2)
The highest severity of the disease was recorded from Wergaba and Gerjele peasant associations. Additionally, peasant associations such as Werebaye, Kara Adisho and Bagedelbo were statistically comparable in recorded disease severity with Wergaba and Gerjele peasant associations with the mean of 41.1%, 39.6% and 37.8%, respectively. In corresponding to the incidence of the disease, the lowest severity of early blight was observed in Selam Bekalsi and Limhat peasant associations with the mean of 12.3% and 11.4%, respectively (Table 2) (Figure 2).
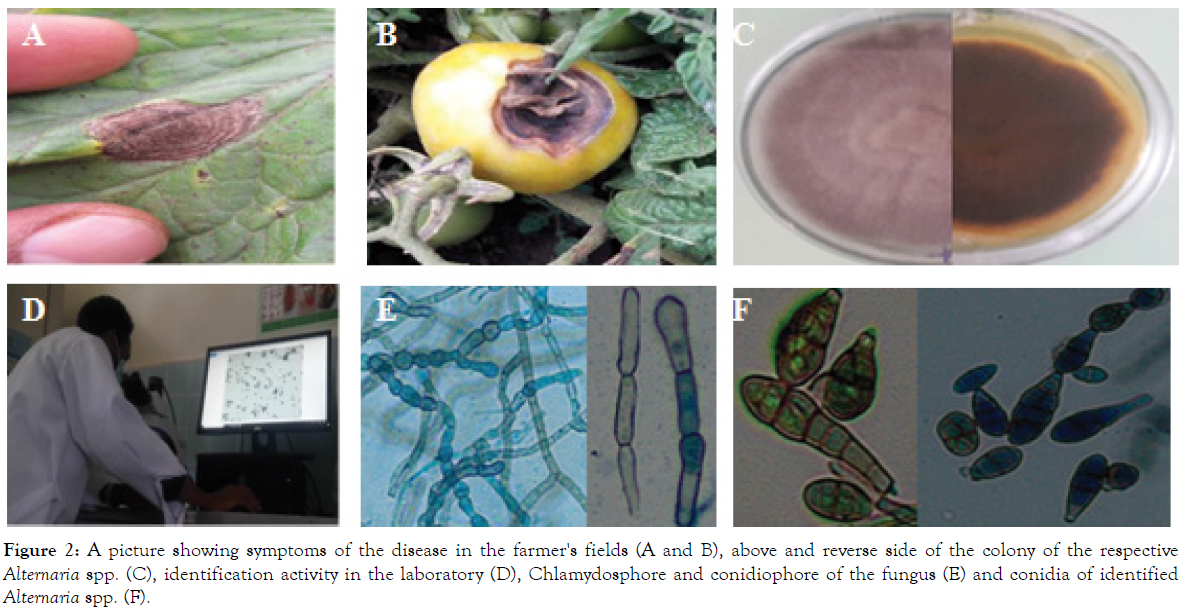
Figure 2: A picture showing symptoms of the disease in the farmer's fields (A and B), above and reverse side of the colony of the respective Alternaria spp. (C), identification activity in the laboratory (D), Chlamydosphore and conidiophore of the fungus (E) and conidia of identified Alternaria spp. (F).
Reaction of tomato varieties to the disease
Results indicated there was a highly significant difference (p<0.001) among tested tomato varieties in reaction to early blight (Table 3). Mean of severity index depicted that all of the tested varieties were categorized into four reaction classes (Table 3). ARP Tomato d2 variety has been shown the lowest mean of percent severity index and significantly different from all other tomato varieties followed by Galilema tomato variety (Table 3). Accordingly, both varieties were indicated a resistant reaction to the disease.
| Varieties | Percent Severity Index (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7DAI | 14DAI | 21DAI | 28DAI | 35DAI | 42DAI | AUDPC | r | R2 (%) | RC | |
| Chali | 11.5de | 13.5f | 20.8de | 25.0de | 25.3e | 28.1f | 490.7d | 0.016** | 91.9 | MR |
| Cochoro | 12.5de | 18.7de | 21.9d | 27.1d | 30.6cd | 34.0cd | 573.8cd | 0.018** | 97.9 | MR |
| Eshet | 10.4e | 15.6ef | 20.8de | 27.1d | 28.6de | 30.5ef | 531.3d | 0.018** | 91.3 | MR |
| Melka shola | 22.9a | 29.2a | 39.8a | 43.4a | 51.0a | 53.0a | 995.7a | 0.025** | 97.0 | HS |
| Melka salsa | 16.7bc | 19.8cd | 29.2c | 38.5b | 41.0b | 43.0b | 745.7b | 0.023** | 94.2 | S |
| Metadel | 18.7b | 23.9b | 28.1c | 31.3c | 33.9c | 36.1c | 672.9bc | 0.014** | 96.6 | MR |
| Fetan | 14.6cd | 18.7de | 20.8de | 22.9ef | 27.9de | 31.3de | 530.9d | 0.014** | 98.5 | MR |
| Bishola | 16.7bc | 22.9bc | 27.7d | 24.3def | 28.8de | 31.8de | 600.6cd | 0.011** | 91.9 | MR |
| Miya | 16.7bc | 22.9bc | 35.4b | 40.2ab | 40.6b | 42.5b | 783.8b | 0.022** | 86.5 | S |
| ARP Tomato | 2.7f | 4.8g | 11.5g | 13.6g | 10.0g | 10.8h | 189.4e | 0.013 | 60.0 | R |
| Galilema | 9.4e | 13.5f | 17.7f | 20.8f | 20.9f | 21.6g | 524.1d | 0.012** | 86.1 | R |
| LSD | 3.4 | 3.9 | 4.0 | 4.1 | 3.5 | 2.7 | 128 | - | - | - |
| CV (%) | 14.7 | 12.5 | 9.6 | 8.3 | 6.8 | 4.9 | 12.4 | - | - | - |
Table 3: Response of tomato varieties against virulent Alternaria isolate under glasshouse conditions.
The obtained results indicate that the highest percent severity index and AUDPC value were recorded from Melka shola tomato variety followed by Melkasalsa and Miya varieties. However, both were significantly different from Melka shola variety (Table 3). Accordingly, Melkashola tomato variety has been shown highly susceptible reaction to the disease. Following Melka shola, Miya and Melkasalsa varieties were demonstrated a susceptible reaction to the disease. In another way, Chali, Cochoro, Eshet, Metadel, Fetan and Bishola tomato varieties were showed a moderately resistant reaction to the disease. However, in surprise, the AUDPC value of Chali variety was less than that of Galilema variety which has shown resistant reaction to the disease. This might indicate the need for more observation dates or another study to determine the exact reaction of these varieties.
Results indicate that the fastest disease infection rate was recorded from Melkashola variety, followed by Melkasalsa and Miya varieties (Table 3) (Figure 3). In contrast, the progression rate of the disease infection was relatively the lowest on Galilema and Bishola varieties. Interestingly, an infection rate of the disease has never been shown a significant difference among the observation dates on ARP Tomato d2 variety. Shortly, the rate of disease infection was so fast on varieties has been shown susceptible reaction to the disease.
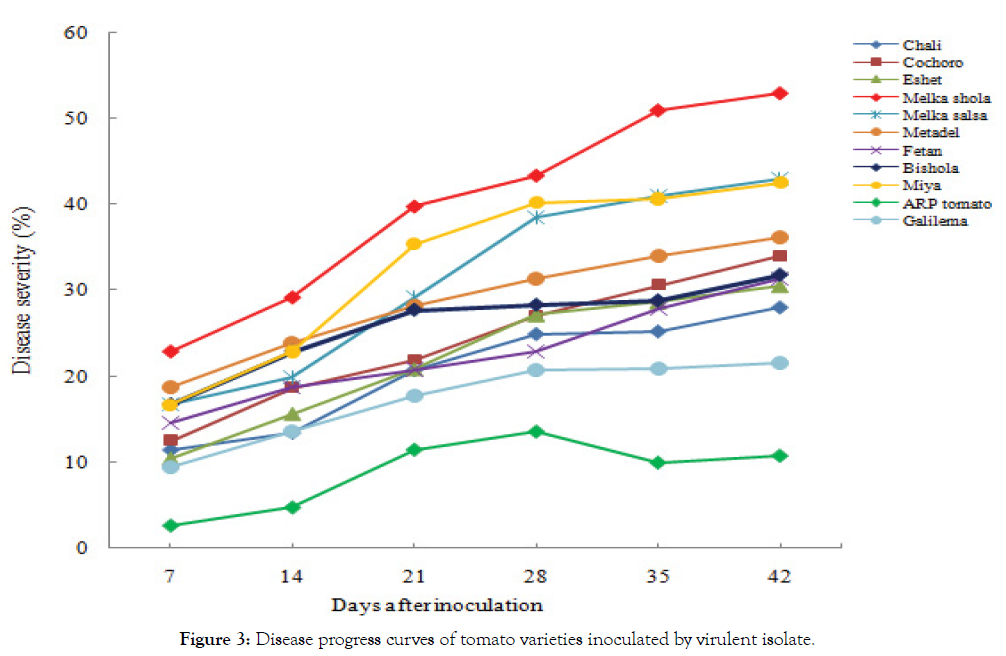
Figure 3: Disease progress curves of tomato varieties inoculated by virulent isolate.
Discussion
In general, the obtained results were consistent with previous findings reported, that the extent of tomato early blight was significantly different from location to location [24,25]. Similarly, present results have coincided with the study of Kamble S, et al. [26] that reported a range of 21% to 56% severity of early blight from different locations. Our results indicated fields free from the occurrence of early blight were observed in Raya Alamata district. This might be possibly due to agronomic factors and the growth stage of the crops on the field. These disease-free fields were irrigated regularly two times per week according to the respondents. In agreement with our result. Rotem J and Palti J [27] reported decreasing the frequency of irrigation with balanced water needs of the crop can help to reduce the risk of disease development. In addition, fields were assessed at the vegetative and flowering growth stage of the plant. Obviously, at these stages, the crop was relatively resistant to early blight [26,28].
The extent of temperature prevailing also might be another fundamental factor to determine the early blight intensity of the study areas. Monthly range of temperature in the Raya Alamata district was 24.30°C-34.10°C with the mean of 30.7°C and 27.20°C-34.30°C with the mean of 32.05°C in January and February months, respectively. Relatively low monthly range of temperature was recorded in Raya Azebo with the mean of 28.35°C and 29.45°C in January and February months, respectively. Previous reports indicated temperature is one of the important factors in the distribution and intensity of early blight [29-31] stated 24-30°C is the optimum temperature range for early blight development. Concurrently, our results revealed mean monthly temperature of the Raya Azebo district was fall in the optimum range of temperature required for early blight disease development. Another remarkable observation during the study has been shown that fields infested by Orobanche weed were highly infected by the disease relatively than infestation-free fields. Infestation of the weed was observed on some peasant associations of the Raya Azebo district. Obviously, Orobanche weed competes for resources and exposes the crop to nutrient deficiency stress that might facilitate early blight infection and development [32]. Hence, this might be the reason for the difference in intensity of the early blight among the peasant associations. In short, a divergence of environmental and agronomic factors might have a lion share of the possible reason for the variations of disease intensity among the districts and peasant associations of the study areas [33].
Apart from the assessing distribution and importance of tomato early blight, we also evaluated some released tomato varieties for their reaction to the disease. Out of 11 tomato varieties tested, ARP tomato d2 and Galilema varieties were showed a resistant reaction to early blight disease. Recently Getachew G, et al. [12] also observed that ARP Tomato d2 variety was relatively resistant to early blight. In agreement with this study, Melkashola and Melkasalsa have been previously reported as susceptible variety to tomato early blight in both field and glasshouse conditions [34]. In another way, disease symptom was observed three days after inoculation on Melkashola and Melkasalsa tomato varieties while the rest of varieties has been shown the symptom after seven days with different extent of disease severity. Concurrently Terna TP, et al. [35], previously reported different duration of days for incidence of the tomato early blight on different tomato varieties after inoculation.
Conclusion
To summarize, tomato early blight was highly prevalent and important in southern Tigray. Incidence and severity of the disease were ranged from 0-94.4% and 0-62.1%, respectively. Encouraging result was found from evaluation of released tomato varieties to the disease reaction. From tested varieties, ARP tomato d2 and Galilema varieties were shown a resistant reaction whereas the rest were showed moderately resistant and susceptible reaction to the disease. Therefore, ARP tomato d2 and Galilema tomato varieties are recommended for further research in the resistance breeding of tomato crops and need to be tested under multi-location environmental conditions. Also, future investigations should be emphasized on the evaluations of host resistance and integrated disease management options to reduce the risk of this disease.
Acknowledgement
We would like to acknowledge the Ethiopian Institute of Agricultural Research for funding this study to be undertaken. Also, Mehoni, Melkassa and Ambo Agricultural Research centers were greatly acknowledged for supporting us by study materials and logistics.
REFERENCES
- Lucatti AF, Heusden AWV, Vos RCD, Visser RG, Vosman B. Differences in insect resistance between tomato species endemic to the Galapagos Islands. BMC Evol Biology. 2013;13(1):1-12.
- FAOSTAT (Food and Agriculture Organization/Statistics). Statistical database of the Food and Agriculture Organization of the United Nations. 2018.
- Derbew B, Ali M, Amina J. Yield and quality of indeterminate tomato (Lycopersicon esculentum Mill.) varieties with staking methods in Jimma. Singapore J Sci Res. 2012;2(2):33 - 46.
- Lemma D. Tomatoes; research experiences and production prospects. Ethiopian Agricultural Research Organization. 2002;43:1-15.
- CSA (Central Statistical Agency). Agricultural sample survey, 2019/2020. Report on area and production of crops (Private peasant holdings, main season). Central Statistical Agency, Addis Ababa.2020.
- Herrera PG, Sanchez-Mata MC, Cámara M. Nutritional characterization of tomato fiber as a useful ingredient for food industry. Innov Food Sci Emerg Technol. 2010;11(4):707-711.
- Worku M, Sahela S. Review on disease management practice of tomato wilt caused Fusarium oxysporum in case of Ethiopia. J Plant Pathol Microbiol. 2018;9(11):1-4.
- Jones JB, Jones JP, Stall R, Zitter T. Infectious antifungal. Plant Physiol. 1991;108:17-27.
- Mehari D, Mohammed Y. Efficacy and economics of fungicides and their application schedule for early blight (Alternaria solani) management and yield of tomato at South Tigray, Ethiopia. J Plant Pathol Microbiol. 2015;6:268.
- Saad A, Kadous E, Tayeb E, Massoud M, Ahmed S, Abou El- Ela A. The inhibitory effect of some antioxidants and fungicides on the growth of Alternaria solani and Fusarium solani in vitro. Middle East J Agri Res. 2014;3(2):123-134.
- Saha P, Das S. Assessment of yield loss due to early blight (Alternaria solani) of tomato. Indian J Plant Prot. 2012;40(3): 195-198.
- Getachew G, Mashila D, Habtamu T, AbuJ. Integrated management of early blight (Alternaria solani) in tomato (Solanum lycopersicum) in Arbaminch Areas, Southwestern Ethiopia. Pest Mgt J Eth. 2018;21:1–21.
- Chaerani R, Groenwold R, Stam P, Voorrips RE. Assessment of early blight (Alternaria solani) resistance in tomato using a droplet inoculation method. J Gen Plant Pathol. 2007;73(2):96-103.
- Mphahlele G. Characterisation and aggressiveness of tomato early blight fungus (Alternaria solani) in Limpopo Province. 2017.
- Van Der Walls J, Korsen L, Avaling T. A review of early blight of potato. African Plant Prot. 2001;7(2):91-102.
- Moges M, Val Selvaraj T, Jebessa M. Influence of some antagonistic bacteria against early blight (Alternaria solani (Ell. & Mart, Jones & Grout.) of tomato (Lycopersicon Esculentum Mill.). Afr J Plant Sci Biotechnol. 2012;6(1):40-44.
- Roylawar P, Panda S, Kamble A. Comparative analysis of BABA and Piriformospora indica mediated priming of defense-related genes in tomato against early blight. Physiol Mol Plant Pathol. 2015;91:88–95.
- Ahmad A, Khaliq I, Zaman M. Prevalence of early blight of tomato and differences among isolates of Alternaria solani in Peshawar division. Asian J Agric Biol. 2014;2(4):263-267.
- Ghosh P, Mandal D, Laha S, Dasgupta M. Dynamic and severity model in management of fungal disease. J Plant Prot Sci. 2009;1:55-59.
- Pandey K, Pandey P, Kalloo G. Resistance to early blight of tomato with respect to various parameters of disease epidemics. J Gen Plant Pathol. 2003;(69):364–371.
- Kumar P. Studies on Alternaria solani causing early blight disease in tomato (Lycopersicon esculentum Mill.). 2017.
- Chaerani C, Pertanian SG, Kardin M, Suhardi S, Sofiari E, van Ginkel RV, et al. Variation In aggressiveness and AFLP among Alternaria Solani isolates from Indonesia. Indones J Agric Sci. 2018;18(2):51-62.
- Lohith MR, Reddy KC, Ramana CV, Rao PV, Reddy KR, Reddy DL. Screening of tomato genotypes against early blight (Alternaria solani) by detached leaf method. In III International Symposium on Tomato Diseases. 2010;914:465-468.
- Abhinandan D, Randhawa HS, Sharma RC. Incidence of Alternaria leaf blight in tomato and efficacy of commercial fungicides for its control. Annals of Biology, India.2004;20(2): 211-218.
- Prasad Y. Studies on variability, pre and post-harvest management of early blight of tomato. Dharwad University of Agricultural Sciences. 2002.
- Kamble S, Sankeshwari S, Arekar J. Survey on early blight of tomato caused by Alternaria solani. Int J Agric Sci. 2009;5(1):317-319.
- Rotem J, Palti J. Irrigation and plant diseases. Annu Rev Phytopathol. 1969;7(1):267-288.
- Meena PD, Chattopadhyay C, Meena SS, Kumar A. Area under disease progress curve and apparent infection rate of Alternaria blight disease of Indian mustard (Brassica juncea) at different plant age. Arch Phytopathol Plant Prot. 2011;44(7):684-693.
- Damialis A, Gioulekas D. Airborne allergenic fungal spores and meteorological factors in Greece: Forecasting possibilities. Grana. 2006;45(2):122–129.
- Escuredo O, Seijo M, Fernandez M, Iglesias I. Effects of meteorological factors on the levels of Alternaria spores on a potato crop. Int J Biometeorol. 2011;55(2):243-252.
- Prasad Y, Naik MK. Evaluation of genotypes, fungicides and plant extracts against early blight of tomato caused by Alternaria solani. Indian J Plant Prot. 2003;31(2):49-53.
- Joel D, Hershenhorn J, Eizenberg H, Aly R, Ejeta G, Rich P, et al. Biology and management of weedy root parasites. Hortitultural Review Westport then New York. 2007;33:267–349.
- Ahmad A, Shafique S, Shafique S. Intracellular interactions involved in induced systemic resistance in tomato. J Hortic Sci. 2014;176:127-133.
- Shiberu T. Evaluation of improved tomato varieties (Lycopersicon Esculentum Mill.) performance against major insect pests under open field and glasshouse conditions. Int J Res Stud Agric Sci. 2016;2(3):1-7.
- Terna TP, Anyam RW, Ekefan EJ. Screening of four local varieties of tomato (solanum lycopersicum l.) for resistance to fungal diseases in a Southern guinea Savannah agro-ecological region of Nigeria. App Sci Report. 2017;20(1):1-10.
Citation: Negesa H, Ayana G (2021) Spatial Distribution of Tomato Early Blight and Reaction of Some Tomato Varieties to the Disease in Southern Tigray, Ethiopia. J Plant Pathol Microbiol 12:584.
Copyright: © 2021 Negesa H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

