Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 3
Solubility Enhancement of Ritonavir: Co-Crystallization
Narala Sagar*, Ampati Rahul and Nanam RajaniReceived: 28-Aug-2019 Published: 29-May-2020, DOI: 10.35248/2153-2435.20.11.621
Abstract
The main objective of this work is to explore co-crystallization approach for increasing solubility of an antiretroviral drug, Ritonavir (RTN). In this study, different co-formers with different functional groups like carboxylic acid andacid amidewere tried in ratio of 1:1, 1:2 and 1:3 (RTN:Co-former) using neat grinding method. Co-formers used were citric acid (CIT) and adipic acid (ADP). The co-crystals formed were characterized by melting point determination, Fourier-transform infrared (FTIR), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and solubility studies. Co-crystals of drug with CIT and ADP showed the improved dissolution profile when compared to the pure RTN. Melting point, DSC, FTIR spectra of co-crystals were different than pure drug and co-formers indicating their interaction. XRD patterns of co-crystals were not completely amorphous but less intense compared to drug alone.
Keywords
Ritonavir; Co-crystallization; Adipic acid; Citric acid; Solubility enhancement
Introduction
Ritonavir (RTN) is a BCS class II drug with low solubility high permeability resulting in less bioavailability. In-order to increase solubility and bioavailability of ritonavir, co-crystallization method was approached using adipic acid and citric acid as coformers. It is antiretroviral drug; the protease inhibitor class used to treat HIV infection and AIDS.
Solubility is of great importance in pharmaceutical systems since a drug must be dissolved before it is absorbed. Solvency is commonly characterized as thermodynamic balance of a solute between two stages. At the point when the medication assimilation process is constrained by disintegration, upgrading solvency can build disintegration rate and along these lines improve bioavailability. Notwithstanding the disintegration rate, the capacity to break up the remedial portion of the medication and the determination of solubilization methods are basic for item improvement. The oral bioavailability of a drug, which is by definition the degree at which its dynamic structure is made accessible at the site of activity after oral organization, is for the most part subject to the solvency of the medication in the gastrointestinal tract, its porousness through the intestinal divider and on impacts of the pharmaceutical plan [1]. According to the biopharmaceutics classification system (BCS), class II drugs are most suitable for bioavailability enhancement through pharmaceutical formulation due to their good permeability which is only limited by their dissolution rate [2].
Cocrystals can be defined in several ways [3,4]. A restrictive definition is that cocrystals are structurally homogeneous crystalline materials containing two or more components present in definite stoichiometric amounts [5]. The cocrystal parts are discrete impartial atomic reactants which are solids at surrounding temperature. In view of this meaning of cocrystals, a pharmaceutical cocrystal implies a cocrystal with one of the segments as an Active Pharmaceutical Ingredient (API) and different parts are called coformers. From the definition, it is unmistakably indicated that an API hydrate isn't a cocrystal; be that as it may, a strong state API hydrate can cocrystallise with a strong coformer to frame a cocrystal [6]. Presently cocrystal approach is a strategy for incredible enthusiasm for the pharmaceutical business. Apart from offering potential improvements in solubility, dissolution rate, bioavailability and physical stability, pharmaceutical cocrystals can enhance other essential properties of the APIs such as flow ability, chemical stability, compressibility and hygroscopicity [7].
The primary difference between co-crystals and salts is that in salts there is a proton transfer from the acidic to the basic functionality of the crystallization partner, or vice versa if applicable, whereas in co-crystals no such transfer takes place. Then again, the principle distinction among co-crystals and solvates is the physical condition of the individual unadulterated segments: on the off chance that one part is in fluid state at room temperature, the gems are assigned as solvates; if the two segments are solids at room temperature, the precious stones are assigned as cocrystals. Till now, pharmaceutical co-crystals are emerging as promising materials in drug discovery and development, particularly in modifying drug properties such as dissolution rate, solubility, bioavailability, stability, hygroscopicity, compressibility and flow ability.
Materials and Methods
All the excipients and active pharmaceutical ingredient (API) used for the development of ritonavir co-crystals were listed below (Table 1):
Table 1: List of materials used.
| S.No | Materials | Supplied by |
|---|---|---|
| 1 | Ritonavir | Raks Pharma Pvt. Ltd.Visakhapatnam, Andhra Pradesh |
| 2 | Adipic acid | Hi-media, Mumbai |
| 3 | Citric acid | Finar, Ahmedabad |
Pre-formulation studies
• Solubility study: The solubility of ritonavir, adipic acid and citric acid was determined.
• Melting point determination: Melting point of ritonavir, citric acid and adipic acid was determined by open capillary method.
• Identification test: Identification of ritonavir, adipic acid and citric acid was carried out by FTIR spectrophotometer.
• Construction of calibration curve: Primary stock solution of 1 mg/ml was prepared. For this accurately weighed amount(100 mg) of ritonavir was transferred into 100 ml volumetric flask and diluted with 0.1 N HCl to make up the volume to 100 ml. Secondary stock was prepared by diluting 10 ml of primary stock solution to 100 ml with 0.1 N HCl to obtain 100 μg/ml solution. From this secondary stock, 10 μg/ml working stock solution was prepared by transferring 10 ml of secondary stock into 100 ml volumetric flask and diluting with 0.1 N HCl up to the mark. Solution of 2,4,6,8,10 ml was taken separately from working stock and make up to 10 ml with 0.1 NHCl to produce 2,4,6,8,10 μg/ml respectively. The absorbance of test was estimated at 244.4 nm utilizing a twofold bar UV-obvious spectrophotometer. The direct relapse conditions were acquired to fit the information of obscure fixation into them and to know the medication focus in the obscure examples and furthermore to realize the medication discharge.
Preparation of ritonavir co-crystals
» Preparation of cocrystals using adipic acid as coformer (dry grinding method): Ritonavir and adipic acid were taken in the ratio 1:1 (A1) and 1:2 (A2)and trituratedseparately in a mortar with pestle for about 60 minutes in clockwise direction until a homogenous mixture is obtained and transferred to an airtight container and stored at room temperature.
» Preparation of cocrystals using citric acid as coformer (dry grinding method): Ritonavir and citric acid were taken in ratio 1:1 (C1), 1:2 (C2) and 1:3 (C3) triturated in a mortar and pestle for about 60 minutes in clockwise direction until a homogenous mixture is obtained and transferred to an airtight container and stored at room temperature.
Evaluation studies
• In vitro dissolution studies: Dissolution studies of the pure drug ritonavir and co-crystal preparations were conducted using type II apparatus (Electro lab, dissolution tester, TDT- 08L), with paddle type at the rotation sped of 50 rpm in 900 ml of 0.1 N HCl which is used as dissolution medium. The temperature was maintained at 37 ± 0.5°C using temperature controller (Electrolab, ETC-11). The disintegration procedure was accomplished for about 90 minutes under sink conditions. The examples of volume 5 ml were gathered at the time frame and supplanted with 5 ml 0.1 N HCl. The examples were dissected in UV-Visible spectrophotometer (Systronics, India). The disintegration profile was analyzed and assessed for measure of medication discharged.
• Determination of drug content: For determining the drug content in the preparation (cocrystal) for the formulation with better dissolution profile, equivalent amount of preparation (cocrystals) containing 10 mg of ritonavir was taken and dissolved in 10 ml of methanol, shaken for 10-15 minutes and the volume was made up to100 ml using SGF. The samples were analyzed using UV-Visible spectrophotometer (Systronics, India) and λmax of 244 nm. All the observations were done in triplicate and the average and standard deviation was calculated.
• Determination of melting point: The melting point of the cocrystals (with better drug release profile) was measured in melting point apparatus by taking the samples in the capillary tubes.
• Fourier-transform infrared spectroscopy (FTIR): IR spectroscopy was performed for ritonavir, adipic aid, citric acid and the cocrystal preparation(formulation with better drug release profile) using a FTIR (Shimadzu, IRTracer-100) spectrophotometer which was employed to characterize the possible interactions between the drug and coformer in solid-state. Samples were prepared using KBr pellet method. Samples of about 2 mg were lightly ground and mixed with 200 mg IR grade dry potassium bromide and then compressed at 10 tonnes in a hydraulic press to form discs. Spectroscopy was done at room temperature (35 ± 5°C).
• Differential scanning calorimetry: The molecular state of the drug in cocrystal formulation was evaluated by performing DSC analysis of pure drug, citric acid and their corresponding co-crystal preparation (formulation with better drug release profile). The DSC curves of the samples were obtained by differential scanning calorimeter (Mettler DSC 823e, Mettler-Toledo, Germany). Average sample weight of 5 ± 2 mg was heated in thermatically sealed aluminum pan over a temperature range of 50°C to 300°C under a constant nitrogen gas flow of 30 ml/min at a heating rate of 10°C/min. The instrument was calibrated with indium (calibration standard, purity > 99.9%) for melting point and heat of fusion.
• Powder X-ray diffraction (P-XRD): Samples containing pure ritonavir, coformer (citric acid) and the cocrystal preparations (formulation with better drug release profile) of ritonavir: citric acid (1:2) was sent for powder X-ray analysis. The diffractogram was obtained using X-ray diffractometry (Bruker AXS D8 Advance) at Karthikeya drugs and pharmaceuticals Pvt, Ltd. The X-ray source used was Cu at a wavelength of 1.5406 Å. Temperature was maintained at -170° to 450°C using low-temperature chamber (Anton Paar, TTK 450). Based on the diffraction pattern, the Relative Degree of Crystallinity (RDC) of the co-crystal preparations ritonavir citric acid was calculated.
Results and Discussions
Solubility
• Ritonavir: Freely soluble in methanol and ethanol, soluble in isopropanol.
• Adipic acid: Slightly soluble in water, freely soluble in ethanol.
• Citric acid: Very soluble in water, freely soluble in ethanol and soluble in ether.
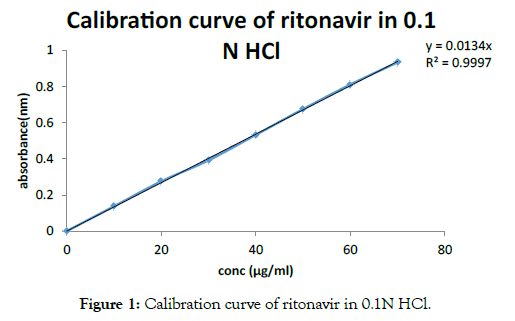
• Construction of calibration curve of ritonavir in 0.1 N HCl using UV-visible spectrophotometry: The standard graph ofritonavir hydrochloride has shown good linearity with regression co-efficient value 0.997 in 0.1 N HCl which suggest that it obeys the Beer-Lambert’s law as seen in Figure 1 and Table 2.
Figure 1: Calibration curve of ritonavir in 0.1N HCl.
Table 2: Calibration curve of ritonavir 0.1N HCl.
| Concentration(μg/ml) | Absorbance(nm) |
|---|---|
| 0 | 0 |
| 10 | 0.1371 |
| 20 | 0.2751 |
| 30 | 0.3918 |
| 40 | 0.5326 |
| 50 | 0.6748 |
| 60 | 0.8106 |
| 70 | 0.9339 |
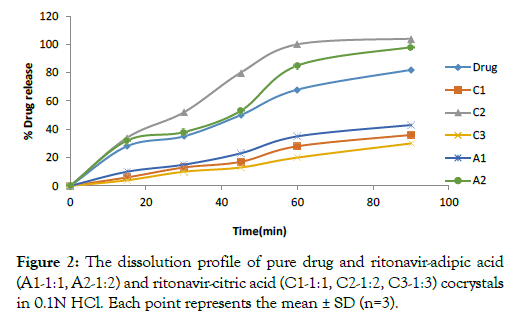
• Drug dissolution study: The Dissolution study was conducted for co-crystal preparations containing adipic acid and citric acid using 0.1 N HCl using USP type II apparatus at 37 ± 0.5°C for 90 mins. The dissolution profile of the co-crystals that contain citric acid showed 100% drug release in 60 minutes for C2 and pure drug showed 82% drug release in 90 minutes (Table 3 and Figure2). This indicates that C2 co-crystals show faster drug release compare to the C1, C3 and pure drug. Whereas, co-crystal formulations that contain adipic acid showed 98% drug release of the drug in 90 minutes for A2 (1:2 ratio) when compared to A1 and pure drug (Table 3 and Figure 2). The drug release profiles of pure drug and all the formulations were captured below:
Table 3: In vitro drug release of pure drug in 0.1 N HCl.
| Time(min) | % Drug release | |||||
|---|---|---|---|---|---|---|
| Drug | C1 | C2 | C3 | A1 | A2 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 28 | 6 | 34 | 4 | 10 | 32 |
| 30 | 35 | 13 | 52 | 10 | 15 | 38 |
| 45 | 50 | 17 | 80 | 13 | 23 | 53 |
| 60 | 68 | 28 | 100 | 20 | 35 | 85 |
| 90 | 82 | 36 | 104 | 30 | 43 | 98 |
Figure 2: The dissolution profile of pure drug and ritonavir-adipic acid (A1-1:1, A2-1:2) and ritonavir-citric acid (C1-1:1, C2-1:2, C3-1:3) cocrystals in 0.1N HCl. Each point represents the mean ± SD (n=3).
• Drug content determination: The drug content was determined for the co-crystals formulations (C2, formulation with better dissolution profile) in triplicates. The values were 98 ± 0.85%given in Table 4. The values indicate good content uniformity for the co-crystal formulations. These results indicate that neat dry grinding method can be used to develop co-crystals of ritonavir.
Table 4: Drug content of cocrystal preparations.
| Cocrystal formulations | Percent drug content |
|---|---|
| C2:ritonavir – citric acid (1:2) | 98 ± 0.85 |
• Melting point: As co-crystallization leads to change in physicochemical properties, melting point of prepared co-crystal (C2), pure drug (ritonavir) and coformer (citric acid and adipic acid) were noted. It was discovered that the dissolving purpose of the co-precious stones demonstrated a critical deviation concerning the liquefying purpose of unadulterated medication and the individual co-formers showing their connection. This test was utilized as a fundamental test for affirmation of co-gem development for additional portrayal (Table 5).
Table 5: Melting points of formulation (C2), pure drug and co-formers.
| S. No | Sample | Melting point (°C) |
|---|---|---|
| 1 | C2 | 100-105 |
| 2 | Ritonavir (pure drug) | 122-124 |
| 3 | Citric acid (coformer) | 149-153 |
| 4 | Adipic acid (coformer) | 151-154 |
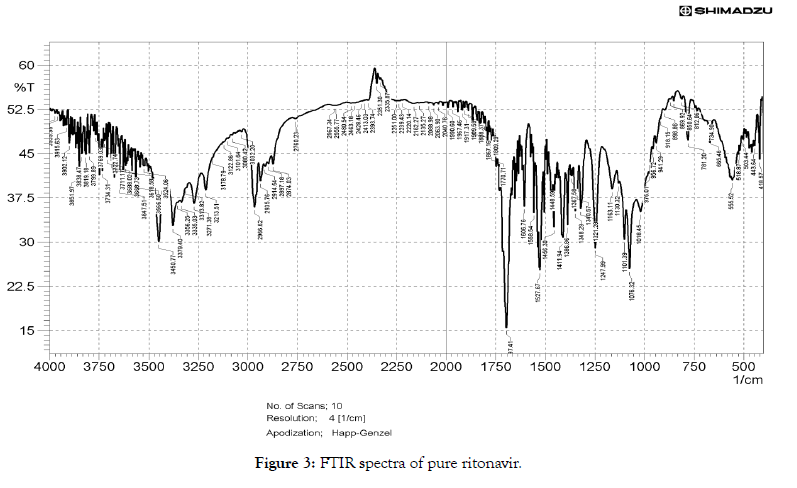
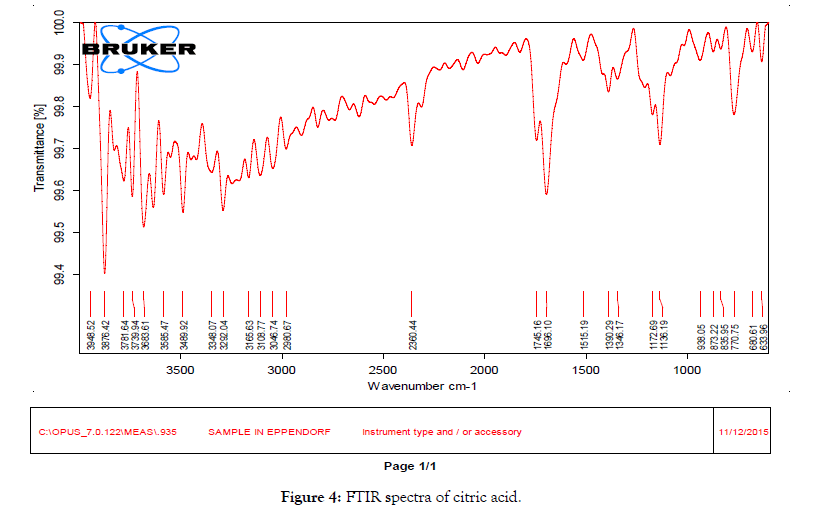
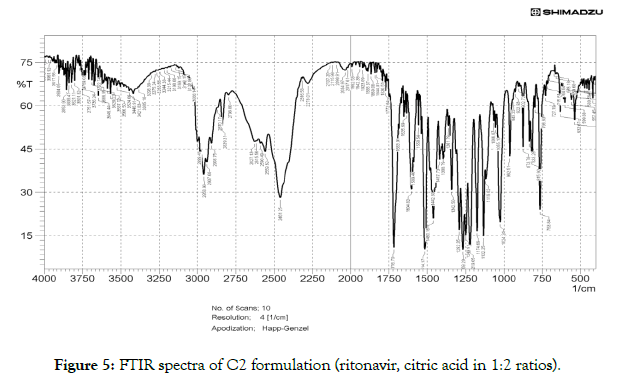
• Fourier transform infrared (FTIR) studies: FTIR is an excellent technique to give an insight into the kind of study the chemical and physical structure changes in the molecular structure of a substance. As shown in Figure 3, the FTIR spectrum of RTN showed presence of C=O amide peak at 1697.41 cm-1C=O ester peak at 1750.04 cm-1 -NH- stretch at 3379.40 cm-1, amide –NH-bend at 1527.67 cm-1. There is a shift in the functional groups present in the spectra of cocrystal (C2) (Figure 4) compared to that of drug, the C=O amide peak is shifted from 1697.41 cm-1 to 1635.69 cm-1 of ritonavir citric acid cocrystals, C=O ester peak shifted to lower frequency from 1750.04 to 1716 cm-1 of ritonavir citric acid cocrystal, -NH-stretch of ritonavir citric acid is found at 3426 cm-1 when compared to pure drug, amide –NH- bend shifted from 1527.67 cm-1 to 1514.17 cm-1. There is a shift in the FTIR frequency of functional groups present in the cocrystals compared to that of drug, showing the presence of the formation of new bonds (Figure 5).
Figure 3: FTIR spectra of pure ritonavir.
Figure 4: FTIR spectra of citric acid.
Figure 5: FTIR spectra of C2 formulation (ritonavir, citric acid in 1:2 ratios).
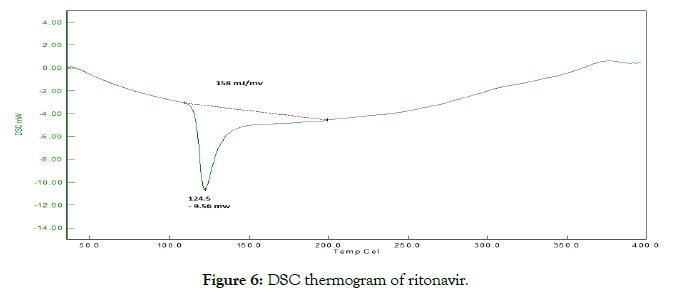
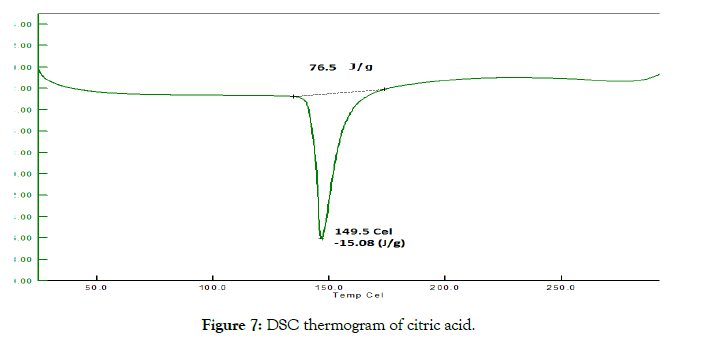
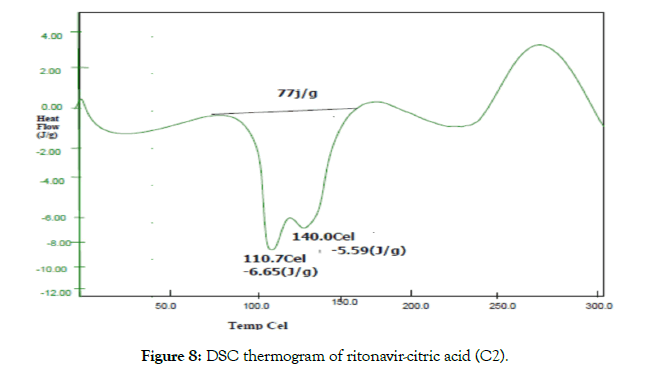
• Differential scanning calorimetry (DSC): The thermotropic behavior and physical state of drug in cocrystals was evaluated by performing DSC analysis. From the DSC thermograms (Figures 6-8), it was observed that the thermograms of the cocrystals were different in pattern and intensity as compared to RTN and co-formers which indicates their interaction. This shift in the melting point is due to the change in crystal lattice of the RTN in presence of co-former, forming a relatively different crystal lattice in co-crystals.
Figure 6: DSC thermogram of ritonavir.
Figure 7: DSC thermogram of citric acid.
Figure 8: DSC thermogram of ritonavir-citric acid (C2).
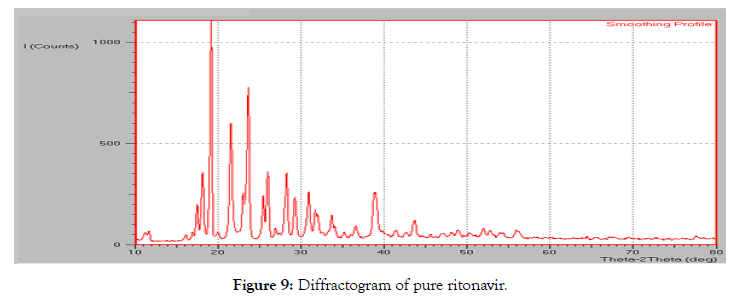
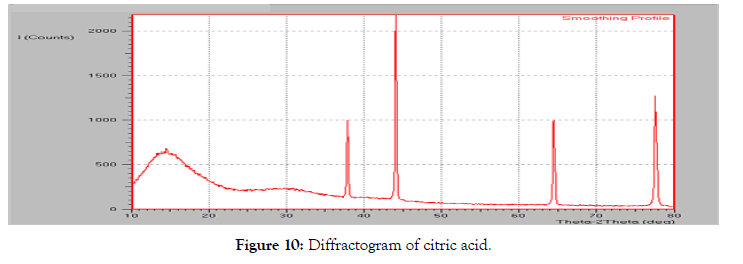
• Powder X-Ray diffraction: The diffraction pattern of the pure ritonavir (Figure 9) showed high intensity peaks at 2-theta values at 18.14°, 19.16°, 21.56°, 23.62°, 25.98° and 28.23°. The diffraction pattern of citric acid showed a singular peak at 44.08áµ?. Two other peaks were observed at 37.85° and 64.43°.
Figure 9: Diffractogram of pure ritonavir.
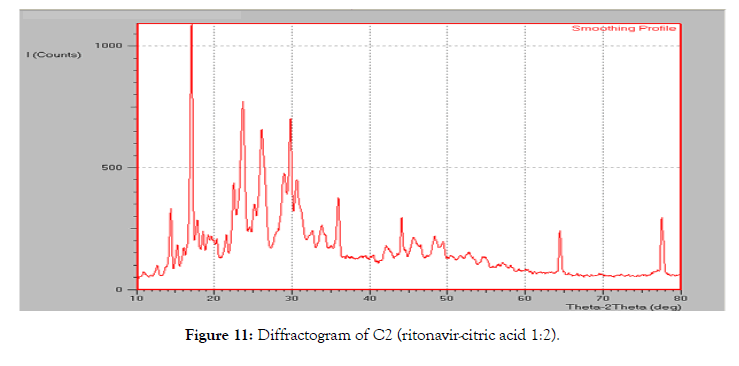
The diffraction pattern of the co-crystal formulation C2 (Figure 10) showed a sharp peak at 17.05°, 26.10°, 29.02°, 29.75° and 77.55° which was unseen in diffraction patterns both the components (pure ritonavir and citric acid) of the complex. The peak obtained at 23.22° which was closer to the peak observed in pure ritonavir, but it was found to be reduced in intensity when compared with pure ritonavir. The other intense peaks of ritonavir were reduced indicating a decrease in the degree of crystallinity (Figure 11).
Figure 10: Diffractogram of citric acid.
Figure 11: Diffractogram of C2 (ritonavir-citric acid 1:2).
The relative degree of crystallinity (RDC) was calculated for the C2 formulation which showed a decrease in the crystallinity in cocrystal formulations when compared to the pure ritonavir. This corresponds to the decrease in the intensity of the ritonavir peaks in co-crystal formulations in the diffraction pattern (Table 6).
Table 6: Relative degree of crystallinity of the co-crystal formulation C2 at different 2θ positions.
| Type of system | 18.14 Pos | 21.56 Pos | 23.62 Pos | 25.98 Pos | 28.23 Pos | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height (cts) | RDC | Height(cts) | RDC | Height(cts ) | RDC | Height(cts) | RDC | Height(cts) | RDC | |
| Pure drug | 213 | - | 381 | - | 505 | - | 222 | - | 211 | - |
| C2 (formulation) | 68 | 0.31 | 175 | 0.45 | 389 | 0.77 | 316 | 1.42 | 186 | 0.77 |
Conclusion
The co-crystals were prepared successfully using different coformers by neat dry grinding method. These co-crystals were characterized by melting point, FTIR, DSC and XRD. These studies indicated formation of new crystal phases due to physical and or chemical interactions between API and co-former. For RTN, dicarboxylic acid co-crystals showed better solubility and dissolution. In vitro dissolution studies were performed, and it shows that there was 100% drug release in 60 minutes for co-crystal formation and pure ritonavir shows 82% release at 90 minutes. The underlying disintegration pace of co-crystals was extensively quicker contrast with unadulterated ritonavir. Improved disintegration productivity and relative disintegration pace of co-crystals than unadulterated medication uncovers those co-crystals as reasonable transporters for improved dissolvability and disintegration pace of unadulterated ritonavir.
Acknowledgements
The authors are grateful to the principal and management of St. Peter’s institute of pharmaceutical sciences for providing the facilities and support for the completion of research work.
REFERENCES
- Jambhekar SS, Breen PJ. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov Today. 2013;18:1173-1184.
- Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413-420.
- Schultheiss N, Newman A. Pharmaceutical co-crystals and their physicochemical properties. Cryst Growth Des. 2009;9:2950-2967.
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201-230.
- Aakeroy CB, Salmon DJ. Building co-crystals with molecular sense and supramolecular sensibility. Cryst Eng Comm. 2005;7:439-448.
- Chieng N, Hubert M, Saville D, Rades T, Aaltonen J. Formation kinetics and stability of carbamazepine-nicotinamide cocrystals prepared by mechanical activation. Cryst Growth Des. 2009;9:2377-2386.
- Lu J, Rohani S. Preparation and characterization of theophylline-nicotinamide co-crystal. Org Process Res Dev. 2009;13:1269-1275.
Citation: Sagar N, Rahul A, Rajani N (2020) Solubility Enhancement of Ritonavir: Co-Crystallization. Pharm Anal Acta 11:621. doi: 10.35248/2153- 2435.20.11.621.
Copyright: © 2020 Sagar N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.