Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 8, Issue 1
Skin Metastases: Revealing a Lung Adenocarcinoma
Leila Nebchi1* and Naamani Chaher22Department of Pathological Anatomy, Mustapha University Hospital Center, Sidi M’Hamed, Algeria
Received: 30-Jan-2023, Manuscript No. JTRR-23-19715; Editor assigned: 02-Feb-2023, Pre QC No. JTRR-23-19715 (PQ); Reviewed: 17-Feb-2023, QC No. JTRR-23-19715; Revised: 23-Feb-2023, Manuscript No. JTRR-23-18715 (R); Published: 03-Mar-2023, DOI: 10.35248/2684-1614.23.8.178
Abstract
Muscle metastases from bronchopulmonary cancer are rare; their revealing character is an exceptional fact. We report the observation of a muscular metastasis of the right arm, revealing a totally asymptomatic bronchial adenocarcinoma. The reason for our patient’s consultation was a painful mass in the right arm. Imaging concluded that there was heterogeneous muscle collection without any erosion of the cortical bone of the humerus. The histological study of the tumor, after surgical excision, concluded in a metastasis of an adenocarcinoma, whose immune histochemical profile oriented towards a broncho-pulmonary or digestive origin. The chest X-ray was normal. Apart from the surgical excision of the muscle metastasis, the treatment included local consolidation radiotherapy associated with palliative chemotherapy. The evolution was marked by the absence of local recurrence, with nevertheless a dissemination of the lung cancer after 7 months of evolution patient deceased. Skeletal muscle metastases from lung cancer are rare and have a poor prognosis.
Keywords
Skeletal muscle; Bronchopulmonary cancer; Chemotherapy
Introduction
Skeletal muscle metastases from lung cancer are rare and their revealing nature constitutes an exceptional fact. We report the case of a skeletal muscle metastasis of the right arm revealing pulmonary adenocarcinoma in a 64-year-old man, totally asymptomatic with a normal chest X-ray. Patient of the 64 year old, with no particular pathological history, smoking 1 packet per day, initially consulted in the orthopedic department for a very painful swelling of the isolated right arm, evolving for 1 month. Physical examination revealed a deep mass in the posterolateral compartment of the right arm, 9 cm in long axis, of firm consistency, painful on palpation (Figure 1).
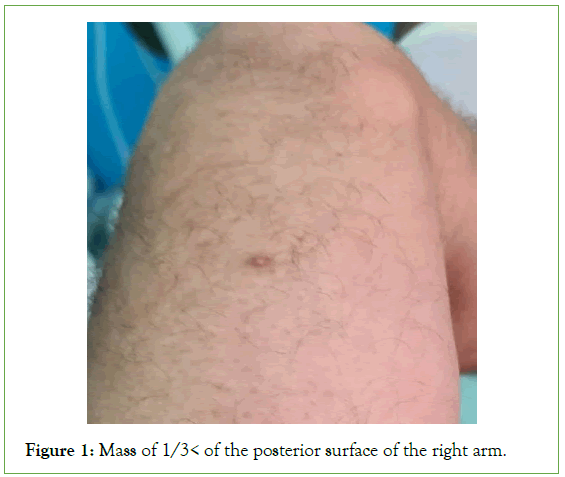
Figure 1: Mass of 1/3< of the posterior surface of the right arm.
Case Presentation
The ultrasound of the right arm showed a juxta-humeral muscle collection of 5 × 9 cm (Figures 2 and 3). The radiograph of the right humerus showed no bone damage (Figure 4). A large surgical biopsy removing the entire lesion en bloc was performed. Macroscopically, it was a friable, yellowish, finely encapsulated tumor of the vastus lateralis muscle (Figures 5-8). Histopathological and immunohistochemical examinations of the surgical specimen concluded that muscle metastasis of an adenocarcinoma could be of pulmonary or digestive origin (Figures 9-14). The chest X-ray was considered normal; the thoraco-abdomino-pelvic scanner (Figure 15).
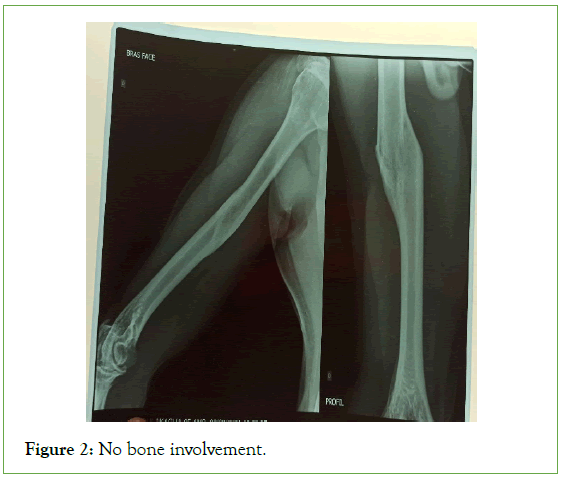
Figure 2: No bone involvement.
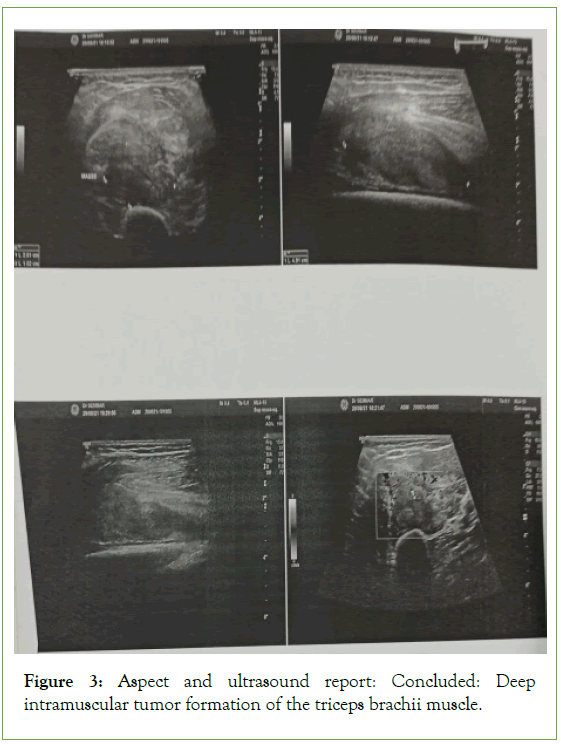
Figure 3: Aspect and ultrasound report: Concluded: Deep intramuscular tumor formation of the triceps brachii muscle.

Figure 4: Deep intramuscular tumor formation of the triceps brachii muscle report.
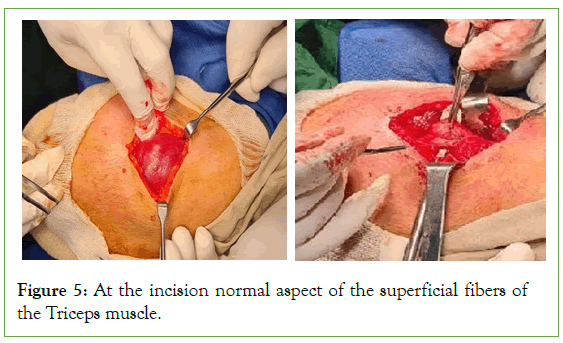
Figure 5: At the incision normal aspect of the superficial fibers of the Triceps muscle.
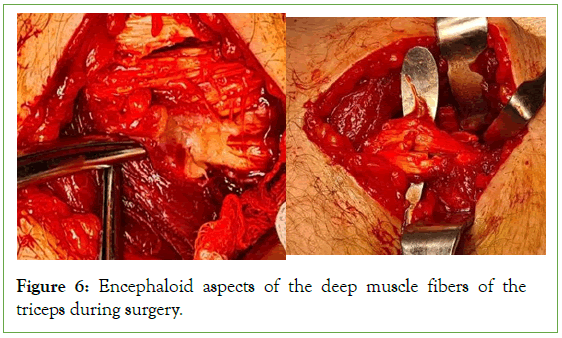
Figure 6: Encephaloid aspects of the deep muscle fibers of the triceps during surgery.
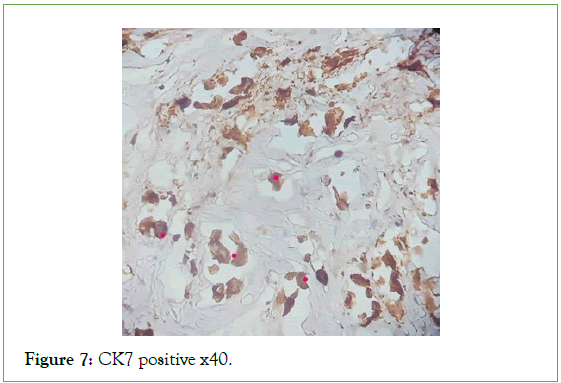
Figure 7: CK7 positive x40.
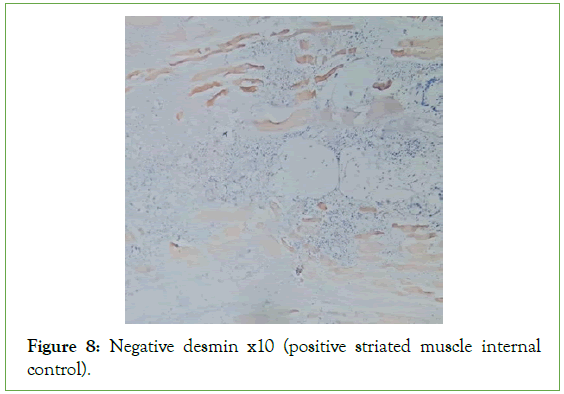
Figure 8: Negative desmin x10 (positive striated muscle internal control).
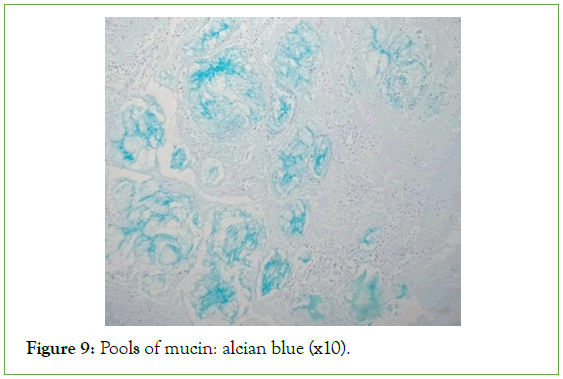
Figure 9: Pools of mucin: alcian blue (x10).
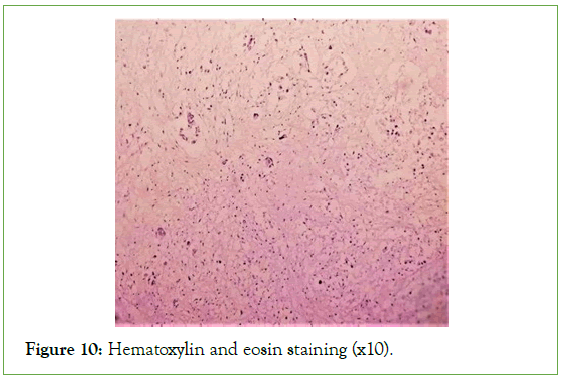
Figure 10: Hematoxylin and eosin staining (x10).
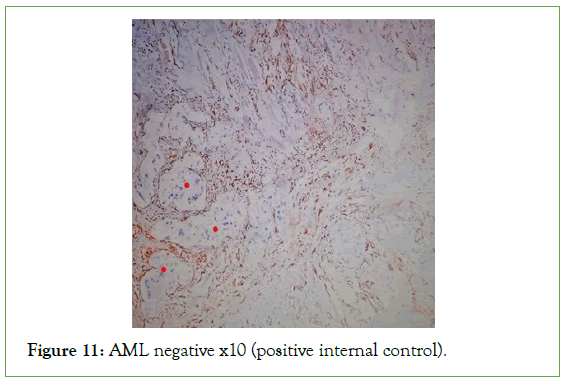
Figure 11: AML negative x10 (positive internal control).

Figure 12: Report of the immunohistochemical study.
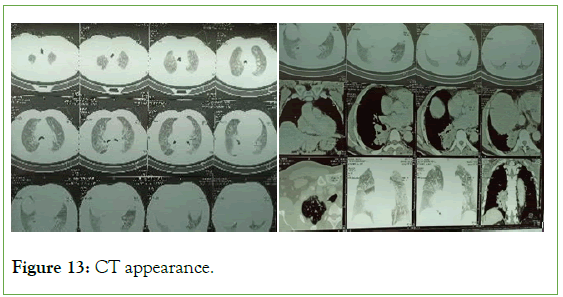
Figure 13: CT appearance.

Figure 14: Thoraco-abdomino-pelvic CT report.

Figure 15: Thoraco-abdomino-pelvic CT report conclusion.
The tumor was classified as T2N0M1, and the patient was receiving palliative chemotherapy based on gemcitabine-cisplatin and radiotherapy. The initial response to treatment was considered satisfactory, with partial regression of the size of the primary tumour. After 7 months of evolution, there was a progression of the disease with metastases in the liver and kidneys, but without recurrence of the muscle metastasis in the deceased patient.
Results and Discussion
Muscle metastases remain rare despite the importance of the striated muscle mass and the richness of its vascularization. But if the incidence of these metastases barely exceeds 1% in clinical series, it is much higher, up to 16%, in autopsy series of cancer patients [1,2]. Muscle mobility and blood flow turbulence would be mechanical factors that can inhibit tumor growth in striated muscle [3]. Similarly, elevated oxygen pressure, release of toxic oxygen free radicals, ability of skeletal muscle to scavenge lactic acid, and acidic muscle pH would be chemical and humoral factors that would protect striated muscle from tumor invasion [4-7]. Muscle metastases have been reported in patients with leukemia, lymphoma, malignant melanoma, thyroid cancer lung cancer and other neoplasias [8-12]. Bronchopulmonary cancer would be the first responsible for this type of metastasis.
Berge et al reported a frequency of muscle metastases of 1% in 747 post-mortem examinations of patients with bronchopulmonary cancer [13]. Histologically, squamous cell carcinoma and adenocarcinoma are the most frequently involved histological types [14,15].
These metastases usually manifest in a known neoplastic context. They are exceptionally revealing of the primary tumour, as in the case of our patient. They are plurifocal in 40% of cases and can theoretically affect all the muscles. However, they are more common in the psoas muscle, diaphragm, and muscles of the abdominal wall, pectoralis, deltoid, thigh muscles, intercostals, sternocleidomastoid, biceps, triceps and backbones. Clinically, these metastases can manifest as pain of varying intensity, a tumor mass syndrome associated or not with extra-articular stiffness. Imaging makes it possible to make the diagnosis of muscle tumor, but without being able to decide on its metastatic character. In ultrasound, muscle metastases appear in the form of heterogeneous hypoechoic images, more or less well limited Computed tomography reveals a mass of spontaneously hypodense tissue density, taking up the contrast heterogeneously, with the possibility of necrotic areas and calcifications. On MRI, these lesions are isodense in T1 sequence, hyperdense in T2 sequence, surrounded by peripheral edema, and their signal is strongly enhanced after injection of contrast product. However, this appearance is nonspecific and can be seen in the event of a primary tumour, abscess, hematoma or intramuscular collection. We did not perform either a CT scan or an MRI of the arms in our patient [16-18]. The anatomopathological diagnosis of muscle metastases can be made by needle biopsy, under ultrasound or CT scanning, or by surgical biopsy, then allowing en bloc excision of the tumour. The treatment of skeletal muscle metastases remains poorly codified. Surgical excision associated with local radiotherapy allows symptoms to be controlled and the quality of life of patients to be improved by reducing or eliminating muscle pain. If the primary tumor is controlled, the association with palliative chemotherapy would allow a relatively prolonged survival. Some observations have reported a partial improvement of symptoms with exclusive radiotherapy. The prognosis of muscle metastases remains pejorative, particularly during the evolution of lung cancer.
Conclusion
Muscle metastases from lung cancer are rare; they are exceptionally revealing. Clinically, these metastases are revealed by a tumor mass syndrome, pain with or without extra- articular stiffness. They are rare for any tumor with a reported incidence greater than one percent. The most common sites of muscle metastases are the thigh muscles, iliopsoas and paraspinous muscles Treatment is poorly codified. However, surgical excision associated with local radiotherapy allows control of symptoms and a certain improvement in the quality of life of patients. Imaging does not offer specific signs. The diagnosis is based on the anatomo-pathological study after surgical excision biopsy.
References
- Di Giorgio A, Sammartino P, Cardini CL, Al Mansour M, Accarpio F, Sibio S, et al. Lung cancer and skeletal muscle metastases. Ann Thorac Surg. 2004; 78(2):709-711.
[Crossref] [Google Scholar] [PubMed]
- Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959; 9(11):757-766.
[Crossref] [Google Scholar] [PubMed]
- Anastasovska V, Balagué F, Waldburger M. Infiltration métastatique musculaire d’un carcinome épidermoïde pulmonaire. Rev du Rhum. 2002; 69(7):755-758.
- Maréchal E, Rivat P, Leclercq R. Unusual shoulder stiffness: Metastasis in the infraspinatus muscle as the first clinical manifestation of lung carcinoma. Rev Chir Orthop Reparatrice Appar Mot. 2001; 87(1):79-83.
[Google Scholar] [PubMed]
- Sridhar KS, Rao RK, Kunhardt B. Skeletal muscle metastases from lung cancer. Cancer. 1987; 59(8):1530-1534.
[Crossref] [Google Scholar] [PubMed]
- Lu HC, Shih TT, Hsu CY, Wu MZ, Li YW. Unusual manifestations of soft tissue metastasis. Clin Imaging.2003; 27(1): 52-54.
[Crossref] [Google Scholar] [PubMed]
- Mohr JP, Kejda-Scharler J. Middle cerebral artery territory syndromes. 2012.
- Di Giorgio A, Sammartino P, Cardini CL, Al Mansour M, Accarpio F, Sibio S, et al. Lung cancer and skeletal muscle metastases. Ann Thorac Surg. 2004; 78(2): 709-711.
[Crossref] [Google Scholar] [PubMed]
- Heyer CM, Rduch GJ, Zgoura P, Stachetzki U, Voigt E, Nicolas V, et al. Metastasis to skeletal muscle from esophageal adenocarcinoma. Scand J Gastroenterol. 2005; 40(8):1000-1004.
[Crossref] [Google Scholar] [PubMed]
- Marioni G, Blandamura S, Calgaro N, Ferraro SM, Stramare R, Staffieri A, et al. Distant muscular (gluteus maximus muscle) metastasis from laryngeal squamous cell carcinoma. Acta Otolaryngol. 2005; 125(6): 678-682.
[Crossref] [Google Scholar] [PubMed]
- Berge T. Cancer in Malmo 1958-1969. An autopsy study. Acta Pathol Microbiol Scand Suppl. 1977; 260:1147.
- Fisher ER, Gingrich RM, Gruhn J, Laing P. Ossifying metastatic carcinoma. Report of a case with comments relative to histogenesis of ectopic ossification. Cancer. 1959; 12(2):257-262.
[Crossref] [Google Scholar] [PubMed]
- Baser S, Fisekci FE, Bir F, Karabulut N. Rhomboideus major muscle metastasis as an initial clinical manifestation of pulmonary adenocarcinoma. Thorax. 2004; (8):728.
[Crossref] [Google Scholar] [PubMed]
- Schmidt GP, Reiser MF, Baur-Melnyk A. Whole-body imaging of the musculoskeletal system: The value of MR imaging. Skeletal Radiol.2007; 36:1109-1119.
[Crossref] [Google Scholar] [PubMed]
- Rosenthal DI. Radiologic diagnosis of bone metastases. Cancer. 1997; 80(S8):1595-1607.
[Crossref] [Google Scholar] [PubMed]
- Bocchino M, Valente T, Somma F, de Rosa I, Bifulco M, Rea G, et al. Detection of skeletal muscle metastases on initial staging of lung cancer: A retrospective case series. Jpn J Radiol 2014; 32:164-171.
[Crossref] [Google Scholar] [PubMed]
- Faure E, Le Pimpec-Barthes F, Dusser D, Riquet M. Skeletal muscle metastases from non-small cell lung. Rev Mal Respi. 2002; 19(1):93-95.
[Google Scholar] [PubMed]
- Perisano C, Spinelli MS, Graci C, Scaramuzzo L, Marzetti E, Barone C, et al. Soft tissue metastases in lung cancer: A review of the literature. Eur Rev Med Pharmacol Sci. 2012; 16:1908-1914.
[Google Scholar] [PubMed]
Citation: Nebchi L, Chaher N (2023) Skin Metastases: Revealing a Lung Adenocarcinoma. J Tum Res Reports. 8:178.
Copyright: © 2023 Nebchi L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

