PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 5
Serum Produced from Expired Fresh Frozen Plasma and Cryosupernatant Supports the Proliferation of Human Cells: Cost-Effective Alternatives to Fetal Bovine Serum
Kazuki Ishiyama1, Mika Ogawa2, Hidefumi Kato3, Kyosuke Takeshita4, Ryuzo Ueda5 and Takayuki Nakayama2*2Department of Clinical Laboratory, Aichi Medical University, Nagakute, Japan
3Department of Blood Transfusion, Aichi Medical University, Nagakute, Japan
4Department of Clinical Laboratory, Saitama Medical Center, Saitama, Japan
5Department of Tumor Immunology, Aichi Medical University, Nagakute, Japan
Received: 24-Apr-2023, Manuscript No. JBDT-23-21115; Editor assigned: 25-Apr-2023, Pre QC No. JBDT-23-21115 (PQ); Reviewed: 15-May-2023, QC No. JBDT-23-21115; Revised: 22-May-2023, Manuscript No. JBDT-23-21115 (R); Published: 29-May-2023, DOI: 10.417 2/2155-9864.23.14.562
Abstract
Background: Fetal Bovine Serum (FBS) is the most common growth factor used in cell culture. However, demands for xeno-free cell culture supplements for clinical use that comply with good manufacturing practice standards are increasing as cellular therapies expand. Human serum has been proposed as an alternative to FBS. Due to limited availability of donors and effective use of medical resources, we attempted to produce serum from expired Fresh Frozen Plasma (FFP) and Cryosupernatant (CS) and tested them as FBS substitutes.
Results: We found that 1-hour incubation of FFP or CS with a physiological level of thrombin and alcium Chloride (CaCl2) depleted virtually all fibrinogen. Comparative proliferation studies with FBS and human Platelet Lysates (hPLs) showed that compared with FBS, FFP-serum exhibited similar or greater proliferative effects on 8 human cell lines: Bone Marrow-Derived Mesenchymal Stromal Cells (BMSC), Adipose Tissue-Derived Mesenchymal Stromal Cells (ADSC), HeLa, 293T, MG-63, HL-60, K562, and Meg-A2 except for Saos-2 cells, whereas CS-serum exhibited weaker proliferative effects on BMSC, ADSC and Saos-2 cells. hPL promoted the growth of BMSC and ADSC cells more vigorously than FFP- and CS-serum.
Conclusions: FFP-and CS-serum can be produced with thrombin and CaCl2 rapidly and in a blood product–sparing manner and function as potent alternatives to FBS for culturing human cells.
Keywords
Xeno-free cell culture supplements; Blood product–sparing; Expired fresh frozen plasma; Cryosupernatant
Introduction
Cellular pluripotent stem cells for regenerative medicine, chimeric antigen receptor T cells for cancer immunotherapy therapies have emerged as new modalities for treating severe diseases lacking effective therapies, such as the use of induced, and Mesenchymal Stromal Cells (MSCs) for anti-inflammation therapy [1]. Ex vivo expansion of cells facilitates their clinical application due to the pooling of a sufficient number of cells of the same quality. Fetal Bovine Serum (FBS) is the most commonly used growth factor for cell culture. However, animal serum can contain various blood-borne pathogens such as viruses and prions, and these contaminants must be avoided in the production of cellular and tissue-based products [2,3]. Additionally, induction of an immune response has been reported in patients receiving MSCs cultured with FBS [4,5]. Thus, demands for xeno-free cell culture supplements for clinical use that comply with Good Manufacturing Practice (GMP) standards are increasing [6].
To date, human Platelets Lysate (hPL) and human serum have been proposed as alternatives to FBS [7]. hPL reportedly promotes ex vivo expansion of MSCs, and human MSCs (hMSCs) cultured with hPL have been used safely in clinical settings [8-10]. The ability of hPL obtained from expired platelet units to promote the expansion of hMSCs was evaluated due to the limited availability of donors and as a means of ensuring the effective use of medical resources and data indicate that hPL supports the growth and osteogenic differentiation of MSCs [11]. However, the ability of hPL to support the growth of other cell types has yet to be established, although several studies have reported the usefulness of human serum [7,12-14].
Currently, GMP-grade human serum can only be procured from a few companies, such as Lonza (Morristown, NJ, USA). Fresh Frozen Plasma (FFP), which is available from local blood banks, consists of serum and coagulation factors. These coagulation factors (mainly fibrinogen) can cause culture medium to gel, however, which inhibits the growth of cells. Thus, fibrinogen-depleted FFP (serum) represents a potential alternative source of human serum. This possibility led us to test whether fibrinogen-depleted plasma is a suitable substitute for FBS. From the perspective of reducing the wasting of blood products, we focused on expired FFP and cryosupernatant (CS). CS is a fraction of plasma that is usually discarded after the cryoprecipitate has been removed [15].
Here, we report a simple and rapid method to remove fibrinogen from plasma. We also demonstrate that serum obtained from expired FFP or CS can be used for cell culture without compromising the growth of most of human cell types.
Material and Methods
Cells and reagents
Roswell Park Memorial Institute (RPMI) 1640, heat-inactivated FBS, and α-Minimal Essential Medium (αMEM) were purchased from Gibco (BRL) Biomanufacturing readiness levels (Carlsbad, CA, USA). Calcium chloride (CaCl2) and thrombin were obtained from Wako Chemicals Ltd. (Osaka, Japan) and Japan Blood Products Organization (Tokyo, Japan), respectively. FFP anticoagulated with Citrate-Phosphate-Dextrose (CPD) was purchased from a Japanese Red Cross blood center. In this study, we used expired FFP that was to be discarded for clinical reasons.
Human adipose tissue–derived MSCs (ADSCs) were established from healthy volunteers who provided informed consent as described elsewhere [16]. The mouse bone marrow–derived stromal cell lines MS-5 and S-17 were gifts from Dr A. C. Berardi (Ospedale Bambin Gesu, Rome, Italy) and Dr K. Dorshkind (UCLA, Riverside, CA) [17,18]. Stromal cell lines were maintained in αMEM containing 10% FBS (Invitrogen, Carlsbad, CA). The human embryonic kidney cell line 293T, the human cervical cancer cell line HeLa, and the mouse osteoblast cell line 7F2 were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The profiles of the promyelocytic leukemia cell line HL-60, the human megakaryocytic leukemia cell line MEG-A2, and the chronic myeloid leukemia cell line K562 are described elsewhere [19-21]. The human osteoblast cell lines MG-63 and Saos-2 were purchased from the ATCC (Manassas, VA, USA) [17,18].
Preparation of hPL
hPL was generated from expired common platelet units as described elsewhere [22]. Platelet units were aliquoted into 50-mL sterilized tubes and stored at -30°C until use. After thawing at 37°C, cell fragments were removed by centrifugation (2,500 rpm 10 min). The supernatant was then collected and supplemented with heparin sodium (final concentration 12.5 U/mL; AY Pharma Co. Inc., Tokyo, Japan).
Effect of CaCl2 and thrombin on fibrin clot formation in FFP
Because FFP is anticoagulated with CPD, a chelator of calcium ions necessary for fibrin clot formation, we neutralized CPD by the addition of CaCl2. After thawing at 37°C, 225 µL of FFP was mixed with 25 µL of CaCl2 at various final concentrations (6.25-100 mM). TC buffer (Sysmex Co. Inc., Hyogo, Japan) was used as a relevant control for CaCl2. The mixture was incubated at 37°C for 60 min and then centrifuged (3,000 rpm 10 min). The fibrinogen content in the supernatant was measured using an automated coagulation analyzer: CP3000TM (Sekisui Medical, Co. Inc., Tokyo, Japan). In another setting, 25 mL of FFP was mixed with thrombin at various final concentrations (0.16–5.0 U/mL) with/without CaCl2 (12.5 mM final concentration) in a 50-mL conical tube. The mixture was incubated at room temperature for 60 min and then photographed. The conical tubes were centrifuged (3,000 rpm 10 min) to remove fibrin clots. The supernatants were incubated at 4°C for 24 h to monitor for formation of additional fibrin clots.
Production of human serum from expired FFP or CS
Based on our findings described below, 500 µL of 1 M CaCl2 and 100 µL of 1 U/mL thrombin were added to each 40 mL of FFP or CS thawed at 37°C, and the mixture was incubated for 1 h at 37°C. After fibrin clots were removed by centrifugation (3,000 rpm 5 min), the pH and fibrinogen, sodium, potassium, chlorine, and calcium content were measured using automatic analyzers: RAPIDPoint500® (Siemens Healthcare, Erlangen, Germany), CP3000® and LABOSPECT® (Hitachi High-Technologies Co. Inc., Tokyo, Japan). Serum produced from expired FFP (hereafter denoted FFP-serum) and serum produced from CS (hereafter denoted CS-serum) was stored at -30°C until use.
In vitro cell proliferation studies with CaCl2 or thrombin
The effects of CaCl2 and thrombin on the proliferation of ADSCs and 7F2 cells were assessed as described previously [23]. Both cell types (2,000 cells in 180 µL of medium/well) were cultured with various concentrations of CaCl2 (final concentration 6.25-200 mM) or thrombin (final concentration 0.036–36.0 U/mL) for 72 h or 48 h, respectively. After the designated incubation times, 10 µL of WST-1 reagent was added to each well, and the absorbance at 450 nm was measured 3h later. The percent increase in cell growth was calculated by dividing the OD value of the cell cultures grown in the presence of each additive by the OD value in the absence of the additive and multiplying by 100. Data shown reflect the mean (± SD) of seven determinations and are representative of three independent experiments.
In vitro cell proliferation studies with FBS, hPL, FFP- serum, and CS-serum
We cultured six types of adherent cells (BMSCs, ADSCs, HeLa, 293T, MG-63, Saos-2) and three types of mouse adherent cells (7F2, MS-5, S-17) with αMEM containing 10% FBS, hPL, FFP-serum, or CS-serum in a 96-well plate (1,000 cells/well). Similarly, we cultured three types of human floating cells (HL-60, K562, Meg-A2) with RPMI containing 10% FBS, hPL, FFP-serum, or CS-serum in a 96-well plate (2,000 cells/well). After a 96-h incubation, cell proliferation was examined by a colorimetric assay as described above. The percentage of cell growth was calculated by dividing the OD value of cell cultures grown in the presence of each additive by the OD value in the presence of FBS and multiplying by 100. Data shown reflect the mean (± SD) of seven determinations and are representative of three independent experiments.
Results
Both CaCl2 and thrombin are required to remove fibrinogen from FFP
Because FFP is anticoagulated with CPD, which chelates calcium ions, we initially attempted to neutralize CPD by adding CaCl2 (Figure 1). (A) Fibrinogen content in FFP was measured after exposure to CaCl2 at varying final concentrations (6.25–100 mM) or control (PBS) for 60 min at 37°C. Data shown reflect the mean (± SD) of triplicate determinations and are representative of three independent experiments.
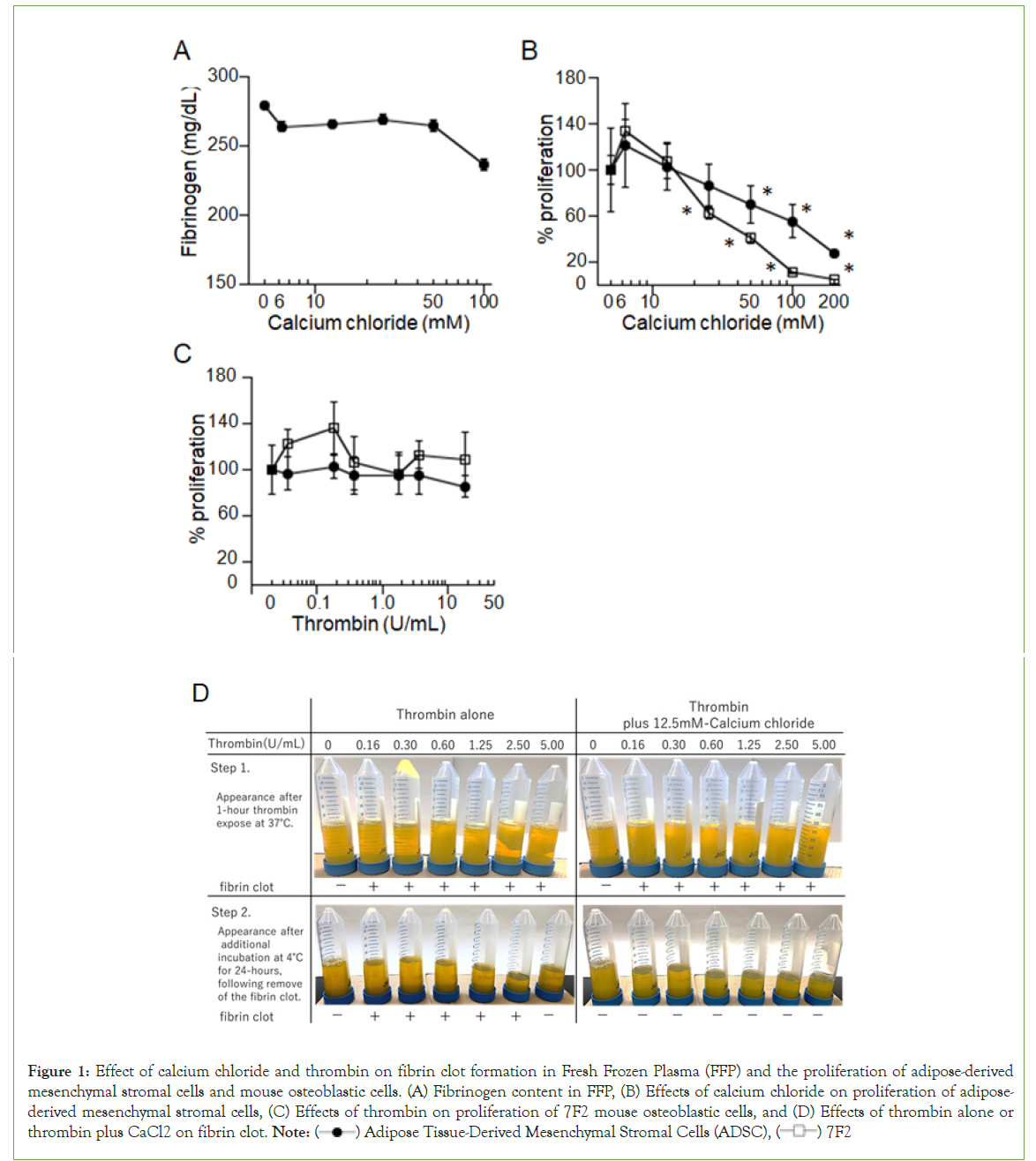
Figure 1: Effect of calcium chloride and thrombin on fibrin clot formation in Fresh Frozen Plasma (FFP) and the proliferation of adipose-derived
mesenchymal stromal cells and mouse osteoblastic cells. (A) Fibrinogen content in FFP, (B) Effects of calcium chloride on proliferation of adiposederived
mesenchymal stromal cells, (C) Effects of thrombin on proliferation of 7F2 mouse osteoblastic cells, and (D) Effects of thrombin alone or thrombin plus CaCl2 on fibrin clot. Note:  Adipose Tissue-Derived Mesenchymal Stromal Cells (ADSC),
Adipose Tissue-Derived Mesenchymal Stromal Cells (ADSC),  7F2
7F2
(B) and (C) Effects of calcium chloride and thrombin on proliferation of adipose-derived mesenchymal stromal cells and 7F2 mouse osteoblastic cells evaluated using a colorimetric assay. Adipose-derived mesenchymal stromal cells (2,000 cells in 180 µL of medium/well) were cultured with various concentrations of CaCl2 (final concentration 6.25–200 mM) or thrombin (final concentration 0.036–36.0 U/ mL) for 72 or 48 h, respectively. After designated incubation times, 10 µL of WST-1 reagent was added to each well, and the absorbance at 450 nm was measured 3h later. The percent increase in ADSC growth was calculated by dividing the OD value of cell cultures grown in the presence of each additive by the OD value in the absence of the additive and multiplying by 100. Data shown reflect the mean (± SD) of seven determinations and are representative of three independent experiments.
(D) Effects of thrombin alone or thrombin plus CaCl2 on fibrin clot formation were evaluated by the external appearance of FFP. Various concentrations of thrombin (final concentration 0–5.0 U/ mL) without/with CaCl2 (final concentration 12.5 mM) were added to FFP, and the mixtures were incubated at 37°C. After 1-hour incubation, fibrin clots were photographed (top row) and then removed by centrifugation. The supernatants were incubated for an additional 24 hours at 4°C, and then images were captured (bottom row). Finally, the fibrinogen content in the mixtures was measured using an automatic device after centrifugation.
However, CaCl2 alone induced slight fibrin clot formation in FFP at a concentration of 100 mM (Figure 1A), and CaCl2 perturbed the proliferation of human ADSCs and mouse 7F2 cells in a dose-dependent manner, which became significant at 100 mM in ADSCs and 25 mM in 7F2 cells (Figure 1B). Thus, we concluded that physiological concentrations of calcium ions were too low to induce fibrin clot formation thoroughly. We then evaluated whether thrombin affected the proliferation of ADSCs and 7F2 cells and found that thrombin alone did not affect the proliferation of ADSCs and 7F2 cells (Figure 1C). Based on these findings, we treated FFP with various final concentrations of thrombin (0–5.0 U/mL) with/ without CaCl2 (final concentration 12.5 mM). As shown in (Figure 1D), thrombin alone induced fibrin clot formation (upper left panel), but not thoroughly, as new fibrin clots appeared during an additional 24-h incubation, except at a thrombin dose of 5.0 U/mL (lower left panel). When treated with both thrombin and CaCl2, fibrinogen was transformed into fibrin clots quickly and thoroughly (upper right panel). After an additional 24 h of incubation, no further fibrin clots were observed (lower right panel).
Characterization of FFP and CS- serum
We produced serum from FFP or CS with thrombin and CaCl2 and characterized the samples by measuring the pH and fibrinogen, sodium, potassium, chlorine, and calcium content. As shown in Table 1, no fibrinogen was detected in the FFP-serum or CS-serum (detection sensitivity 50 mg/mL). There were minimal differences in the contents of sodium ions, potassium ions, and chlorine ions, but not calcium ions, between the intact FFP and corresponding serum or intact CS and corresponding serum (Table 1).
| PH | Fibrinogen (mg/dL) | Sodium (mEq/L) | Potassium (mEq/L) | Chloride (mEq/L) | Calcium (mmol/L) | |
|---|---|---|---|---|---|---|
| FFP | 7.465 ± 0.042 | 263.0 ± 43.2 | 170.8 ± 3.6 | 3.16 ± 0.05 | 78.0 ± 1.6 | 1.91 ± 0.02 |
| FFP-serum | 7.468 ± 0.029 | N.D | 174.7 ± 1.5 | 3.22 ± 0.08 | 113.0 ± 2.1 | 15.9 ± 0.56 |
| CS | 7.404 ± 0.048 | 90.3 ± 10.6 | 158.8 ± 3.0 | 3.11 ± 0.10 | 82.6 ± 3.3 | 1.96 ± 0.04 |
| CS-serum | 7.398 ± 0.044 | N.D | 159.6 ± 3.1 | 3.13 ± 0.10 | 110.0 ± 3.7 | 13.4 ± 0.31 |
Note: FFP-Fresh Frozen Plasma, CS-Cryosupernatant, N.D-Not Detected
Table 1: Fibrinogen and electrolyte profiles of serum derived FFP and CS.
FFP and CS-serum support the proliferation of human cells
We compared the proliferation of six lines of human adherent cells, three lines of human floating cells, and three lines of murine adherent cells cultured with FBS, hPL, FFP-serum, or CS-serum (Figure 2). After the designated incubation times, 10µL of WST-1 reagent was added to each well, and then the absorbance at 450 nm was measured. The percent increase in cell growth was calculated by dividing the OD value of cell cultures grown in the presence of hPL, FFP, or CS by the OD value in the presence of FBS and multiplying by 100. Data shown reflect the mean (± SD) of four determinations and are representative of three independent experiments. BMSC- Human Bone Marrow-Derived Stromal Cell; ADSC- Human Adipose Tissue- Derived Stromal Cell; HeLa- Human Cell Line Derived From Cervical Cancer; 293T, human cell line derived from embryonic kidney; MG63 and Saos-2, human cell lines derived from osteosarcoma; HL-60, human promyelocytic leukemia cell line; K562, human multipotential leukemia cell line; Meg-A2, human megaloblastic leukemia cell line; 7F2, mouse osteoblastic cell line; MS-5 and S-17, mouse stromal cell lines.
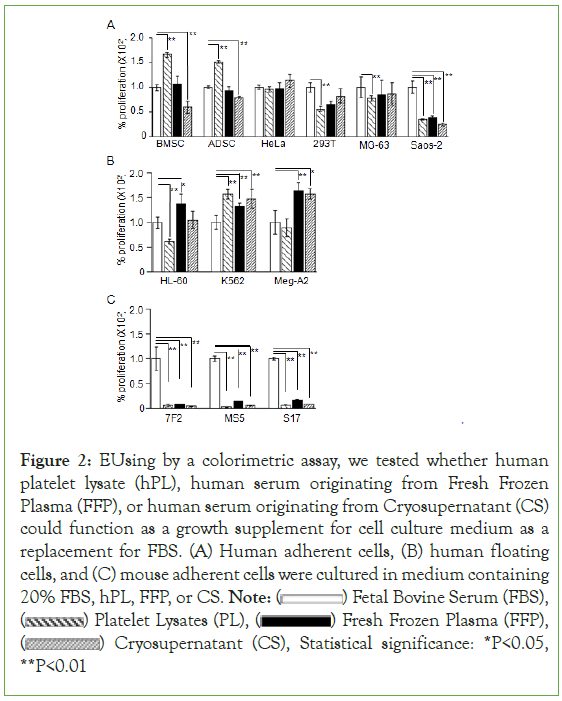
Figure 2: EUsing by a colorimetric assay, we tested whether human
platelet lysate (hPL), human serum originating from Fresh Frozen
Plasma (FFP), or human serum originating from Cryosupernatant (CS)
could function as a growth supplement for cell culture medium as a
replacement for FBS. (A) Human adherent cells, (B) human floating
cells, and (C) mouse adherent cells were cultured in medium containing
20% FBS, hPL, FFP, or CS. Note:  Fetal Bovine Serum (FBS),
Fetal Bovine Serum (FBS),  Platelet Lysates (PL),
Platelet Lysates (PL),  Fresh Frozen Plasma (FFP),
Fresh Frozen Plasma (FFP),  Cryosupernatant (CS), Statistical significance: *P<0.05, **P<0.01
Cryosupernatant (CS), Statistical significance: *P<0.05, **P<0.01
FFP-serum exhibited a similar or stronger proliferative effect compared with FBS in all human cells except Saos-2, whereas CS-serum exhibited a weaker proliferative effect in BMSCs, ADSCs, and Saos-2 cells (Figure 2A,2B). hPL promoted the growth of BMSCs and ADSCs more vigorously than FFP- and CS-serum (Figure 2A). Neither FFP- serum nor CS-serum exhibited a proliferative effect in mouse cells (Figure 2C).
Discussion
Because FFP is anticoagulated with CPD, a chelator of calcium ions essential for blood clotting, we tried to neutralize CPD and activate the blood coagulation cascade by adding CaCl2 to the plasma. However, we found that CaCl2 alone did not induce fibrin clot formation even at supra-physiological concentrations (Figure 1A, 1B). It was reported that FV, FVII, and FVIII are the most labile factors in plasma and degrade over time [24,25]. These data and our findings suggested that addition of thrombin would be necessary to transform fibrinogen into fibrin directly and induce fibrin clot formation. As expected, thrombin alone induced fibrin clot formation thoroughly but required more than 24 h, whereas thrombin with CaCl2 at a physiological concentration required only 1 h to induce fibrin clot formation (Figure 1D). Additionally, we did not observe any harmful effects of thrombin on human ADSCs or 7F2 cells (Figuire 1C), as it has been reported that antithrombin quickly inactivates thrombin in plasma [26]. Thus, we concluded that using both thrombin and CaCl2 would be a time- saving and safe method to produce serum from FFP. Similarly, we successfully produced serum from CS by adding thrombin and CaCl2 (data not shown).
We found that FFP- and CS-serum produced with thrombin and CaCl2 could be used as a growth supplement to replace FBS in culturing human adherent and floating cells, except Saos-2 cells. Both types of serum were particularly effective in comparison with FBS for culturing human floating cell lines: HL-60 (not with serum from CS), K562, and Meg-A2. hPL exhibited a superior proliferative effect on human BMSCs and ADSCs (Figure 2A), as reported previously [27]. However, FFP-and CS-serum only minimally supported the growth of murine cells (Figure 2C). This discrepant result could be related to the non-reactivity of some human cytokines, such as IL-3, against murine cells, even though the proportion of mouse genes with a single identifiable orthologue in the human genome is approximately 80% [28,29]. These results and other evidence suggest that FFP and CS-serum represent potent alternatives to FBS for culturing human cells, particularly hematological cells. When large-scale MSC culture is necessary for clinical application, hPL should be used instead of FFP or CS-serum.
The response of MG-63 cells to hPL, FFP-serum, and CS-serum differed from that of Saos-2 cells (Figure 2A), even though both cell lines were originally established from osteoblastoma. FFP-serum and CS-serum contained an increased concentration of calcium ions (Table 1). However, calcium ions reportedly promote the proliferation of mouse osteoblasts, suggesting that increased calcium content should not perturb the proliferation of Saos-2 cells [30,31]. Anti-A antibodies and Anti-B antibodies reportedly induced the lysis of certain cells via activation of complement [32,33].
Conclusion
According to the ATCC website, Saos-2 cells express B antigen; however, it is unknown which antigen is expressed by MG63 cells, and this could be related to the difference in proliferation between Saos-2 and MG63 cells in medium containing hPL, FFP-serum, or CS-serum. Medium containing heat-inactivated serum is reportedly ideal for in vitro culture of osteosarcoma cells. Thus, FFP-serum and CS-serum should be heat-inactivated for culturing cells in bone repair applications using osteoblasts differentiated from MSCs.
Collectively, our data provide evidence that FFP-serum and CS-serum can be produced rapidly and in a blood product-sparing manner and used as potent alternatives to FBS for culturing human cells.
Acknowledgements
We are grateful to Ms. Satoko Kato and Ms. Yukiji Ando for their technical and secretarial help.
Sources of Funding
This study was supported in part by a Japanese Grant-in-Aid for Scientific Research [(C) 20K08721] to T. Nakayama.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bordignon C, Carlo-Stella C, Colombo MP, De Vincentiis A, Lanata L, Lemoli RM, et al. Cell therapy: achievements and perspectives. Haematologica. 1999;84(12):1110-1149.
[Google Scholar] [PubMed]
- Nuttall PA, Luther PD, Stott EJ. Viral contamination of bovine foetal serum and cell cultures. Nature. 1977;266(5605):835-837.
[Crossref] [Google Scholar] [PubMed]
- Kozasa T, Aoki H, Nakajima N, Fukusho A, Ishimaru M, Nakamura S. Methods to select suitable fetal bovine serum for use in quality control assays for the detection of adventitious viruses from biological products. Biologicals. 2011;39(4):242-248.
[Crossref] [Google Scholar] [PubMed]
- Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89(3):776-779.
[Crossref] [Google Scholar] [PubMed]
- Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49(3):152-156.
[Crossref] [Google Scholar] [PubMed]
- Sheu J, Klassen H, Bauer G. Cellular manufacturing for clinical applications. Dev Ophthalmol. 2014;53:178-188.
[Crossref] [Google Scholar] [PubMed]
- Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27(9):2331-2341.
[Crossref] [Google Scholar] [PubMed]
- Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228-236.
[Crossref] [Google Scholar] [PubMed]
- Lucchini G, Introna M, Dander E, Rovelli A, Balduzzi A, Bonanomi S, et al. Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant. 2010;16(9):1293-1301.
[Crossref] [Google Scholar] [PubMed]
- Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
[Crossref] [Google Scholar] [PubMed]
- Jonsdottir-Buch SM, Lieder R, Sigurjonsson OE. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8(7):68984.
[Crossref] [Google Scholar] [PubMed]
- Kocaoemer A, Kern S, Kluter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25(5):1270-1278.
[Crossref] [Google Scholar] [PubMed]
- Canovas D, Bird N. Human AB serum as an alternative to fetal bovine serum for endothelial and cancer cell culture. ALTEX. 2012;29(4):426-428.
[Crossref] [Google Scholar] [PubMed]
- Heger JI, Froehlich K, Pastuschek J, Schmidt A, Baer C, Mrowka R, et al. Human serum alters cell culture behavior and improves spheroid formation in comparison to fetal bovine serum. Exp Cell Res. 2018;365(1):57-65.
[Crossref] [Google Scholar] [PubMed]
- O'Shaughnessy DF, Atterbury C, Bolton MP, Murphy M, Thomas D, Yates S, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11-28.
[Crossref] [Google Scholar] [PubMed]
- Nishiwaki S, Nakayama T, Saito S, Mizuno H, Ozaki T, Takahashi Y, et al. Efficacy and safety of human adipose tissue-derived mesenchymal stem cells for supporting hematopoiesis. Int J Hematol. 2012;96(3):295-300.
[Crossref] [Google Scholar] [PubMed]
- Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17(2):145-153.
[Crossref] [Google Scholar] [PubMed]
- Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138(4):1082-1087.
[Crossref] [Google Scholar] [PubMed]
- Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980;77(5):2936-2940.
[Crossref] [Google Scholar] [PubMed]
- Abe A, Emi N, Kato H, Adachi K, Murate T, Saga S, et al. Establishment and characterization of an immature human megakaryoblastic cell line, MEG-A2. Leukemia. 1995;9(2):341-349.
[Crossref] [Google Scholar] [PubMed]
- Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, et al. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer. 1976;18(4):421-431.
[Crossref] [Google Scholar] [PubMed]
- Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16(2):170-180.
[Crossref] [Google Scholar] [PubMed]
- Sugimoto K, Miyata Y, Nakayama T, Saito S, Suzuki R, Hayakawa F, et al. Fibroblast Growth Factor-2 facilitates the growth and chemo-resistance of leukemia cells in the bone marrow by modulating osteoblast functions. Sci Rep. 2016;6:30779.
[Crossref] [Google Scholar] [PubMed]
- Downes KA, Wilson E, Yomtovian R, Sarode R. Serial measurement of clotting factors in thawed plasma stored for 5 days. Transfusion. 2001;41(4):570.
[Crossref] [Google Scholar] [PubMed]
- Neisser-Svae A, Trawnicek L, Heger A, Mehta T, Triulzi D. Five-day stability of thawed plasma: solvent/detergent-treated plasma comparable with fresh-frozen plasma and plasma frozen within 24 hours. Transfusion. 2016;56(2):404-409.
[Crossref] [Google Scholar] [PubMed]
- Abildgaard U. Binding of Thrombin to Antithrombin III. Scand J Clin Lab Invest. 1969;24(1):23-27.
[Crossref] [Google Scholar] [PubMed]
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2):3-22.
[Crossref] [Google Scholar] [PubMed]
- Fung MC, Hapel AJ, Ymer S, Cohen DR, Johnson RM, Campbell HD, et al. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984;307(5948):233-237.
[Crossref] [Google Scholar] [PubMed]
- Mouse GSC, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520-562.
[Crossref] [Google Scholar] [PubMed]
- Kanatani M, Sugimoto T, Fukase M, Fujita T. Effect of elevated extracellular calcium on the proliferation of osteoblastic MC3T3-E1 cells:its direct and indirect effects via monocytes. Biochem Biophys Res Commun. 1991;181(3):1425-1430.
[Crossref] [Google Scholar] [PubMed]
- Sugimoto T, Kanatani M, Kano J, Kaji H, Tsukamoto T, Yamaguchi T, et al. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells. J Bone Miner Res. 1993;8(12):1445-1452.
[Crossref] [Google Scholar] [PubMed]
- Chan JH, Dua HS, Powell-Richards A, Jones DR, Harris IM. Effect of ABO blood group mismatching on corneal epithelial cells: an in vitro study. Br J Ophthalmol. 2001;85(9):1104-1109.
[Crossref] [Google Scholar] [PubMed]
- Bruserud O, Tronstad KJ, Berge R. In vitro culture of human osteosarcoma cell lines: a comparison of functional characteristics for cell lines cultured in medium without and with fetal calf serum. J Cancer Res Clin Oncol. 2005;131(6):377-384.
[Crossref] [Google Scholar] [PubMed]
Citation: Ishiyama K, Ogawa M, Kato H, Takeshita K, Ueda R, Nakayama T (2023) Serum Produced from Expired Fresh Frozen Plasma and Cryosupernatant Supports the Proliferation of Human Cells: Cost-Effective Alternatives to Fetal Bovine Serum. J Blood Disord Transfus. 14:562.
Copyright: © 2023 Ishiyama K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

