Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Screening for Ecologically Competent, Nutritional Characteristics and Symbiotically Effective Chickpea Nodulating Mesorhizobium spp. Isolated from Acidic Soils of Ethiopia
Atsede Muleta1, Kassahun Tesfaye2, Teklehaimanot Haileselassie2, Douglas R. Cook3 and Fassil Assefa12Department of Biotechnology, P.O Box 1176, Ethiopia
3Department of Plant Pathology, University of California Davis, One Shields Ave, Davis, CA, USA
Received: 20-Feb-2021 Published: 23-Aug-2021
Abstract
Background: Nitrogen fixations are limited in acidic soil due to the sensitivity of legume, rhizobia, and the symbiosis to low pH. However, legumes and their rhizobia show different responses to soil acidity. Objective: The experiment was conducted to screen indigenous Mesorhizobium isolates for their ecological competitiveness and symbiotically effectiveness for enhancing nitrogen fixation in chickpea production. Methodology: A total of 81 genetically diverse indigenous Mesorhizobium spp. were screened for low pH tolerance and their potential to ecological adaptations under in vitro conditions and their symbiotic effectiveness on two chickpea varieties under greenhouse conditions. Results: 62 (77%) strains grew well at low pH 5, and 47 (75.8%) of them were phosphate solubilizers. The species displayed marked differences in their eco-physiological characteristics such as the utilization of different carbon and nitrogen sources, pattern of tolerance to salinity, temperature, Mn2+ and Al3+ toxicity, heavy metals, and inherent resistance to antibiotics. They also displayed significant (p<0.01) differences in their nodulation features (nodule number, nodule dry weight) and yield characters (shoot dry weight) on Natoli and DZ-ck-2011s-2-0042 chickpea varieties. Based on their symbiotic effectiveness (SE), five strains, namely a.15star (ANI95 groups 5C), a.117L2 (ANI95 groups 2D), a.71 (ANI95 groups 4B), a.40L2 (ANI95 groups 8A), and a.200M (ANI95 groups 3A) showed the best performance on both varieties, even out-performed over the commercially available local strain Cp41 and tolerance to different in vitro ecological conditions. Conclusion: Ethiopian acidic soils harbored symbiotically effective, ecologically competent, and phosphate solubilizing Mesorhizobium species. Thus, these strains could be recommended as prospective commercial inoculants provided they can be tested in field trials in acidic soils.
Keywords
DZ-ck-2011 s-2-0042 varity; Natoli varity; Symbiotic effectiveness
Introduction
Soil acidity is one of the most important factors that affect nitrogen fixation and the production of leguminous crops, because it increases Aluminum (Al) and Manganese (Mn) toxicity and hampers Calcium (Ca) and Phosphorus (P) uptake by plant [1]. Jaiswal et al. [2] have reported that phosphorus deficiency and Aluminium (Al) toxicity in acid soils severely affect growth and symbiotic nitrogen fixation in legumes. These stresses limits the persistence and survival of rhizobia strains in the soil, and affect the exchange of molecular signals between rhizobia and their hosts, thus reducing nodulation. However, legumes and their specific endosymbiontic rhizobia exhibit varied responses to acidity and effective symbiosis on the host under acidic stress depends upon the strain and legume variety [3]. Thus, legume production can be improved with the selection of acid-tolerant varieties, effective and competitive strains of rhizobia and liming in acidic soil [4].
Phosphorus deficiency is one of the constraints of legume production in soil acidic conditions. It is interesting to note that rhizobia are one of the most effective phosphates solubilizing rhizobacteria that can improve phosphorus nutrition in the soil [5]. Therefore, acid-mediated P deficiency in acidic soils could be alleviated by inoculating phosphate solubilizing bacteria [6]. Thus, isolating and characterizing of symbiotically effective chickpea rhizobia with phosphate solubilization and other multiple growth properties from acidic soils is crucial for inocula production to enhance legume production [5,7].
Chickpea is one of the most important leguminous crops nodulated by specific group of rhizobia under the genus Mesorhizobium, to fix atmospheric nitrogen and improve soil fertility. It is estimated that chickpea fixes inorganic nitrogen with suitable rhizobial partner to utilizable form to plants to the tune of 90-180 kg/ha/ yr [8]; depending upon the host variety, symbiotic and ecological competence of Mesorhizobium strains [9,10]. Inoculation of chickpea with ecologically fit mesorhizobial strains improved nodulation, growth and yield components of chickpea varieties under adverse conditions [11], which is partly measured by their in vitro ability to utilize different carbon and nitrogen substrates, their inherent resistance to different antibiotics and tolerance to environmental factors such as acidity (pH), temperature and salinity (salt) [12].
According to [13], chickpea is one of the successful leguminous crops better adapted to grow and fix nitrogen in acidic soils. Some strains such as Mesorhizobium loti have shown a high degree of acid tolerance in laboratory media, being able to grow at pH values as low as 3.0 [13,14]. Imen et al. have demonstrated that Mesorhizobium can have a dual purpose of effective symbiotic association for nitrogen fixation and phosphate solubilization to enhance chickpea production in acidic soils. Mesorhizobia isolates having nitrogen fixing as well as high P solubilizing capability have great value for sustainable yield enhancement.
Ethiopia is the major chickpea producing country in Africa [15]. However, it is estimated that about 43% of the total cultivated land area is affected by soil acidity [16]. It has been reported that soil acidity mainly in the central and western parts of the country limits chickpea and other legume crops production [17,18]. Under the circumstances, nitrogen and phosphorus are deficient in most highland acidic soils of Ethiopia.
In Ethiopia, several studies were undertaken on eco-physiological and symbiotic properties of chickpea nodulating rhizobia [19-23]. Most of these studies identified (5-10%) chickpea rhizobia isolates combined tolerance to different in vitro stress conditions and nutritional versatility with symbiotic effectiveness comparable to Nitrogen fertilizer control plants. Other studies also showed effective symbiosis and P solubilization by rhizobia nodulating faba bean [24,25], and soybean [26] from acidic soils. However, there is still a dearth of information on the pattern of ecological competitiveness, symbiotic effectiveness and phosphate solublization of the different taxonomic groups of Mesorhizobium spp. from acidic soils of Ethiopia. Therefore this study was initiated to screen low pH tolerant, phosphate solubilizing, heterotrophically competent, and symbiotically effective strains from eight indigenous Mesorhizobium species under in vitro laboratory and greenhouse conditions.
Methodology
Sources of Mesorhizobium Isolates
The study included 81 mesorhizobial strains were retrieved from roots nodule of chickpeas, collected from acidic soils in central, western southern and northern parts of Ethiopia. They were isolated on Yeast extract agar media, genetically identified into the genus Mesorhizobium spp (Table 1) [27] and deposited in culture collection centers of the Plant Pathology Laboratory, University of California Davis and Department of Microbial, Cellular and Molecular Biology, Addis Ababa University.
| Sample strain | ANI95 Groups | NCBI Accession |
Isolation site | Altitude (masl) | Latitude | Longtiude | |
|---|---|---|---|---|---|---|---|
| a.15star | 5C | SAMN09232704 | Arisi | 2362 | 8.016972N | 39.85008E | |
| a. 90 | 10A | SAMN09232756 | West Shewa | 2219 | 8.386444N | 38.22611E | |
| a. 55 | 11A | SAMN09232745 | South Wollo | 1752 | 11.71503N | 39.6526E | |
| a. 152 | SAMN09232702 | Gurage | 1974 | 8.287641N | 38.53556E | ||
| a. AR452 | 2D | SAMN09232765 | East Wellega | 1582 | 9.002881N | 36.74009E | |
| a.117L2 | SAMN09232685 | Asosa | 1399 | 9.763652N | 34.79209E | ||
| a.144s | SAMN09232699 | West Wellega | 1905 | 8.646222N | 34.84814E | ||
| a.138w | SAMN09232697 | North Wollo | 1416 | 12.08462N | 39.6654E | ||
| a.35star | SAMN09232763 | East Wellega | 1552 | 9.011324N | 36.74068E | ||
| a.AR1 | 2E | SAMN09232759 | West Shewa | 2237 | 8.995119N | 38.49062E | |
| a.64 | SAMN09232749 | West Wellega | 2016 | 8.577056N | 34.73936E | ||
| a.71 | 4B | SAMN09232751 | West Shewa | 2231 | 8.054306N | 39.87258E | |
| a.89 | SAMN09232754 | ,, | 2254 | 9.023472N | 38.51169E | ||
| a.111 | SAMN09232683 | Bale | 2014 | 7.021806N | 40.69303E | ||
| a.8star | SAMN09232755 | Arisi | 2326 | 7.985000N | 39.97147E | ||
| a.AR7 | 1D | SAMN09232774 | Asosa | 1567 | 10.03125N | 34.57175E | |
| a.222 | SAMN09232724 | West Wellega | 2016 | 8.577056N | 34.73936E | ||
| a.302star | 1C | SAMN09232733 | West Shewa | 2259 | 9.008362N | 38.4611E | |
| a.66 | SAMN09232750 | South Wollo | 1461 | 10.57622N | 39.92128E | ||
| a.200M | 3A | SAMN09232714 | East Wellega | 1582 | 9.002881N | 36.74009E | |
| a.200s | SAMN09232715 | ,, | 1676 | 11.49973N | 39.61389E | ||
| a.16star | 8A | SAMN09232707 | Arisi | 2325 | 8.000917N | 39.93356E | |
| a.40L2 | SAMN09232738 | S/W/Shewa | 2248 | 8.585578N | 38.24065E | ||
| a.104 | SAMN09232682 | Arisi | 2338 | 8.064417N | 39.96378E | ||
| a.161 | SAMN09232705 | ,, | 2334 | 8.002722N | 39.91611E | ||
| a.45 2 | SAMN09232742 | S/W/Shewa | 2014 | 8.021667N | 38.09672E | ||
| Cp41 (reference strain) | - | ||||||
Table 1: Mesorhizobium strains and origin of culture collection used in ecological adaptations and symbiotic characterization.
Screening for Low pH Tolerance and Phosphate Solubilization of Mesorhizobium Strains
All the strains were screened for acid tolerance on medium consists of 300 μM MgSO4.7H2O, 300 μM CaCl2, 100 μM Fe EDTA. 10 μM KCI, 1μM MnCl2.4H2O, 0.4 μM ZnSO4.7H2O, 0.1 μM CuCl2.2H2O, 0.02 μM Na2MoO4.2H2O, 0.02 μM Co(N03)2.6H2O, 500 μM KH2P04,500 μM K2HPO4, arabinose (5.0 g), galactose (5.0 g), (1.1 g) Na glutamate, biotin (0.1 mg), thiamine (1.0 mg), (0.005%) bromothymol blue, (15g) agar, and 1L distilled water and adjusted to pH 5 containing high Mn (1.0 mM) and Al (50 μM), and low P (5 μM) and Ca (50 μm) [28]. The isolates were also prescreened for P solubilization on Pikovskaya medium containing inorganic tri-calcium phosphate. The medium contained; g/l (glucose 10, yeast extract 0.5, Ca3 (PO4)2 (5.0), (NH4)2SO4 (0.5), NaCl (0.2), KCl (0.2), MgSO4.7H2O (0.1), MnSO4.2H2O (0.002), FeSO4.7H2O (0.002), and agar 15. One hundred μl of the inocula (109 cfu/mL) were spotted on the medium and incubated at 28° for 7-10 days. Isolates that formed clear zones around the colony were considered as phosphate solubilizers and the solubilization index (SI) was calculated according to Edi-Premono et al., [29].
SI= Colony diameter + Halo zone diameter
Colony diameter
Eco-Physiological and Nutritional Characteristics of Selected Mesorhizobial Strains
All tests, except carbon and nitrogen utilization, were carried out on YEMA plates by inoculating with 10 μl of inoculums suspension (109 cfu/ml) and incubated at 28°C for 5 days against inoculated control unless stated otherwise. All tests were carried out in triplicates and results were recorded visually as ‘’+’’ for growth and ‘’- ‘’ absence of growth.
Salt, pH and Temperature Tolerance
Salt and low pH tolerance was determined on YEMA plates containing 1 to 5% (w/v) NaCl concentrations and the medium adjusted to pH (4 and 4.5), respectively [30]. Temperature tolerance was evaluated by inoculating them on YEMA plates under incubation temperatures (4, 10, 15, 20, 37, 40 and 45°C) [31].
Intrinsic Antibiotic and Heavy Metal Resistance
The intrinsic antibiotic and heavy metal resistance of strains were determined on solid YEMA medium containing filter sterilized antibiotics or heavy metals. The stock solutions of the antibiotics or heavy metals were sterilized using (0.22 μm Millipore) membrane filters and added to YEMA. The antibiotics used were (μg. mL-1): ampicillin (5 and 10), chloramphenicol (5 and 10), erythromycin (10 and 20), nalidixic acid (5 and 10), streptomycin (40 and 80), rifampicin (5, 10), neomycin (5 and 10) and tetracycline (5 and10). Similarly, the heavy metals used were CoCl2 (25,100), CuCl2.2H2O (50, 100), NiSO4 (50,100), and ZnCl2 (50,100), K2Cr2O7 (50, 100) [32]. Acidity related Al3+ and Mn2+ tolerance was tested at two different Al concentrations KAl(SO4)3 (50 μM and 100 μM) and two Mn concentrations MnCl2 (75 μM and 100 μM) using Keyser and Munns (KM) agar medium under acidic conditions at pH 5 [33].
Nutritional Versatility of Strains on Different Carbon and Nitrogen Substrates
Different carbohydrates were added as described by Amarger et al. [30] at a final concentration of 1gL-1 of the basal medium containing (gl/1): K2HPO4, 1; KH2PO4, 1; FeCl3.6H2O, 0.01, NH4SO4, 1NaCl2, 0.1; MgSO4.7H2O, 0.2; and agar, 15. The following filter sterilized (0.22 μm millipore) heat liable carbon sources; Citric acid, D-sorbitol, D-glucose, D-galactose, xylose, trehalose were added after autoclaving; and heat stable α-lactose, D-fructose, glycerol, α-cellulose, sucrose, and maltose were autoclaved with the basal medium. Filter sterilized L-tryptophan, methionine, L-tyrosine, leucine, riboflavin; DL-β-phenylalanine, L-arginine, glutamic acid, L-lysine, L-serine, glycine, and thiamine were used as a sole nitrogen source to a final concentration of 0.5 gL-1 on the basal medium containing (gL-1): K2HPO4, 1; KH2PO4, 1; FeCl3. 6H2O, 0.01; MgSO4.7H2O, 0.2; CaCl2, 0.1; NH4 (SO4)2, 1; and agar, 15; from which (NH4)2(SO4) was omitted and mannitol was added after autoclaving [30]. All plates were incubated at 28°C for 5 days.
Symbiotic Characterization Mesorhizobia Strains
The experiment was carried out at Debre Zeit Agricultural Research Center (DZARC), Debre Zeit, Ethiopia. The study was undertaken in a pot experiment using a sterile sand culture under greenhouse condition. Each Mesorhizobium strain was grown on YEMB and incubated at 28°C for 5 days. Seeds of chickpea cultivars called ‘Natoli and DZ-ck-2011 s-2-0042’ were surface sterilized with 4% sodium hypochlorite for 3 min, then rinsed with five changes of sterile distilled water and allowed to germinate on water agar 1.5 % w/v at 250C for three days.
The germinated seeds were planted in alcohol swabbed plastic pots (3 kg capacity) containing washed and autoclaved sterilized river sand. Five seeds were planted pot-1, individually flooded with 1 ml of the culture suspension (10-9 cfu/ml) and thinned down to three plants pot-1 after 5 days of emergence (DAE). The experiment was laid out with three replications for each treatment using randomized complete design with 12 h photoperiod, day temperature (28 ± 20C) and night temperature (17 ± 30C), by including uninoculated but nitrogen-fertilized (1% w/v KNO3) pots as positive (TN) control and uninoculated non- nitrogen-fertilized (T0) pots as negative controls. The pots were irrigated with nitrogen-free plant growth nutrient solution CRS (Center for Rhizobium Studies, Australia once a week and with sterile distilled water every three days, respectively. The pH of the nutrient solution was adjusted to pH5.
Plants were harvested after 8 weeks of planting to record the number of nodules plant-1 (NN), nodule dry mass (NDW); shoot dry weight (SDW). The percent symbiotic effectiveness (SE) was calculated according to [34]. The percent symbiotic effectiveness of the isolates was expressed as a percentage of the shoot dry biomass of each treatment compared with the shoot dry biomass of the positive control (with N). Finally, symbiotic effectiveness was rated as highly effective (HE) when the percentage of effectiveness >80%, effective, (E) between 50 and 80%, and of low effectiveness (LE) between 35 and 50%. Strains were considered ineffective when the percentage effectiveness was less than 35%. The data were analyzed by one-way analysis of variance (ANOVA) using the general linear model procedure of the SAS software package (SAS/STAT; version 9.3) and mean values were separated according to Duncan’s multiple range test at p = 0.05.
Results and Discussion
Screening of Mesorhizobia Strains for Low pH Tolerance and Phosphate Solubilization
Chickpea nodulating Mesorhizobium strains were screened based on low pH tolerance and 62 (76%) selected strains showed growth on low pH 5 medium (data not shown), indicating the presence of low pH tolerant indigenous strains. Other studies also showed the presence of low-pH tolerant chickpea rhizobia in Portugal [13] and Morocco [31]. The data showed that the growth of isolated strains varied ranging from none to profuse; indicating the strains of a given species varies in their pH tolerance. Such differences in tolerance to acidity among strains have been reported previously for various Rhizobium [26,33,35] indicated that Rhizobium strains that survived in the acid soil cannot grow on a nutrient medium with a pH as low as that of the soil from which the strains were isolated. According to Icgen et al. [36], chickpea rhizobia strains displayed a tendency to neutralize the pH of the medium when grown freely in media adjusted to different low pH values, and this might account for the success in acidic conditions. Overall, the present work shows that in vitro evaluation of strain growth under pH stress may also be a useful method for finding rhizobial isolates adapted to different soil pH (Table 2).
| Sample strains | ANI95 Groups | Relative species | Low pH tolerance | Temperature tolerance | NaCl % tolerance | Soil pH of the isolation site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 4 | pH 4.5 | 10 oC | 15 oC | 20 oC | 37 oC | 40 oC | 45 oC | 1% | 2% | 3% | 4% | 5% | ||||
| a.AR1 | 2E | M. plurifarium STM8773T (CCNB01000001) |
- | + | - | - | + | + | + | + | + | + | + | + | + | 5.9 |
| a.64 | - | + | + | + | + | + | + | + | + | + | - | - | - | 5.3 | ||
| a.AR452 | 2D | - | + | - | - | + | + | + | - | + | + | - | - | - | 5 | |
| a.138W | + | + | - | - | + | + | + | + | + | + | + | - | - | 5.1 | ||
| a.144S | - | + | - | - | + | + | + | + | + | + | - | - | - | 4.7 | ||
| a.117L2 | + | + | + | + | + | + | + | + | + | + | + | - | - | 4.9 | ||
| a.35star | + | + | - | - | + | + | - | - | + | + | - | - | - | 4.8 | ||
| a.AR7 | 1D | M. loti strain UFLA01-765T (NZ_LPWA00000000) |
- | + | - | + | + | + | + | + | + | + | + | - | - | 5.3 |
| a.222 | - | + | + | + | + | + | + | + | + | + | - | - | - | 5.2 | ||
| a.66 | - | + | - | + | + | + | + | - | + | + | + | + | + | 5.9 | ||
| a.302star | 1C | - | + | - | + | + | + | + | - | + | + | + | + | + | 5.4 | |
| a.200M | 3A | M.sp.WSM3876 T (NSGA01000001) |
- | + | + | + | + | + | + | + | + | + | - | - | - | 4.4 |
| a.200s | - | + | - | - | + | + | + | - | + | + | + | - | - | 5.2 | ||
| a.71 | 4B | M.amorphae CCNWGSO123-pacbio T(NZ_CP015318) |
- | + | - | - | + | + | + | + | + | + | - | - | - | 5.1 |
| a.89 | - | + | - | - | + | + | + | - | + | - | - | - | - | 5.1 | ||
| a.111 | - | + | + | + | + | + | + | - | + | - | - | - | - | 5.5 | ||
| a.8star | - | + | - | - | + | + | + | + | + | + | - | - | - | 5.2 | ||
| a.40L2 | 8A | M.australicum WSM2073T (NC_019973) | + | + | - | + | + | + | + | - | + | + | + | - | - | 5.9 |
| a.16star | - | + | - | + | + | + | + | + | + | + | + | - | - | 5 | ||
| a.104 | - | + | - | + | + | + | + | - | + | + | + | + | - | 5.3 | ||
| a.161 | + | + | - | - | + | + | + | - | + | + | + | + | + | 5.7 | ||
| a.45 2 | + | + | - | - | + | + | + | - | + | + | + | + | + | 5 | ||
| a.152 | 11A | M. opportunistum WSM207T (CP002279) |
+ | + | - | - | + | + | + | - | + | + | + | + | - | 5.2 |
| a.55 | + | + | - | - | + | + | + | - | + | + | + | + | + | 5.1 | ||
| a.15star | 5C | M.ciceri T (NZ-CM002796) |
- | - | + | + | + | + | + | - | + | + | + | + | + | 5.7 |
| a.90 | 10A | M. sp. LSJC280BOO T(NZ_AYVL00000000) |
+ | + | - | + | + | + | + | - | + | + | + | + | - | 4.9 |
| 9 (35%) | 25 (96%) | 6 (23%) |
13 (50%) | 26 (100%) | 26 (100%) | 25 (96%) | 11 (42%) | 26 (100%) | 24 (92%) | 16 (62%) | 10 (39%) | 7 (27%) | ||||
Table 2: Eco-physiological characteristics of selected chickpea nodulating mesorhizobia strains grown YEMA medium and incubated for 5-7 days.
A large number of the mesorhizobia strains 47(76%) were capable of solubilizing insoluble inorganic phosphate sources (tri-calcium phosphate) with solubilization index (SI) ranging from 1.3 to 3.1 (Table 3) of which Mesorhizobium a.138w (ANI95 groups 2D) showed the largest solubilization index (SI = 3.2), followed by Mesorhizobium a.117L2 (ANI95 groups 2D) and a.71 (ANI95 groups 4B) (SI = 3.0). Previous studies in Ethiopia also proved that chickpea rhizobia have phosphate solubilizing characteristics with solubilizing efficiency (SI) of 0.5 to 1.3 [19,20]. This data showed the number of (population density) (76%) and the solubilization indices of the mesorhizobial strains isolated from acidic soils was higher than the ones previously reported (30-44%) from other relatively high pH soils in Ethiopia [19,20] and 70% in India. According to Peix et al., [5] chickpea rhizobia, are the best phosphate solubilizing of all root nodule bacteria on plates, better mobilize phosphate from TCP and significantly increase growth of the host plant.
| Sample strains | ANI95 Groups | Relative species | Soil acidity related metal (Al and Mn toxicity tolerance at pH 5 | Phosphate solubilization activity (S.I.) | |
|---|---|---|---|---|---|
| Al3+ | Mn2+ | ||||
| 100 μM /ml | 100 μM /ml | ||||
| a.AR1 | 2E | M plurifarium STM8773T (CCNB01000001) |
+ | - | 1.8 |
| a.64 | + | + | 2.9 | ||
| a.AR452 | 2D | - | - | 2.3 | |
| a.138W | - | - | 3.1 | ||
| a.144S | - | + | 2.8 | ||
| a.117L2 | + | + | 3 | ||
| a.35star | - | - | 2.7 | ||
| a.AR7 | 1D | M.loti strain UFLA01-765T (NZ_LPWA00000000) |
- | - | - |
| a.222 | - | - | 2.5 | ||
| a.66 | - | - | 2.5 | ||
| a.302star | 1C | - | - | 2.5 | |
| a.200M | 3A | M.sp.WSM3876 T (NSGA01000001) |
+ | + | 1.9 |
| a.200s | - | - | 1.3 | ||
| a.71 | 4B | M.amorphae CCNWGSO123-pacbio T(NZ_CP015318) |
+ | + | 3 |
| a.89 | - | - | 2.5 | ||
| a.111 | - | - | 2.7 | ||
| a.8star | - | - | 2.9 | ||
| a.40L2 | 8A | M.australicum WSM2073T (NC_019973) | - | + | 2.5 |
| a.16star | - | - | 2.7 | ||
| a.104 | - | + | 2.3 | ||
| a.161 | - | - | 1.7 | ||
| a.45 2 | - | - | 1.7 | ||
| a.152 | 11A | M.opportunistum WSM207T (CP002279) |
+ | + | 2.2 |
| a.55 | + | + | 2 | ||
| a.15star | 5C | M.ciceri T(NZ-CM002796) | - | - | - |
| a.90 | 10A | M.sp. LSJC280BOO T(NZ_AYVL00000000) |
- | + | 2.5 |
| 7(27%) | 10 (38.5%) | ||||
Table 3: Soil acidity related metal (Al3+ and Mn2+ toxicity) tolerance at pH 5 of selected chickpea nodulating mesorhizobia strains.
Eco-Physiological Characteristics of Selected Mesorhizobium Strains
The mesorhizobial strains were prescreened for tolerance to pH 5, of which 26 strains were selected for further analysis. The strains were tested for in vitro tolerance at lower pH, 9 and 25 strains were grown at pH 4 and 4.5, respectively (Table 2). Studies also indicated that Mesorhizobium loti grown in medium at pH values as low as 4 [14] and other chickpea mesorhizobia isolates are able to grow at strongly acidic pH 3 [13]. The result indicated that tolerance to acidity is more strain specific than species specific [30]. Interestingly, a.15star (ANI95 groups 5C) showed growth at pH 5 but did not grow at pH (4, 4.5) and thus might be considered a moderate acidophile. Similarly, Brigido et al. [13] and Laranjo and Olivera, [12] have shown the isolates that belong to the group of M. ciceri with preference for tolerance at pH 5, which suggest that a species-related tolerance to acidity. The data did show a negative correlation (r=-0.09,-0.26, p=0.05) between origin of isolation soil pH and pH of isolation medium which is contrary to reports that show a positive correlation between chickpea rhizobia tolerance to different pH values and the origin-soil pH [13,30,38]. According to Brigado et al. [39], adaptation to acid pH by chickpea rhizobia is due to the presence of chaperones genes in their cells which are important for survival during acid stress.
All the strains were able to grow between 20 and 37°C and showed variations below and above these values, where 6(23%) strains were resistant to 10°C; whereas 11 strains (42.3%) were able to grow at 45°C (Table 2), which is relatively different from temperature sensitive chickpea rhizobia (40-45°C) isolated from Ethiopia [22] and China [10]. Conversely, studies from Turkey [40], and Morocco [31] showed chickpea rhizobia were able to grow at high temperature (>400C), which could be related to local adaptation. Mesorhizobium a.117L2 (ANI95 groups 2D) and a.200M (ANI95 groups 3A) strains showed wide range of temperature tolerance (10- 450C) than the other strains (Table 2). Similarly, Laranjo and Olivera [12] and Rai et al. [41] have shown pattern of tolerance to high temprature by Mesorhizobium plurifarium and Mesorhizobium loti strains, respectively. According to Rodrigues et al [37], adaptation of tolerance to high temperature by rhizobia strains in chickpea rhizobia is due to overproduction of a set of proteins, termed heat shock proteins (HSPs), which are important for survival during stress conditions.
Chickpea rhizobia strains displayed high diversity in their salt tolerance (Table 2). All strains were tolerant to 1% NaCl, but 24 (92%) of the strains survived at 2% NaCl, and fewer isolates 7 (27%) were able to grow on YEMA medium containing 5% NaCl. This result is comparable to previous finding in Ethiopia that indicates a wide range of variation in salt tolerance to 1-5% (w/v) NaCl concentration [21]. The most tolerant strains were a.AR1 (ANI95 groups 2E), a.30s (ANI95 groups 1C), a.66 (ANI95 groups 1C), a.161 (ANI95 groups 8A), a.452 (ANI95 groups 8A), a.55 (ANI95 groups 11A) and a.15star (ANI95 groups 5C) that were able to grow at 5% NaCl. Studies also showed that Mesorhizobium ciceris, Mesorhizobium plurifarium and Mesorhizobium loti strains were tolerant to high NaCl concentration [12,41]. According to Zahran [42] salt tolerant rhizobia are endowed with the capacity to tolerate osmotic stress that is mainly associated with their capacity to accumulate low molecular weight organic solutes in their cells.
Acidity-Al3+/Mn2+ Tolerance
The strains showed variable responses to Al3+ and Mn2+ toxicity which is often associated with acidic soils (Table 3). Thus, 7(27 %) and 10 (38%) of the Mesorhizobium strains displayed tolerance to Al3+ and Mn2+toxicity at a concentration of 100 μM /ml at pH 5, respectively. The data showed that strains a.117L2 (ANI95 groups 2D), a.64 (ANI95 groups 2E), a.152 (ANI95 groups11A), a.55 (ANI95 groups 11A), a.200M (ANI95 groups 3A) and a.71 (ANI95 groups 4B) were the most tolerant strains that grew well at all tested concentration of Al3+ and Mn2+. The current result also confirmed the earlier report of Ayanaba et al. [33] who stated that, there is relationship between acid-Al sensitivity of isolates with their colony texture, as large-mucoid rhizobial colonies were more resistance than dry-pinpoint colonies. Previous studies in Ethiopia [19] and Morocoo [31] also showed relatively same pattern of resistance to Mn2+, and sensitivity to Al3+ in chickpea rhizobia isolates. According to Jaiswal et al. [2], Al3+ toxicity and acidity itself is probably more important limiters of rhizobial growth than Mn2+ toxicity in acid soils.
Intrinsic Antibiotics (IAR) and Heavy Metals Resistance (HR)
The Mesorhizobium strains showed variations in inherent antibiotic resistance in that almost all the strains were resistant to nalidixic acid, kanamycin, ampicillin, chloramphenicol and erythromycin with the exception of Mesorhizobium a.71 (ANI95 groups 4B) and a.89 (ANI95 groups 4B) that were sensitive to erythromycin (Table 4). Three Mesorhizobium strains, a.71 (ANI95 groups 4B), a.55 (ANI95 groups 11A) and a. AR7 (ANI95 groups 1D) were resistant to streptomycin and a. AR7 strain from M. genospeces 1D group were resistant to tetracycline. Mesorhizobium a.15star (ANI95 groups 5C) and a.AR7 (ANI95 groups1D) showed multiple antibiotic resistances by growing on media containing 7-8 of the antibiotics tested (Table 5). This is similar to a previous report that showed chickpea rhizobia such as M. ciceri strains tolerant to 70-80%) tested antibiotics [23]. Other studies in Ethiopia also showed that chickpea rhizobia were relatively resistant to nalidixic acid and erythromycin; and sensitive to streptomycin and tetracycline [21]. On the contrary, chickpea rhizobia from Morocoo [31] and Turkey [40] were sensitive to ampicillin, chloramphenicol and kanamycin, indicating large variability in antibiotic resistance of chickpea endosymbionts.
| Sample strains | ANI95 Groups | Relative species | Intrinsic antibiotics resistance (IAR) | Heavy metals resistance (HR) |
|---|---|---|---|---|
| a.AR1 | 2E | M plurifarium STM8773T (CCNB01000001) |
Rif, Kan, Amp, Chl, Er, Nal | Al, Cu, Zn, Cr |
| a.64 | Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn | ||
| a.AR452 | 2D | Kan, Amp, Chl, Er,Nal | Al, Mn, Zn, Cr | |
| a.138W | Kan, Amp, Chl, Er,Nal | Al, Mn, Cu, Zn, Cr | ||
| a.144S | Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.117L2 | Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.35star | Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.AR7 | 1D | M.loti strain UFLA01-765T (NZ_LPWA00000000) |
Stre, Tetr, Rif, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr |
| a.222 | Kan, Amp, Chl, Er, Nal | Al, Mn | ||
| a.66 | Rif, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.302star | 1C | Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | |
| a.200M | 3A | M.spWSM3876T (NSGA01000001) |
Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Zn |
| a.200s | Kan, Amp, Chl, Er, Nal | Al, Mn, Zn, Cr | ||
| a.71 | 4B | M.amorphae CCNWGSO123-pacbioT(NZ_CP015318) | Stre, Neo, Kan, Amp, Chl, Nal | Al, Mn |
| a.89 | Rif, Neo, Kan, Amp, Chl, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.111 | Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.8star | Kan, Amp, Chl, Er,Nal | Mn | ||
| a.40L2 | Rif, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr | ||
| a.16star | 8A | M.australicum WSM2073T (NC_019973) | Rif, Kan, Amp, Chl, Er, Nal | Al, Cu, Zn, Cr |
| a.104 | Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn | ||
| a.161 | Rif, Kan, Amp, Chl, Er, Nal | Al, Cu, Zn, Cr | ||
| a.45 2 | Rif, Kan, Amp, Chl, Er, Nal | Al, Cu, Zn | ||
| a.152 | 11A | M.opportunistum WSM207T (CP002279) |
Neo, Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr |
| a.55 | Stre, Kan, Amp, Chl, Er, Nal | Al, Mn, Zn, | ||
| a.15star | 5C | M.ciceri T(NZ-CM002796) | Rif, Neo,Kan, Amp, Chl, Er, Nal | Al, Mn, Cu, Zn, Cr |
| a.90 | 10A | M.sp. LSJC280BOO T(NZ_AYVL00000000) |
Kan, Amp, Chl, Er, Nal | Al, Mn,Cu, Zn |
Table 4: Intrinsic Antibiotic resistance (IAR), Heavy metals tolerance of selected chickpea nodulating mesorhizobia strains.
The mesorhizobial strains showed different response to heavy metals (Table 4). Accordingly, all strains failed to grow on Co and Ni (data not shown), whereas most strains (65-96%) were tolerant to aluminum, zinc, manganese, copper and chromium. The data showed that 12 (46) % of the strains showed a wide spectrum of heavy metal resistance growing on five of the heavy metals tested. The study in Ethiopia also showed that chickpea rhizobia were resistant to Mn, Al, and Zn. A study from Morocco also showed 20-60% of the chickpea rhizobia were resistant to Al, Zn, and Cu. This generally indicates that there is inherent antibiotic and heavy metal resistance by strains based upon the origin of isolation and the type of chemicals they had been exposed to in the soil.
Pattern of Carbon and Nitrogen Source Utilization
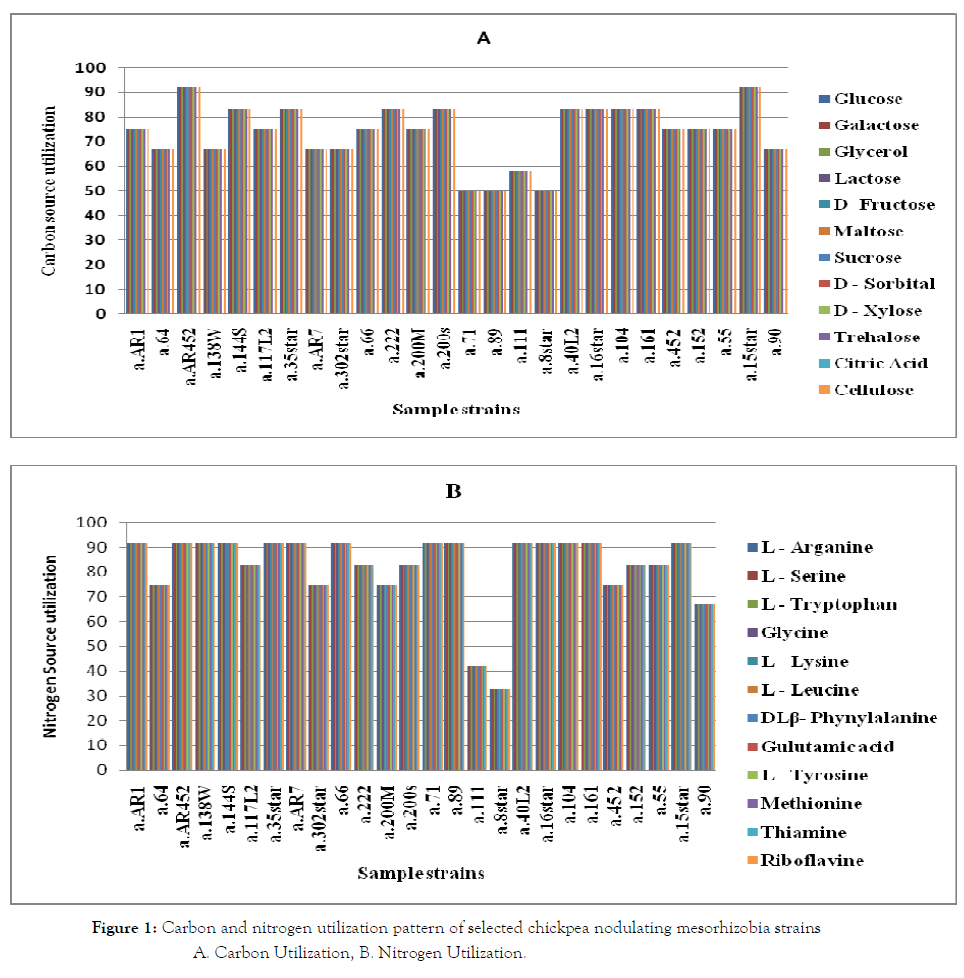
Most of the chickpea mesorhizobia strains were able to utilize many of the carbon substrates tested and failed to grow on citric acid (Figure 1). Mesorhizobium a.15star (ANI95 groups 5C) and a.AR452 (ANI95 groups 2D) strains were the most versatile of all the strains that were able to grow on more than 90% of the carbon sources; whereas Mesorhizobium strains a.71, a.89 and a.8star (ANI95 groups 4B) group limited to grow on 50% of the tested carbon substrates. This result is concurrent with findings of Sirag and Assefa [23] that showed most M. plurifarium and M. ciceri strains from Ethiopia were versatile in carbohydrate utilization than the other groups.
Figure 1. Carbon and nitrogen utilization pattern of selected chickpea nodulating mesorhizobia strains
A. Carbon Utilization, B. Nitrogen Utilization.
Chickpea rhizobia strains also exhibited diversity in utilizing different amino acids and vitamins as sole N-sources (Figure 1). All strains were grown on all the tested N-sources whereas fewer strains failed to grow on L-lysine and glycine. Unlike that of carbon sources, 14 (54%) of the strains utilized more than 90% of the nitrogen substrates, whereas a.8star (ANI95 groups 4B) strain utilized only 33% of the tested N sources substrates, indicating that most Mesorhizobium strains were more versatile in N utilization than C utilization. Other studies in Ethiopia also showed the same pattern of carbon and nitrogen utilization of chickpea rhizobia. On the contrary, some M. ciceri and M. plurifarium strains utilized citrate and lysine unlike to the present finding.
This implies that chickpea rhizobia display large variability in carbon and nitrogen substrate utilization that may be related to differences in strains/genotype or local adaptations [43, 44].
Symbiotic Effectiveness of Mesorhizobium Strains
The greenhouse trial of selected strains on a sand culture under low pH 5 showed considerable variations among the tested mesorhizobial strains in shoot dry mass, nodule number, nodule dry mass per plant and symbiotic effectiveness (SE) compared to the control (p ≤ 0.01) (Table 5). The inoculated strains induced nodules on both chickpea varieties ranging from 10 NN/plant with Mesorhizobium a.AR1 (M. genospecies ANI95 groups 2E) to 98 NN/ plant with Mesorhizobium a.64 (ANI95 groups 2E) (mean= 61 NN/ plant) on Natoli variety; and from 12.5 NN/plant for Mesorhizobium a.90 (ANI95 groups 10A) to 85 NN/plant with Mesorhizobium a.89 (ANI95 groups 4B) (mean= 38 NN/plant) on DZ-ck-2011 s-2-0042 variety.
| Sample strains | ANI95 Groups | Relative species | Shoot dry weight plant-1 (g) | Symbiotic effectiveness (SE %) | Number of nodules plant-1 (NN/plant) | Nodule dry weight plant-1 (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natoli | DZ-2012-CK-2011s-2-0042 | Natoli | DZ-2012-CK-2011s-2-0042 | Natoli | DZ-2012-CK-2011s-2-0042 | Natoli | DZ-2012-CK-2011s-2-0042 | |||||||||
| a.15star | 5C | M.ciceri T(NZ-CM002796) | 1.53±0.319b | 1.21±0.025ab | 96 | HE | 94 | HE | 58±0.278l | 36±0.591h | 89±11.342fe-g | 90±3.22cb-d | ||||
| a. 90 | 10A | M.sp. LSJC280BOO T(NZ_AYVL0000000) |
0.90±0.419e | 0.93±0.034c | 58 | E | 73 | E | 56±0.867n | 29±1.212k | 102±12.58bdc-e | 82±3.37f | ||||
| a. 55 | 11A | M.opportunistum WSM207T(CP002279) | 0.80±0.441f | 0.69±0.035e | 40 | LE | 60 | E | 62±0.912k | 45±1.272f | 89±13.23fe-g | 91±3.551cb | ||||
| a. 152 | 0.70±0.051g | 0.82±0.052d | 45 | LE | 61 | E | 74±1.059i | 45±1.478f | 70±15.35j-k | 77±4.127g | ||||||
| a.AR1 | 2E | M plurifarium STM8773T (CCNB01000001) |
0.63±0.513h | 0.67±0.043f | 48 | LE | 54 | E | 10±1.057s | 17.7±1.474n | 71±15.33j-k | 68±4.113h | ||||
| a.64 | 1.26±0.505d | 0.82±0.043d | 78 | E | 66 | E | 98±1.045a | 36±1.464h | 119±15.14a | 102±4.059a | ||||||
| a.117L2 | 2D | 1.53±0.466b | 1.00±0.037b | 96 | HE | 81 | HE | 89±0.963b | 37±1.342h | 109±13.96ba-c | 99±3.752ab | |||||
| a. AR452 | 0.90±0.455e | 0.97±0.036c | 57 | E | 73 | E | 55±0.941o | 39±1.317g | 66±13.66k | 101±3.664a | ||||||
| a.144s | 0.80±0.506f | 0.66±0.041f | 51 | E | 52 | E | 82±1.025e | 21.3±1.472m | 86±14.78fih-g | 67±4.095h | ||||||
| a.138w | 0.80±0.514f | 0.67±0.048f | 51 | E | 54 | E | 52±1.055p | 12.7±1.473o | 49±15.30l | 57±4.104i | ||||||
| a.71 | 4B | M.amorphae CCNWGSO123pacbioT(NZ_CP015318) | 1.50±0.474b | 1.22±0.037a | 95 | HE | 96 | HE | 80±0.979f | 57±1.366dc | 87±14.20fih-g | 82±3.812f | ||||
| a.89 | 1.30±0.480d | 0.71±0.038e | 79 | E | 57 | E | 48±0.992q | 85±1.387a | 72±14.39j-k | 76±3.869g | ||||||
| a.AR7 | 1D | M.loti strain UFLA01-765T (NZ_LPWA00000000) |
0.70±0.503g | 0.72±0.042e | 45 | LE | 60 | E | 74±1.040i | 33±1.456i | 104±15.08bd-c | 87±4.037de | ||||
| a.222 | 0.70±0.501g | 0.73±0.045e | 42 | LE | 59 | E | 78±1.047g | 52±1.469e | 88±15.18feh-g | 86±4.061e | ||||||
| a.302star | 0.93±0.485e | 0.73±0.038e | 59 | E | 59 | E | 58±1.003m | 31±1.398j | 83±14.54jih-g | 89±3.915ce-d | ||||||
| a.66 | 1C | 0.80±0.480f | 0.81±0.039d | 48 | LE | 65 | E | 75±1.012h | 56±1.412d | 70±14.67j-k | 82±3.915f | |||||
| a.200M | M.spWSM3876T (NSGA01000001) |
1.40±0.499c | 1.20±0.038ba | 86 | HE | 82 | HE | 83±1.025d | 39±1.439g | 116±14.95ba | 100±3.991a | |||||
| a.200s) | 3A | 0.90±0.501e | 0.81±0.040d | 56 | E | 67 | E | 84±1.031c | 58±1.448c | 100±15.02fed-c | 92±4.012cb | |||||
| a.16star | M.australicum WSM2073T (NC_019973) | 0.80±0.493f | 0.83±0.037d | 51 | E | 67 | E | 71±1.050j | 39±1.424g | 91±15.23fed-g | 77±3.941g | |||||
| a.40L2 | 8A | 1.40±0.496c | 1.00±0.039b | 86 | HE | 80 | HE | 82±1.019e | 73±1.436b | 109±14.87ba-c | 93±3.974b | |||||
| a.104 | 0.90±0.508e | 0.84±0.046d | 55 | E | 68 | E | 46±1.052r | 37±1.461h | 74±15.38jih-k | 92±4.082cb | ||||||
| CP41Reference strain | M. abyssinica | 0.60±0.542h | 0.70±0.051e | 37 | LE | 55 | E | 58±1.060m | 25±1.481l | 75±15.38jih-k | 69±4.128h | |||||
| Nitrogen | 1.60±0.611a | 1.24±0.041a | 100 | 100 | ||||||||||||
| Control | 0.20±0.630i | 0.30±0.040g | 13 | 23 | ||||||||||||
| CV | 2.59 | 2.44 | 1.31 | 1.27 | 0.861 | 1.95 | 9.57 | 2.66 | ||||||||
| Mean | 0.98 | 0.85 | 63.3 | 66.9 | 61.3 | 37.7 | 80 | 77 | ||||||||
| Significance | *** | *** | *** | *** | *** | *** | ||||||||||
Table 5: Symbiotic characteristics of chickpea nodulating mesorhizobia strains at pH 5.
The nodule dry mass was within the range of 49 mg/plant and 119 mg/ plant (mean=80 mg/ plant) with Natoli variety, and 57 to 102 mg/plant (mean=77 mg/plant) on DZ-ck-2011 s-2-0042 host variety, respectively (Table 5). The strains also induced the accumulation of shoot dry weight ranging from 0.63 g/plant to 1.53 g/plant (mean= 0.98 g/plant) on Natoli variety; and 0.66 g/ plant to 1.22 g/plant (mean= 0.85 g/plant) on DZ-ck-2011 s-2-0042 variety (Table 5).
This study indicated that the number of nodule and dry mass under low pH condition was much lower than recorded in other studies which agreed with reports from [45,46]. The average nodule dry mass obtained from both varieties were also much lower than 120 mg/per plant reported by Jida and Assefa [19], 200 mg/plant by Tena et al. [21] and 212 mg/per plant from chickpea which was reported by Brigido from moderately acidophilic mesorhizobia. The low nodule dry mass could also be attributed to direct pH stress on the plant, limiting nutrient uptake and subsequent dry matter accumulation [42].
On the basis of relative shoot dry matter accumulation in reference to N fixing and control plants, 5 (24%) strains were highly effective (HE) and 10 (48%) strains were effective (E) on both varieties. However, all the Mesorhizobium strains were highly effective (HE) and effective (E) on DZ-ck-2011s-2-0042 variety, but only 24% and 48 % of these strains were highly effective (HE) and effective (E), respectively on Natoli variety; and Mesorhizobium strains, a.55 (ANI95 groups 11A), a.AR7 (ANI95 groups 1D), a.66 (ANI95 groups 1C), a.152 (ANI95 groups 11 A), a.AR1 (ANI95 groups 2E) and a.222 (ANI95 groups 1D) strains were lowly effective on Natoli variety, indicating variations in their symbiotic compatibility and interaction between rhizobia and the host genotypes [11,46,47]. In all cases, the Natoli variety showed higher nodule number per plant, nodule dry weight, shoot dry weight, but not higher values in symbiotic effectiveness than the DZ-2012-CK-2011s-2-0042 variety. Alemu and Lule [18] have indicated that chickpea genotypes showed differential response to acidic pH condition.
In general, combined evaluation of ecological competitiveness (in vitro) and symbiotic effectiveness data showed that five strains namely; a.117L2 (ANI95 groups 2D), a.15star (ANI95 groups 5C), a.71 (ANI95 groups 4B), a.40L2 (ANI95 groups 8A) and a.200M (ANI95 groups 3A) performed better than the other strains, and even out-performed over the commercially available local strain Cp41 on both plant varieties at pH 5 under greenhouse conditions. This is concurrent with a report that M. ciceri strains from Portugal were effective strains with (SE) > 75%, whereas M. ciceri and M. plurifarium strains from Ethiopia were highly effective on both varieties.
In this experiment, the most highly effective strains were obtained from pH 5 condition compared to other investigator reports on acidic soil in Ethiopia. Kenasa et al. [24] have revealed that rhizobial isolates of faba bean collected from acidic soils of Wollega, western Ethiopia were effective on faba bean, whereas, Muleta et al. [26] have demonstrated that rhizobia strains of soybean collected from acidic soils of Ethiopia were effective from which, only 4% of the soya bean strains were found to be highly effective. It is likely that symbiotic performance of the strains is significantly dependent on pH condition. Thus, this result underlines the importance for a local screening of symbiotically effective and ecologically competent mesorhizobia isolates from acidic soil to enhance grain yield of the chickpea crop in acidic soil.
Conclusion
In the present study it can be concluded that Ethiopian acidic soils harbored symbiotically effective chickpea nodulating Mesorhizobium spp. These strains were also ecologically competent and heterotrophic versatile in carbon and nitrogen utilization, with a wide range of in vitro tolerance to various stress conditions, including low pH, Mn2+ and Al3+ toxicity, salinity, high temperature, heavy metals and antibiotics indicating their potential to effectively nodulate and fix nitrogen under field conditions.
The strains showed variations in their symbiotic effectiveness in nitrogen fixation on the two host varieties showing better performance on DZ-ck-2011s-2-0042 variety. Five strains: a.15star (ANI95 groups 5C), a.117L2 (ANI95 groups 2D), a.71 (ANI95 groups 4B) and a.40L2 (ANI95 groups 8A), and a.200M (ANI95 groups 3A) were relatively superior in their symbiotic performance, compatibility with both chickpea varieties, and can be recommended as prospective commercial inoculants provided they can be tested in field trials in acidic soils.
Funding
This work was supported by USAID-PEER Program Cycle-4, administered by the U.S. National Academies of Sciences, Engineering, and Medicine (NASEM).
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the Department of Microbial, Cellular and Molecular Biology, Addis Ababa University, Department of Plant Pathology, University of California Davis, USA and Debre Zeit Agricultural Research Center are acknowledged for their supports.
REFERENCES
- Hungria M, Vargas MAT. Environmental factors affecting nitrogen fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res 2000; 65:151-164.
- Jaiswal SK, Naamala J, Dakora FD. Nature and mechanisms of aluminum toxicity, tolerance, and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 2018; 54:309–318.
- Guo Y, Ling NY, Jianguo H. Effects of liming and Sinorhizobium inoculation on growth, nodulation and nutrient concentrations of Lucerne in acid soil purplish soil in China. Trap Grasslands 2009; 43: 112-117.
- Graham PH. Stress tolerance in Rhizobium and Bradyrhizobium, and nodulation under adverse soil conditions. Can J Microbiol 1992; 38: 475–484.
- Peix A, Rivas-Boyero AA, Mateos PF, Rodriguez-Barrueco C, Martinez-Molina E, Velazquez E. Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol Biochem 2001; 33:103-110.
- Singh S, Gupta G, Khare E, Behal KK, Arora NK. Phosphate Solubilizing Rhizobia Promote the Growth of Chickpea under Buffering Conditions.Inter J Pure App Biosci, 2014; 2: 97-106.
- Glick BR. Plant Growth-Promoting Bacteria : Mechanisms and Applications. Scientifca2012;10:6064-963401.
- Werner D. Production and Biological Nitrogen Fixation of Tropical Legumes. In: Werner D and Newton W E. (eds) Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment, Springer, Netherlands, 2005; pp. 1-13.
- Aslam M, Ahmad HK, Ullah H, Ayaz M, Ahmad E, Sagoo AG, et al. Nodulation grain yield and grain protein contents as affected by Rhizobium inoculatioin and fertilizer placement in chickpea cultivar bittle-98.Sarhad J Agric 2010; 26: 467-474.
- Zhang J, Yang X, Guo C, de Lajudie P, Singh RP, Wang E, et al. Mesorhizobium muleiense and Mesorhizobium gsp. nov. are symbionts of Cicer arietinum L. in alkaline soils of Gansu, Northwest China. Pl Soil 2017; 410: 103–112.
- Ben Romdhane SM, Aouani M, Trabelsi P, De Lajudie, Mhamdi R. Selection of high nitrogen-fixing rhizobia nodulating chickpea (Cicer arietinum L.) for semi-arid Tunisia. J Agro and Crop Scienc 2008; 194: 413-420.
- Laranjo M, Oliveira S. Tolerance of Mesorhizobium type strains to different environmental stresses. Anton. Leeuw Int JG 2011; 99:651–662.
- Brigido C, Alexandre A, Laranjo M, Oliveira S. Moderately acidophilic Mesorhizobia isolated from chickpea. Lett Appl Microbiol 2007; 44:168–174.
- Jarvis BDW, Van Berkum P, Chen WX, Nour SM, Fernandez MP, Cleyet-Marel JC. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium meditrraneum, and Rhizobium tianshanense to Mezorhizobium gen. nov., Int J Syst Bacteriol 1997; 47: 895-898.
- FAOSTAT (2019). Statistical Database of the United Nation Food and Agriculture Organization (FAO) Statistical Division. Rome.
- Agegnehu G, Yirga C, Erkossa T. Soil Acidity Management. Ethiopian Institute of Agricultural Research (EIAR). Addis Ababa, Ethiopia 2019; Pp: 1-50.
- Deressa A, Wakene CN, Geleto T. Inventory of Soil Acidity Status in Crop Lands of Central and Western Ethiopia. Utilization of diversity in land use systems: Sustainable and organic approaches to meet human needs Tropentag, October 9-1 Witzenhausen 2007.
- Alemu B, Lule D. Yield and agronomic performances of desi type chickpea genotypes against acidic soil of Western Ethiopia. J Agric Biotech Sustain Dev 2018; 10:116-121.
- Jida M, Assefa F. Phenotypic diversity and plant growth promoting characteristics of Mesorhizobium species isolated from chickpea (Cicer arietinum L.) growing areas of Ethiopia. Afr J Biotechnol 2012; 11: 7483-7493.
- Muleta D, Assefa F, Screening for symbiotically effective and ecologically competitive chickpea rhizobial inoculants from Ethiopian soils. Ethiop J Biol Sci2015; 14: 1–18.
- Tena W, Wolde-Meskel E, Degefu T, Walley F. Genetic and phenotypic diversity of rhizobia nodulating chickpea (Cicer arietinum L.) in soils from southern and central Ethiopia. Can J Microbiol 2017; 63: 690–707.
- Gebremedhin W, Assefa F, Moses T, Cargele M. Nutritionally Versatile, Abiotic Stress Resistant and Symbiotically Effective Chickpea (Cicer arietinum L.) Root Nodulating Rhizobial isolates from Eastern, Southeastern and Southern Ethiopia.Electro J Biol 2018; 14: 87-99.
- Siraj T, Assefa F. Mesorhizobium ciceri and Mesorhizobium prulifarium are the dominant symbiotically effective strains on Natoli and Arerti chickpea host varieties. Ethiop J Biol Sci 2018; 17: 19–35.
- Kenasa G, Jida M, Asefa F. Characterization of phosphate solubilizing faba bean (Vica faba L.) nodulating rhizobia isolated from acidic soils of wollega. J Sci Techno Art Research 2014; 3: 11-17.
- Tsegaye D. Evaluation of biological nitrogen Fixing and phosphate solubilizing Rhizobia of Faba bean (Vica faba L.) from acidic soils of Ethiopia. Ph.D. Dissertation. Haramaya University 2015; p.78.
- Muleta D, Ryder MR, Denton MD. The potential for rhizobial inoculation to increase soybean grain yields on acid soils in Ethiopia. Soil Sci Plant Nutri 2017; 63:441-45.
- Greenlon A, Chang PL, Mohammed Z, Muleta A, Carrasquilla-garcia N. Global-level population genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria. Proc Natl Acad Sci USA 2019; 116: 15200–15209.
- Gemell L, Roughley R, Reed M, Hartley EJ. Screening of Rhizobium leguminosarum bv. Trifolii for adaptation to acid and neutral soils using a selective agar medium. Soil Biol Biochem 1993; 25:1463–1464.
- Edi–Premono MA, Moawad X, Vleck PLG. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indonesian J Crop Sci 1996; 11:13 -23.
- Amarger N, Macheret V, Laguerre G. Rhizobium gallicum sp. nov. and Rhizobium sp. nov. from Phaseolus vulgaris nodules. Int J Syst Bacteriol 1997; 47: 996–1006.
- Maâtallah J, Berraho EB, Muñoz S, Sanjuan J, Lluch C. Phenotypic and molecular characterization of chickpea rhizobia isolated from different areas of Morocco. J Appl Microbiol 2002; 93: 531–540.
- Howieson JG, Dilworth MJ. Working with rhizobia. Australian Center for International Agricultural Research (ACIAR) Monograph No. 173. Canberra, Australia 2016.
- Ayanaba A, Asanuma S Munns DN. An agar plate method for the rapid screening of Rhizobium for tolerance to acid aluminum stress. Soil Sci Soc Am J 1983; 47:256-258.
- Purcino HMA, Festin PM, Elkan GH. Identification of effective strains of Bradyrhizobium for Archis Pintoli. Trop Agri2000; 77:226-231.
- Asanuma S, Ayanaba A. Variation in acid-Al tolerance of Bradyrhizobium japonicum strains from African soils. Soil Sci Plant Nutr 1990; 36: 309–317.
- Icgen B, Ozcengiz G, Alaeddinoglu NG. Evaluation of symbiotic effectiveness of various Rhizobium cicer strains. Res Microbiol2002; 153: 369–372.
- Rodrigues C, Laranjo M, Oliveira S. Effect of heat and pH stress in the growth of chickpea mesorhizobia. Curr Microbiol 2006; 53: 1–7.
- Alexandre A, Brígido C, Laranjo M, Rodrigues S, Oliveira S. A survey of chickpea rhizobia diversity in Portugal reveals the predominance of species distinct from Mesorhizobium ciceri and Mesorhizobium mediterraneum. Microb Ecol 2009; 58:930–941.
- Brígido C, Oliveira S. Most Acid-Tolerant Chickpea Mesorhizobia Show Induction of Major Chaperone Genes upon Acid Shock. Microbol Ecol 2013; 65:145–153.
- Küçük C, Kivanç M. Preliminary characterization of Rhizobium strains isolated from chickpea nodules. Afr J Biotechnol 2008; 7: 772-775.
- Rai R, Dash PK, Mohapatra T, Singh A. Phenotypic and molecular characterization of indigenous rhizobia nodulating chickpea in India. Indian J Experi Biol 2012; 50:340-350.
- Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol Biol Rev 1999; 63: 968-989.
- SAS Institute. Inc. The SAS System for Windows. Release 9.3. SAS Institute, Inc., Cary, N.C. 2012.
- Nour SM, Fernandez MP, Normand P, Cleyet-Maret JC. Rhizobium ciceri sp. nov. consisting of strains that nodulate chickpea (Cicer arietinum L). Int J Syst Bacteriol 1994; 44: 511-522.
- Angelini J, Taurian T, Morgante C, Ibanez F, Castro S, Fabra A. Peanut nodulation kinetics in response to low pH. Plant Physiol Biochem 2005; 43: 754–759.
- Keneni G, Bekele E, Assefa F, Imtiaz M, Debele T, Dagne K, et al. Phenotypic diversity for symbio-agronomic characters in Ethiopian chickpea (Cicer arietinum L.) germplasm accessions. Afr J Biotechnol 2012; 11:12634- 12651.
- Imen H, Neila A, Adnane B, Manel B, Mabrouk Y, Saidi M, et al. Inoculation with Phosphate Solubilizing Mesorhizobium Strains Improves the Performance of Chickpea (Cicer aritenium L.) Under Phosphorus Deficiency. J Plant Nutri 2015; 38: 1656-1671.
Citation: Muleta A, Tesfaye K, Selassie THH, Cook DR, Assefa F(2021) Screening for Ecologically Competent, Nutritional Characteristics and Symbiotically Effective Chickpea Nodulating Mesorhizobium spp. Isolated from Acidic Soils of Ethiopia. J Microb Biochem Technol.13:482. DOI: 10.35248/1948-5948.21.13.482.
Copyright: 2021 Atsede M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported by USAID-PEER Program Cycle-4,administered by the U.S. National Academies of Sciences,Engineering, and Medicine (NASEM).