Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 3
Sars-Cov2 Spike and Telomerase RNA’s Compared to Arrive at an Explanation for Increased Ageing in Alveolar Cells in Severe COVID-19
Han Geurdes*Received: 08-Apr-2021 Published: 29-Apr-2021, DOI: 10.35248/2155-9597.21.12.396
Abstract
In this letter we investigate if SARS-CoV-2 RNA is involved in the increased ageing of alveolar cells. Our in silico study is explorative. With the results we are able to outline experiments with AEC2 repair of bleomycin damaged alveolar cells. If AEC2 repair capability is diminished by spike RNA then perhaps this result provides a first step on a route to treat immortal lung cancer cells.
Keywords
Cov2; COVID-19; RNA; Virus
Introduction
In a recent study of COVID-19, a (statistical) relation was found between severity of COVID-19 illness and a decrease in length of peripheral blood lymphocyte telomeres [1]. COVID-19 is caused by the SARS-CoV-2 virus which introduces its single strand RNA via the ACE2 and TMPRSS2 receptors into the cell [2]. SARS-CoV-2 causes from mild flu-like symptoms in approximately 80% of the cases to a severe lung and multi-organic failure which can result in death of a significant percentage of patients [1].
Telomeres are chromosome ends to protect against rearrangement and the DNA broken strand repair system of the cell [3]. When cells divide and DNA is replicated, the telomeres become shorter [4]. This is a normal consequence of cellular division and is a molecular mechanism of cellular ageing [5].
Here we will focus on Alveolar Epithelial Cells (AEC) and note a certain parallelism between the effect of severe COVID-19 on the lungs and Idiopathic Pulmonary Fibrosis (IPF). The first thing we may observe is that lung alveolar integrity is related to telomere length and the activity of the enzyme telomerase [6]. If the number of telomeres goes below the Hayflick limit, the cell enters the senescence and mortality stage [7].
Telomerase is a ribonucleoprotein complex to maintain telomeres [8]. Telomerase is synthesized in stem/progenitor cells but its de novo synthesis in ordinary cells is suppressed. When an ordinary cell escapes mortality it becomes a cancer cell. Secondly, IPF is an illness with increased prevalence in advanced age with the hallmark of activation of AEC and epithelium driven accumulation of lung connective tissue [9]. The age factor suggests a role for telomeres and telomerase. Telomeres are therefore center stage here. Note e.g. also in early life, length of telomeres is dynamic and e.g. telomere reduction in skin cells is caused by UV radiation [10,11].
Thirdly there are two kinds of AEC. AEC1, responsible for oxygen processing and the progenitor cells AEC2. The latter produce surfactant and can transform, when necessary, into AEC1 [12]. The ability to go from AEC2 to AEC1 is in need of telomerase [12]. We note that human AEC2 has ACE2 receptors so AEC2 is vulnerable to SARS-CoV-2 infection [1].
Methods
Here, ways to compare RNA sequences are designed to establish a distance measure for “similar”. In this step the secondary structure of the hTR is of importance [13,14]. We developed in our computational lab, a method based on multidimensional scaling descriptive statistics of similarities among objects [15,16]. The elementary objects here are the (NTs) nucleotides; A, U(T), C and G. In RNA the NTs are connected by ribose phosphor sugar repetitive elements No [16]. In the present analysis we will only look at the sequences containing the NTs to determine δi,j. Each NT at a certain position contribute to the ψ .
The first characteristic is pairwise comparison of quantum Helium approximate wave function ψ solutions of, Hψ = Eψ , and H the Hamiltonian. i.e.
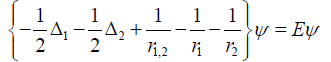
Each atom pair in the NT molecule is treated as though their
outer electrons are in a Helium “atom” with Hamiltonian as given
[17,18]. In the potential energy term, 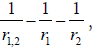 the r1,2 represent
the distance between the two electrons and the rk with k=1,2,
the distance between the electron and the (to those two) positive
charged plane of the NT molecule. The E is the eigenvalue (energy)
and the nabla’s
the r1,2 represent
the distance between the two electrons and the rk with k=1,2,
the distance between the electron and the (to those two) positive
charged plane of the NT molecule. The E is the eigenvalue (energy)
and the nabla’s 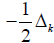 represent the quantum kinetic energy in
the He Hamiltonian. Mass and Planck constant are in unity units.
Use is made of Hückel coefficients to identify the different atoms
in the NT molecule. Later computations can e.g. try to introduce
the LCAO molecular orbital method in determining the δi,j [17].
represent the quantum kinetic energy in
the He Hamiltonian. Mass and Planck constant are in unity units.
Use is made of Hückel coefficients to identify the different atoms
in the NT molecule. Later computations can e.g. try to introduce
the LCAO molecular orbital method in determining the δi,j [17].
A further qualitative (dis)similarity is based on H bridges between the NTs. This concept is also employed to determine a matrix for second order configuration influence. The second order structure matrix is believed to hold the (pseudo)knots that are relevant to the architecture of the RNA in the telomerase complex. Obviously, the complementarity computations in the matrix overestimate the architectural form. However, the biochemical relevant architecture is present as a sub matrix.
In addition, per three NTs a four dimensional Euclidean distance
computation was performed. Each NT represents a dimension in
4 space. Another point was a scaled qualitative categorization of
similarity of three NTs in their amino acid effect. Finally, data from
ATR-IR spectra (pubChem) were used for the 4 NTs to establish a
similarity and to connect to a more semi-empiric set of data. Here
axes of different NTs had to be transformed into each other in
order to make the comparison. A normalization of 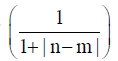 was employed for the n-th and m-th NT in the two to be compared
sequences.
was employed for the n-th and m-th NT in the two to be compared
sequences.
Finally, RNA sequences are compared modulo the implicit restrictions and theoretical assumptions. The modeling details can be found in Geurdes [17]. In the present case we first employed classical MDS and subsequently isoMDS in R. Then the second order configuration matrix was employed to the two axes and a subsequent isoMDS provides the result projection in a two dimensional space.
In this space the 75% Euclidean radius of the circle around the origin (0,0), i.e., R75(0,0), is a measure of similarity of the two RNA sequences. 75% of the points lie within the perimeter. Its rationale is that the coordinates sum to (0,0) in the projection. Furthermore, for computational convenience, the comparison is based upon 4 separate comparisons, each of size 271, with start points 1, 100, 199 and 268 (Figure 1). Here, rotational freedom around the “out of plane” axis through (0,0) is employed to obtain the configuration with the smallest R75(0,0). In the in silico experiments we employed S spike data from GenBank: MT419837.1 and GenBank: U86046.1 for hTR.
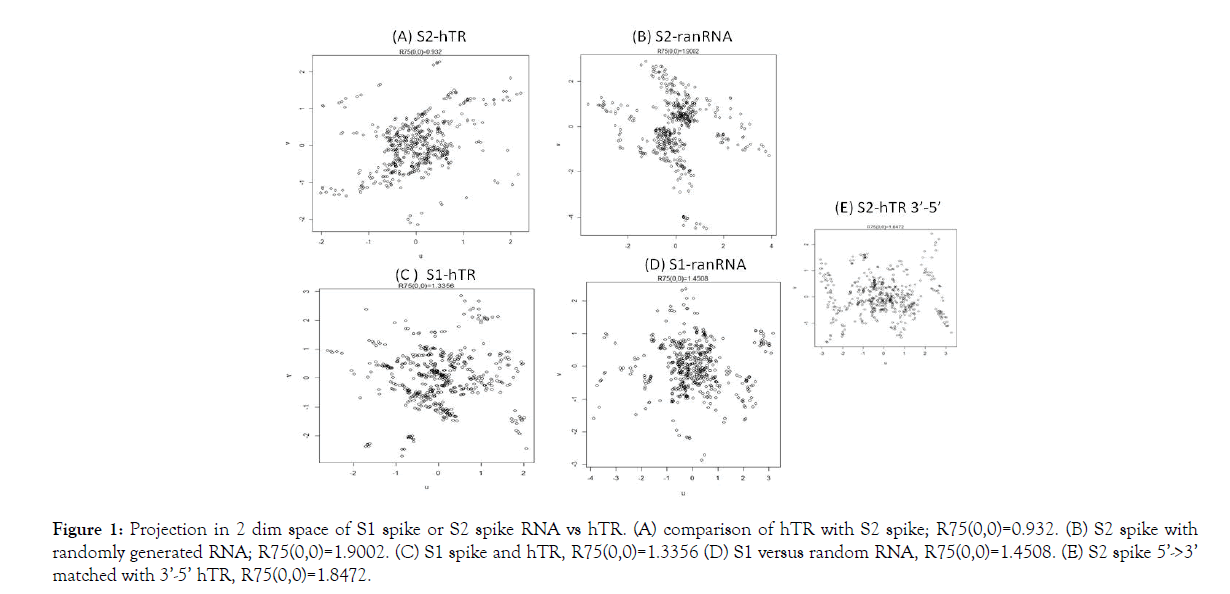
Figure 1: Projection in 2 dim space of S1 spike or S2 spike RNA vs hTR. (A) comparison of hTR with S2 spike; R75(0,0)=0.932. (B) S2 spike with randomly generated RNA; R75(0,0)=1.9002. (C) S1 spike and hTR, R75(0,0)=1.3356 (D) S1 versus random RNA, R75(0,0)=1.4508. (E) S2 spike 5’->3’ matched with 3’-5’ hTR, R75(0,0)=1.8472.
Results and Discussion
In figure, the results of computation are displayed. The comparison occurs: 5’->3’. Only in (E) we have S2 5’->3’ vs hTR 3’->5’. Before presenting the result of our computations, an alternative explanation is given. Fibrosis can be caused by ACE2 blocking [19]. If the merging of the virus with the AEC2 membrane destroys the ACE2 enzyme, it most likely will induce multiple divisions in order to maintain the ACE2 enzyme function at a required level. Shortening of telomeres are expected in that case. Below we will explain telomere shortening in severe COVID-19 as follows.
With the use of a 2 dimensional projection, the match with randomly generated RNA in Figures 1B and 1D, for both S1 and S2 produces a larger R75(0,0) than with biological RNA. Further, S2 has the lowest R75(0,0) value. Moreover, reversal of the direction of comparing 5’->3’ S2 RNA with 3’->5’ hTR gave a relatively high R75(0,0), viz. Figures 1E and 1A. Modulo the assumptions in our model, we then b can conclude that our in silico experiments indicate that the S2 part of the SARS-CoV-2 spike mRNA (start at RNA position: 21563+2028=23591 is 1 S2 RNA) best fit the hTR of telomerase. Therefore, the in vitro experiment that we propose is to have AEC2 cells that are able to assemble telomerase and to introduce in those cells SARS-CoV-2 S2 spike RNA.
A possible experimental set up is in vitro bleomycin-induced Lung Epithelial Cell (LEC) apoptosis. Let us concentrate on AEC2 cells. Bleomycin causes an initial increase, and then a reduction, in telomerase activity [19]. The influence of S2 spike RNA on telomerase synthesis can therefore be quantified by looking at the first 24 h telomerase increase in LEC of the AEC2 kind [20]. We predict that S2 spike RNA treated AEC2 will produce less telomerase activity in this peak of 24h. The reduction in the activity can be quantified with G-quadruplex-intercalating porphyrin telomerase inhibitor. Further, the telomerase activity can also be quantified with PCR based Telomere Repeat Amplification Protocol (TRAP) [20]. Detection of telomerase activity is briefly presented [20]. An in vivo experiment can be modeled as AEC2 transplantation [21]. Experiment where AEC2 and S2 RNA treated AEC2 can give a difference in recovery grade from bleomycine induced lung injury [22].
Finally we note that if the S2 RNA lowers the telomerase activity then a first step on the road to a possible treatment of proliferation of immortal lung cells could be found. Telomerase activity was found to be absent in most normal human somatic cells but present in over 90% of cancerous cells [7]. Immortalization is the hallmark of malignant transformation and a premalignant phenotype [23].
Conclusion
If S2 RNA has the predicted hampering effect on telomerase synthesis and can be delivered to the cancer cells, then the immortality of this type of cells is destroyed. Perhaps that the increase of caveolin-1 secretion of immortalized AEC2, can be a sign for a possible parasite vector delivering the S2 RNA.
Acknowledgment
The author wishes to thank Ad Popper, Director, Xilion BV.
REFERENCES
- Sanchez-Vazquez R, Guío-Carrión A, Zapatero-Gaviria A, Martínez P, Blasco MA. Shorter telomere lengths in patients with severe COVID-19 disease. Aging (Albany NY). 2021;13(1):1-15.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade long structural studies of SARS coronavirus. J Virol. 2020;94(1):e00127.
- Wu RA, Upton HE, Vogan JM, Collins K. Telomerase mechanism of telomere synthesis. Annu Rev Biochem. 2017;86(1):439-460.
- Martinez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and teleomere-binding proteins. Nat Rev Cancer. 2011;11(3):161-176.
- Mir SM, Tehrani SS, Goodarzi G, Jamalpoor Z, Asadi J, Khelghati N, et al. Shelterin complex at telomeres: implications in ageing. Clin Interv Ageing. 2020;15(2):827-839.
- Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, et al. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol. 2009;296(1):L57-L70.
- Cong Y-S, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66(3):407-425.
- Chen R, Zhang K, Chen H, Zhao X, Wang J, Li L, et al. Telomerase deficiency causes alveolar stem cell senescence-associated low-grade inflammation in lungs. J Biol Chem. 2015;290(52):30813-30829.
- Pardo A, Selman M. Lung fibroblasts, ageing and idiopathic pulmonary fibrosis, Ann Am Thoracic Soc. 2016;13(5):S417-S421.
- Bertucci EM, Mason MW, Rhodes OE, Parrott BB. The ageing DNA methylome reveals environment-by-ageing interactions in a model teleost. BiorXiv. 2021;10(1); 265-320.
- Stout GJ, Blasco MA. Telomere length and telomerase activity impact the UV sensitivity syndrome xeroderma pigmentosum. Cancer Res. 2013;73(6):1844-1854.
- Parra E, Pincelli MS, Teodoro WR, Velosa APP, Martins V, Rangel MP, et al. Modeling pulmonary fibrosis by abnormal expression of telomerase/apoptosis/collagen V in experimental usual interstitial pneumonia. Braz J Med Biol Res. 2014;47(7):567-575.
- Zhang Q, Kim N-K, Feigon J. Architecture of human telomerase RNA. Proc Natl Acad Sci U S A. 2011;108(51):20325-20332.
- Hu X, Kim J-K, Yu C, Jun H-I, Liu J, Sankaran B, et al. Quality-control mechanism for telomerase RNA folding in the cell. Cell Rep. 2020;33(13):108568.
- Cox TF, Cox MAA. Multidimensional scaling (2nd edn) CRC Press, Boca Raton, USA 2001.
- Ripley BD. Pattern recognition and neural networks (1st edn) Cambridge University Press, Cambridge, United Kingdom 1996.
- Geurdes H. Approximative He Hamiltonian in descriptive multidimensional scaling statistics of RNA contained information with application to SARS-CoV-2 S spike RNA and 7SLRNA. 2021.
- Lowe JP. Quantum chemistry (1st edn) Elsevier, Amsterdam, The Netherlands 1978.
- Verdechia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76(1):14.
- Fridlender ZG, Cohen PY, Golan O, Arish N, Wallach-Dayan S, Breuer R. Telomerase activity in bleomycin-induced epithelial cell apoptosis and lung fibrosis. Eur Respr J. 2007;30(2):205.
- Bryan TM, Englezou A, Dunham MA, Reddel RR. Telomere length dynamics in telomerase positive immortal human cell populations. Exp Cell Res. 1998;239(2):370.
- Alvarez-Palomo B, Sanchez-Lopez LI, Moodley Y, Edel MJ, Serrano-Mollar A. Induced pluripotent stem cell-derived lung alveolar epithelial type II cells reduce damage in bleomycin-induced lung fibrosis. Stem Cell Res Ther. 2020;11(2):213.
- Milyavsky M, Shats I, Erez N, Tang X, Senderovich S, Meerson A, et al. Prolonged culture of telomerase-immortalized human fibroblasts leads to a premalignant phenotype. Canc Res. 2003;63(21):7147.
Citation: Geurdes H (2021) SARS-CoV-2 Spike and Telomerase RNA’s Compared to Arrive at an Explanation for Increased Ageing in Alveolar Cells in Severe COVID-19. J Bacteriol Parasitol. 12: 396.
Copyright: © 2021 Geurdes H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

