Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 9, Issue 5
Safety Comparison of Atomextine and Lisdexamfetamine: Using Pharmacovigilance Database and Tools
Mohammed Aboukaoud*Received: 04-May-2021 Published: 25-May-2021, DOI: 10.35248/2329-6887.21.9.315
Abstract
Background Our study is the first to utilize the OpenVigil 2.1, a universal novel web-based pharmacovigilance analysis tool to analyze real-life safety of ADHD medications with special emphasis on newer medications and psychiatric adverse events. Thorough knowledge of adverse events for each medication reveals safety signals and support treatment guidelines and clinical management of the events. Methods we analyzed adverse event cases documented in the OpenVigil 2.1 from mid-2003 until February 2020. The medical dictionary for regulatory activities (MedDRA browser English version 20.0) was used to group lower level terms with a common preferred term to be searched for in the database. The proportional reporting ratio (PRR) and the reporting odd ratio (ROR) were used to quantitate the strength of the association between reported adverse events for atomoxetine, lisdexamfetamine, amphetamine, methylphenidate (instant release, intermediate acting, long acting). Results During the period we evaluated a total of 38,412 cases reported for methylphenidate, atomoxetine, lisdexamfetamine, amphetamine. We found a significantly lower risk for depressed mood (ROR 0.026, 95%CI 0.016-0.042) and tics (ROR 0.48, 95%CI 0.30-0.76) reported for atomoxetine compared with methylphenidate. Lisdexamphetamine did not seem to decrease the risk of psychiatric events. On the contrary, lisdexamfetamine carried a six fold risk for suicidal adverse events compared with other drugs in the data and a twofold risk of suicidal adverse events and tics compared with methylphenidate. Conclusions we conclude that Atomoxetine is a good choice for patients with comorbid tics or depression or for patients who develop depressed mood on stimulants. There is a great overlap between ADHD symptoms and stimulant/non stimulant adverse events, therefore prior to medication switch, therapeutic response should be evaluated carefully. Data in the literature on lisdexamfetamine remains scarce. Owing to signals on tic disorders and suicidal adverse events, we highly recommend studying these issues further with RCT and Cohort studies.
Keywords
ADHD, Lisdexamfetamine, Methylphenidate, Atomoxetine, Suicidal Adverse events, Tic disorders
Introduction
Epidemiology
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed psychiatric disorders, characterized by impulsivity, hyperactivity, and inattention [1,2]. Up to 7.1% of primary school-aged children and 3.4% adults are diagnosed with ADHD worldwide [1]. Recent population-based observational studies show a growing incidence of adult onset ADHD suggesting that the disorder might not necessarily begin in childhood [3]. A rampant increase in prescription Ritalin® use in the last four years has been documented for the general Israeli population [4]. The health maintenance organizations (HMOs), ‘Maccabi’ and ‘Clalit’ reported about a million prescriptions provided both to adults and children in Israel for 2016 [4, 5].
ADHD displays itself differently in adults than in children. The main symptoms reported by parents to ADHD in children include learning difficulty in class, high rate of physical activity and restlessness (manifested as recurrent fidgeting). Some parents notice disability in forming and maintaining order and organization in their child [6]. Adults mainly report more internal and less obvious symptoms such as a feeling of aggravation in situations that require long-term stillness. Adults also report difficulty in concentrating especially in a noisy environment, difficulty in learning from lectures as well as poor reading-writing skills and cumbersome time management. Common to all patients’ with ADHD, symptoms are persistent and interfere with functioning or development and carry a substantial social price.
Pathophysiology
The etiology of ADHD is complex and multifactorial featuring interplay between genetic and environmental influences. Studies suggest structural alterations in several brain cortical regions and their connections with the striatum and cerebellum [7-9].
Children with ADHD display impaired functional connectivity between anterior and posterior brain regions, such as hypo connectivity of the dorsal attention network with the default-mode network (responsible for mind-wandering). The latter may not be fully deactivated during goal-directed cognitive tasks resulting in symptoms of inattention.
On the contrary, hyper connectivity of the orbitofrontal cortex (involved in executive control) with reward-motivation regions had been documented [10]. Misalignment of these network connections may help explain mind-wandering, lack of motivation, impulse control and impaired executive functioning in ADHD.
Hyper or hypo connectivity are reflections of the genetic imbalance in catecholamine metabolism and response in the cerebral cortex. Either too little or too much catecholamine release in the prefrontal cortex weakens the cognitive control of behavior and attention [9]. Genome-wide association studies and candidate gene research suggest a plausible genetic hypothesis for ADHD as a mixture of dominant and recessive major genes, including dopamine receptor genes, serotonin transporter genes, glutamate receptors and others [11,12].
Psycho-stimulant medications comply with the dopamine deficit hypothesis and catecholamine theory which proposed that subtle abnormalities in the dopamine and norepinephrine neurotransmitter systems might account for the array of ADHD symptoms [13]. Recent studies conclude that the dopamine and norepinephrine transporter may not be altered in ADHD patients [13-15], however, psycho-stimulants such as Methylphenidate increase extracellular levels of dopamine and norepinephrine by inhibiting their transporters [16].
ADHD Management
When choosing a medication, the following should be taken into account: patient/guidance preference, comorbidities, adverse events and coverage (pharmacokinetic profile) [17].
Although treatment guidelines agree that stimulants should be the first choice, there are several differences among the recommendations. Recently, lisdexamfetamine (L-lysine- dextroamphetamine) a prodrug of dextroamphetamine offering efficacy at 14 hours post- dose in adults has been registered by the Israeli Ministry of Health [18]. Table 1 summarizes the main advantage and disadvantage of long-acting lisdexamfetamine compared to immediate release formulations.
| Advantages | Disadvantages |
|---|---|
| Lower risk of abuse | Offers less flexibility in time to initial effect |
| May enhance adherence | Flatter pharmacokinetic profile (MPH SR) |
| Less prone to having peak/trough effects | More expensive |
Table 1: Advantages and disadvantages of long-acting formulations compared to short-acting ones.
Purpose of the Study
Novel web-based pharmacovigilance analysis tools which provides disproportionality analysis for a wide range of drugs on the market, include the OpenVigil 2.1 and is freely accessible [19,20]. The OpenVigil 2.1 software provides intuitive user interfaces, powerful algorithms, and highly configurable output of findings, both individual reports and counts, in various output formats [19].
Our study is the first to make practical and effective use of a large post marketing database that documents reports worldwide and utilizes signal detection methods to compare psychiatric and nonpsychiatric adverse events for different treatments in ADHD. There are limited data on psychiatric events following drugs treatment of ADHD that were assessed in our study. Moreover, the safety of atomoxetine and lisdexamfetamine, the new generation of ADHD medications are scarcely reported.
Objectives
The primary objective of the study was to implement the most common methods of signal detection and assessment used in pharmacovigilance.
Specific objectives
1. To provide safety signals on psychiatric adverse events in newer ADHD medications; atomoxetine and lisdexamfetamine.
2. To provide a follow up for safety of senior medications, amphetamine and methylphenidate.
Psychiatric events were of special interest especially suicidal AEs, as well as insomnia and lack of appetite.
Methods
We analyzed adverse event cases documented in the OpenVigil 2.1 from mid-2003 until February 2020. The OpenVigil 2.1 is a novel web-based pharmacovigilance analysis tool which mainly uses the open FDA online interface of the Food and Drug Administration (FDA) to access U.S. American and international pharmacovigilance data from the Adverse Event Reporting System (AERS), the WHO Uppsala Monitoring Centre (international) and other countries [20]. Reports of adverse events are submitted by healthcare professionals (such as physicians, pharmacists, nurses and others), consumers (such as patients, family members, lawyers and others), and drug manufacturers and marketers. Drug manufacturers, distributors, and license holders, are legally bound to actively collect and report all adverse effects related to their products.
Individual Case Safety Reports
Individual case safety reports (ICSRs) defined by the Guideline of good pharmacovigilance practice (GVP) include the minimum data elements; an identifiable reporter, an identifiable patient, an adverse reaction and a suspect medicinal product [21].
Adverse drug reactions analyzed were in accordance with the World Health Organization (WHO) definition: “any response to a drug, which is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis, or therapy or modification of physiologic function” [22]. We expanded the definition to include reports on lack of therapeutic efficacy with or without reference to an associated adverse drug reaction.
Adverse Drug Reaction Coding and Search
The medical dictionary for regulatory activities (MedDRA browser English version 20.0) that was developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provided the related terms for adverse event of interest [23]. There are five levels to the MedDRA hierarchy, arranged from very specific to very general. The OpenVigil database was then searched by available lower level terms (most specific level- how an event may be reported in practice) and preferred terms (distinct medical descriptor of a symptom/sign) [24]. Lower level terms with a common preferred term were grouped as such: anger-aggression, depression-depressed mood, fatigue-somnolence, anxiety- tension-nervousness, excessive blinking-movement disorder-involuntary muscle contraction (Tics), suicidal behavior-suicidal ideation-suicidal thoughts.
To capture as many cases as possible, ADHD drugs were identified using the terms for the generic and brand names of these medications; Strattera (Atomoxetine), Vyvanse (Lisdexamfetamine), Adderal (Amphetamine), Methylphenidate (Ritalin, Ritalin LA, Ritalin SR).
Statistical Analysis
The frequency of adverse events by MedDRA term as a proportion of all adverse event reports for methylphenidate (and formulations), atomoxetine, lisdexamfetamine and amphetamine within each of the datasets was calculated.
Disproportionality Analysis
The proportional reporting ratio (PRR) and the reporting odds ratio (ROR) were used to quantitate the strength of the association between reported adverse events for different pharmacotherapies for ADHD. First, we compared the proportion of cases reporting adverse events with each ADHD drug to the proportion of cases reporting the event with all other medications. Second, for events that are highly related to the disease, the comparator was narrowed to other ADHD medications instead of all other drugs. The same pharmacovigilance database was used as a comparator to provide the events as required for these disproportionality analyses. An adverse event signal is detected for a PRR ⩾2.0 with an associated Yates corrected χ2 value of ⩾4.06 and for the ROR if the lower bound of the 95% two-sided confidence interval exceeds 1 [25,26]. All analyses were performed using SPSS Statistics (IBM® Corporation, summers, NY) version 20 [27].
Signals were compared to Methylphenidate. Since lisdexamfetamine is additionally prescribed for binge eating disorders a comparison for suicidal AEs was made with topiramate. To further account for any indication bias, we examined the signals of lisdexamfetamine before and after 2015, the year lisdexafetamine was approved for binge eating disorder. Data were also obtained from the EudraVigilance database and compared with the OpenVigil 2.1 database to examine reproducibility of some of our results.
Results
During the period we evaluated a total of 38,412 cases (19,380, 13,642, 3881 and 1509 cases reported for methylphenidate, atomoxetine, lisdexamfetamine, amphetamine, respectively). Nervous system and psychiatric adverse events, as well as drug ineffectiveness and growth retardation were of special interest in this study. Figure 4 shows the prevalence of adverse events for each drug. Apathy, tics and growth retardation carry the lowest frequency in the database. It is clear that drug ineffectiveness report frequency is almost equal in all medications. A peak was noticed for fatigue and somnolence with atomoxetine, yet a relatively low value is noticed for depressed mood.
Figure 4 shows no. of adverse events reported as a percentage of all adverse events reported for the drug of interest.
Atomoxetine, Amphetamine and Lisdexamfetamine are further analyzed to evaluate the strength of association of the event compared with Methylphenidate. Reports with a count >3 or those that possessed a relatively high frequency and substantial clinical impact interest us the most.
There was a significantly lower risk for depressed mood (ROR 0.026, 95%CI 0.016- 0.042) and tics (ROR 0.48, 95%CI 0.30- 0.76) reported with atomoxetine compared with methylphenidate (Table 3). But a higher risk was found for anxiety, suicidal AEs, apathy and aggression.
Lisdexamfetamine did not seem to decrease the risk of psychiatric events. On the contrary, about a twofold risk was found for anxiety, aggression, suicidal AEs and tics with lisdexamfetamine. In addition a statistically significant, weak increased risk of depressed mood was noticed.
Amphetamine does not seem to differ than Methylphenidate in most of the psychiatric events. However, a weak lower risk of suicidal AEs was noticed (ROR 0.68, 95%CI 0.48- 0.97).
Further adverse events were analyzed in Table 4. Atomoxetine and lisdexamfetamine carry a greater risk for reports on lack of appetite; wheras amphetamine lowers the risk of lack of appetite (ROR 0.43, 95%CI 0.28-0.67). Atomoxetine has a significantly greater risk of fatigue and somnolence, whereas lisdexamfetamine does not seem to differ than methylphenidate. Lisdexamfetamine possesses a 3 fold risk for insomnia reports; however, atomoxetine lowers the risk of insomnia. Amphetamine does not seem to differ from Methylphenidate. There was a significantly greater risk for headaches with Atomoxetine.
Table 4 shows the Reporting Odds Ratio (ROR) calculated as odds for Event of interest-Drug of interest over the odds for Event of interest-Methylphenidate and the 95% CI.
The signals described as ROR for depressed mood, insomnia, tics, anger/aggression and suicidal behavior/ideation were compared (In Figures). Two methods of disproportionality analysis were investigated; one included all drugs in the database and the other included ADHD drugs. The signals with Proportional Reporting Ratio (PRR) are accounted for separately in the supplementary material (Table 2).
| PRR | 95%CI | ROR | 95%CI | X2 | ||
|---|---|---|---|---|---|---|
| Atomoxetine | 0.026 | 0.016 to 0.042 | 0.025 | 0.015 to 0.040 | 598, p-value < 0.00001 | |
| Depressed mood/ | Lisdexamfetamine | 1.9 | 1.67 to 2.16 | 1.95 | 1.67 to 2.28 | 72.8, p-value< 0.00001 |
| Depression | Amphetamine | 1.65 | 1.31 to 2.07 | 1.68 | 1.21 to 2.14 | 17.6, p-value= 0.000027 |
| Methylphenidate | 2.9 | 2.80 to 3.00 | 2.99 | 2.61 to 3.41 | 276.7, p-value< 0.00001 | |
| Atomoxetine | 1.48 | 1.40 to 1.56 | 1.5 | 1.36 to 1.66 | 61.8, p-value < 0.00001 | |
| Suicidal behaviour/ | Lisdexamfetamine | 1.24 | 1.08 to 1.42 | 1.25 | 1.05 to 1.47 | 8.20, p-value 0.004191 |
| ideation | Amphetamine | 0.55 | 0.39 to 0.77 | 0.54 | 0.38 to 0.76 | 12.9, p-value 0.000327 |
| Methylphenidate | 0.67 | 0.63 to 0.72 | 0.66 | 0.60 to 0.73 | 61.7, p-value is < 0.00001 | |
| Atomoxetine | 1.05 | 0.99 to 1.12 | 1.06 | 0.95 to 1.16 | 1.10, p-value 0.294656 | |
| Lisdexamfetamine | 2.09 | 1.86 to 2.34 | 2.36 | 2.08 to 2.67 | 195.5, p-value is < 0.00001 | |
| Insomnia | Amphetamine | 0.87 | 0.67 to 1.12 | 0.86 | 0.66 to 1.13 | 1.14, p-value = .284695. |
| Methylphenidate | 0.65 | 0.61 to 0.69 | 0.63 | 0.57 to 0.70 | 81.3, p-value is < 0.00001 | |
| Tics | Atomoxetine | 0.44 | 0.31 to 0.63 | 0.44 | 0.28 to 0.68 | 13.8, p-value 0.000204 |
| Lisdexamfetamine | 1.94 | 1.33 to 2.84 | 1.94 | 1.22 to 3.09 | 8.23, p-value= 0.00412 | |
| Amphetamine | 1.25 | 0.57 to 2.74 | 1.25 | 0.55 to 2.86 | 0.30, p-value= 0.58715 | |
| Methylphenidate | 1.34 | 1.15 to 1.56 | 1.34 | 0.94 to 1.92 | 2.61, p-value= 0.10624 | |
| Atomoxetine | 1.37 | 1.30 to 1.44 | 1.4 | 1.28 to 1.53 | 55.7, p-value< 0.00001 | |
| Lisdexamfetamine | 1.44 | 1.29 to 1.61 | 1.48 | 1.30 to 1.68 | 37.2, p-value < 0.00001 | |
| Anger/Agression | Amphetamine | 0.53 | 0.39 to 0.71 | 0.51 | 0.38 to 0.69 | 20.0, p-value < 0.00001 |
| Methylphenidate | 0.68 | 0.65 to 0.72 | 0.66 | 0.61 to 0.72 | 82.5, p-value < 0.00001 | |
| Atomoxetine | 1.12 | 1.05 to 1.19 | 1.12 | 1.01 to 1.25 | 4.83, p-value= 0.02808 | |
| Lisdexamfetamine | 1.32 | 1.16 to 1.51 | 1.34 | 1.15 to 1.56 | 14.5, p-value= 0.00014 | |
| Anxiety/Nervousness | Amphetamine | 1.06 | 0.83 to 1.35 | 1.06 | 0.82 to 1.37 | 0.20, p-value = 0.65548 |
| Methylphenidate | 0.8 | 0.75 to 0.84 | 0.79 | 0.71 to 0.87 | 20.9, p-value < 0.00001 | |
Table 2: Signal detection analysis of methylphenidate, amphetamine, atomoxetine and lisdexamfetamine vs other ADHD drugs comparator.
| Reference = MPH | ROR | 95%CI | P-value | |
|---|---|---|---|---|
| Atomoxetine | 0.03 | 0.016 - 0.042 | < 0.0001 | |
| Depressed mood | Lisdexamfetamine | 1.19 | 1.01 - 1.39 | 0.034 |
| Amphetamine | 1.09 | 0.85 - 1.39 | 0.5 | |
| Atomoxetine | 1.22 | 1.09 - 1.36 | 0.0005 | |
| Anxiety | Lisdexamfetamine | 1.47 | 1.25 - 1.72 | < 0.0001 |
| Amphetamine | 1.2 | 0.92 - 1.55 | 0.18 | |
| Atomoxetine | 1.53 | 1.39 - 1.68 | < 0.0001 | |
| Aggression | Lisdexamfetamine | 1.76 | 1.54 - 2.02 | < 0.0001 |
| Amphetamine | 0.65 | 0.48 - 0.88 | 0.0054 | |
| Atomoxetine | 1.6 | 1.43 - 1.78 | < 0.0001 | |
| Suicidal AEs | Lisdexamfetamine | 1.53 | 1.30 - 1.81 | < 0.0001 |
| Amphetamine | 0.68 | 0.48 - 0.97 | 0.034 | |
| Atomoxetine | 0.48 | 0.30 - 0.76 | 0.0019 | |
| Tics | Lisdexamfetamine | 1.55 | 0.96 - 2.50 | 0.073 |
| Amphetamine | 1.09 | 0.47 - 2.50 | 0.85 | |
| Atomoxetine | 1.52 | 1.11 - 2.08 | 0.0095 | |
| Apathy | Lisdexamfetamine | 0.93 | 0.53 - 1.65 | 0.81 |
| Amphetamine | 0.34 | 0.084 - 1.39 | 0.13 |
Table 3: Measurement of Psychiatric events associated with Atomoxetine, Lisdexamfetamine and Amphetamine. Results presented as ROR with Methylphenidate the comparator drug.
| Reference = MPH | ROR | 95%CI | P-value | |
|---|---|---|---|---|
| Atomoxetine | 1.21 | 1.07 - 1.36 | 0.0024 | |
| Lack of appetite | Lisdexamfetamine | 1.66 | 1.40 - 1.96 | < 0.0001 |
| Amphetamine | 0.43 | 0.28 - 0.67 | 0.0002 | |
| Atomoxetine | 3.04 | 2.79 - 3.32 | < 0.0001 | |
| Fatigue/Somnolence | Lisdexamfetamine | 1.16 | 0.99 - 1.36 | 0.065 |
| Amphetamine | 1.55 | 1.25 - 1.92 | 0.0001 | |
| Atomoxetine | 1.33 | 1.19 - 1.48 | < 0.0001 | |
| Insomnia | Lisdexamfetamine | 2.68 | 2.34 - 3.07 | < 0.0001 |
| Amphetamine | 1.11 | 0.85 - 1.47 | 0.4372 |
Table 4: Measurement of other events associated with Atomoxetine, Lisdexamfetamine and Amphetamine. Results presented as ROR with Methylphenidate the comparator drug.
The signal for depressed mood disappeared in atomoxetine. However, the signal significantly increased by up to three folds with methylphenidate compared to other ADHD medications (Figure 1).
Regarding insomnia the signal for atomoxetine, amphetamine and methylphenidate disappeared after narrowing the comparator, however, the signal for lisdexamfetamine decreased, still holding a twofold risk compared to other ADHD medications (Figure 2).
The signal for tics was not affected with lisdexamfetamine, holding a twofold risk after narrowing the comparator. The signal for methylphenidate disappeared when compared to other ADHD medications (Figure 3).
In the case of aggression the signal for amphetamine and methylphenidate disappeared. However, for atomoxetine and lisdexamfetamine, the signal remained with a lower intensity; ROR was reduced from 11 fold to a 1.5 fold risk (Figure 4).
Atomoxetine and lisdexamfetamine had the highest signals for suicidal behavior. The signal remained after narrowing the comparator, with both drugs carrying a twofold risk for this event. On the contrary, signals for amphetamine and methylphenidate disappeared when compared to other ADHD medications. According to our data, Amphetamine seems to have the lowest risk of suicidal behavior compared to other ADHD drugs (Figure 5).
Figure 1-5 shows the Reporting Odds Ratio (ROR) calculated as odds for Event of interest- Drug of interest over the odds for Event of interest-All drugs in the database, compared with the ROR calculated as odds for Event of interest-Drug of interest over the odds for Event of interest- Other ADHD drugs. This allows us to account for ADHD symptoms as possible confounders. The 95% CI and PRR values are provided separately in the supplementary material (Table 2).
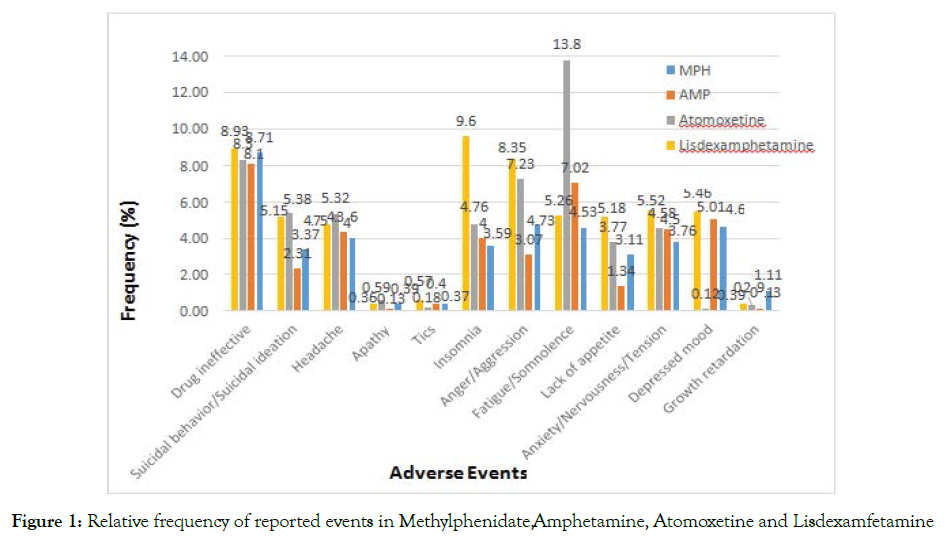
Figure 1. Relative frequency of reported events in Methylphenidate, Amphetamine, Atomoxetine and Lisdexamfetamine
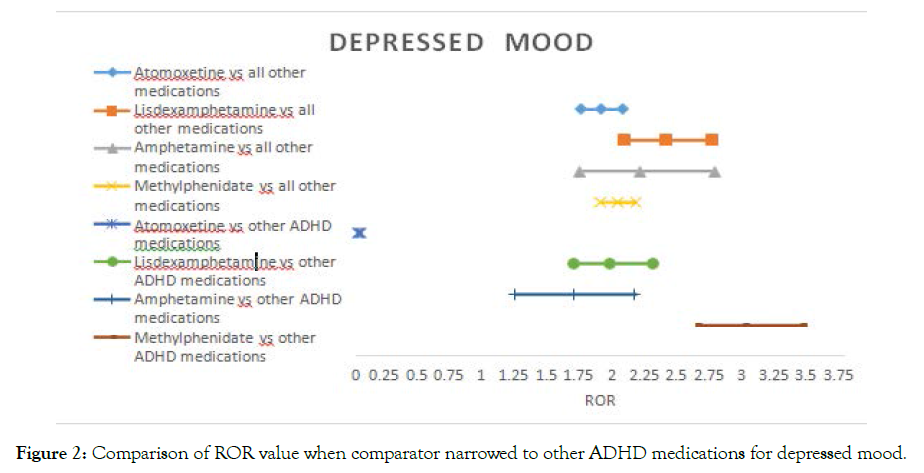
Figure 2. Comparison of ROR value when comparator narrowed to other ADHD medications for depressed mood.
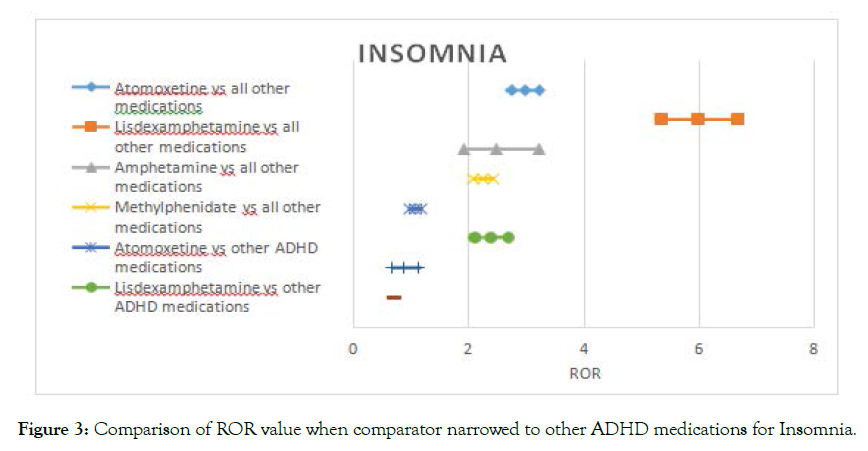
Figure 3. Comparison of ROR value when comparator narrowed to other ADHD medications for Insomnia.
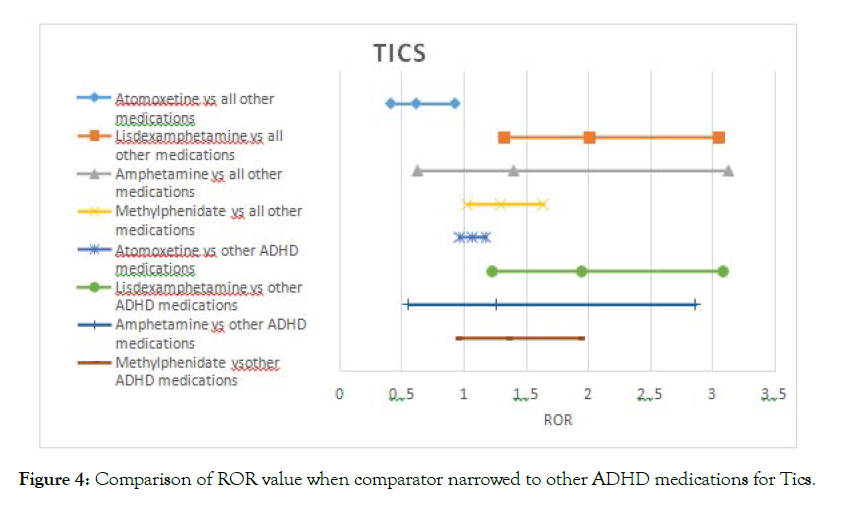
Figure 4. Comparison of ROR value when comparator narrowed to other ADHD medications for Tics.
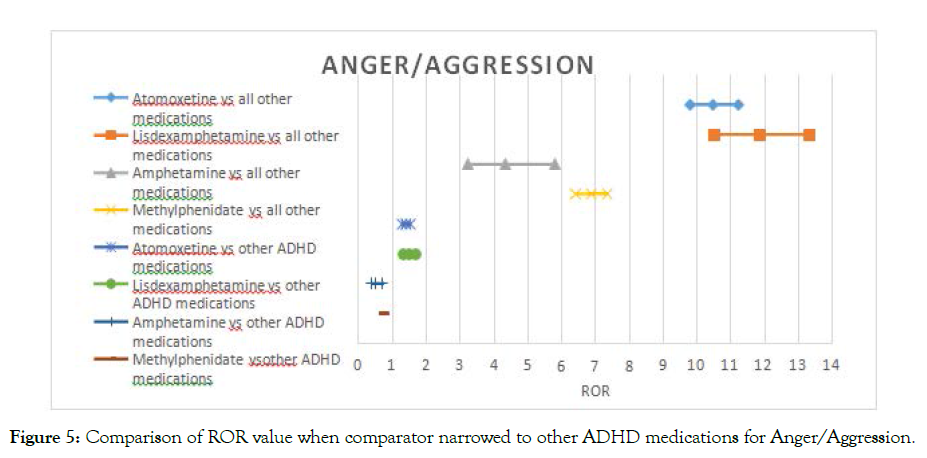
Figure 5. Comparison of ROR value when comparator narrowed to other ADHD medications for Anger/Aggression.
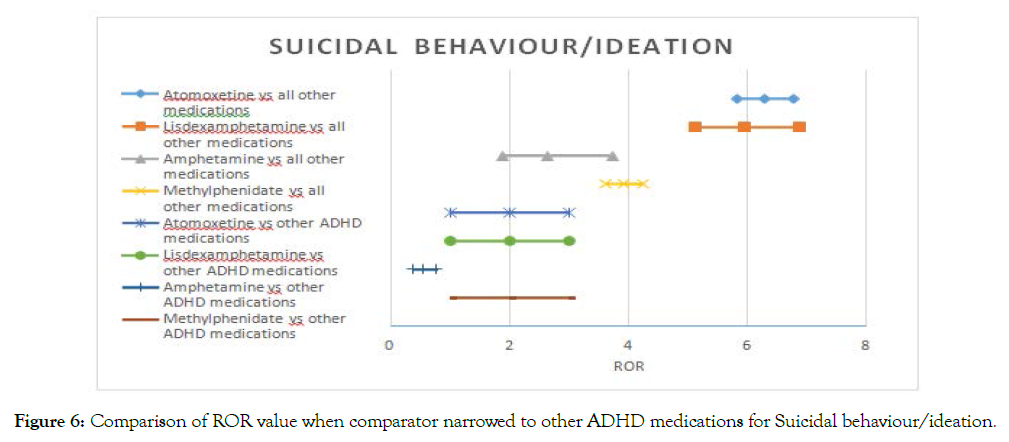
Figure 6. Comparison of ROR value when comparator narrowed to other ADHD medications for Suicidal behaviour/ideation.
Table 5 shows the Reporting Odds Ratio (ROR) calculated for lisdexamfetamine compared with methylphenidate and topiramate from the Openvigil and Eudravigilance database.
| Ref.=MPH | Ref.=Topiramate | |||||
|---|---|---|---|---|---|---|
| ROR | 95%CI | P-value | ROR | 95%CI | P-value | |
| Openvigil | 1.53 | 1.30-1.81 | < 0.0001 | 1.77 | 1.57-1.99 | < 0.0001 |
| Eudravigilance | 2.08 | 1.78-2.43 | < 0.0001 | 2.8 | 2.38-3.30 | < 0.0001 |
Table 5: Lisdexamfetamine Suicidal AEs compared with Topiramate and Methylphenidate from the EudraVigilance and Openvigil.
Table 6 shows the Reporting Odds Ratio (ROR) calculated as odds for Event of interest-Drug of interest over the odds for Event of interest-All drugs in the database before and after lisdexamfetamine was indicated for binge eating disorder.
| ROR up to 2015 | ROR after 2015 |
|---|---|
| 5.84 95%CI (5.07-6.73) P-value<0.0001 | 6.995 95%CI (6.12-8.00) P-value<0.0001 |
Table 6: ROR for Lisdexamfetamine Before and After 2015.
Discussion
The main findings of the study to be discussed in this section include a significant reduction in depressed mood and tics for atomexetine. We also found that the new generation amphetamine, lisdexamfetamine does not reduce the risk of psychiatric events; on the contrary, an increased reporting risk for tics and suicidal adverse events were noted.
Adverse Events
Psychiatric events, such as depressed mood may occur at any time of the prescribed dose [29]. The listed events in table 3 are also considered rebound symptoms of ADHD and therefore it is not always clear whether psychiatric adverse events, such as depressed mood, irritability, nervousness and fatigue are pure adverse events [28, 29].
Atomoxetine has shown a considerably lower risk of depressed mood compared to methylphenidate. The signal has completely disappeared when the comparator was narrowed to other ADHD drugs. Suggesting that depressed mood may be a symptom of ADHD rather than an adverse event. Improvement in depressive symptoms in ADHD comorbid patients has been documented in the literature with atomoxetine, approving that depression is not a pure adverse event of this drug. A 2017 systematic review of RCTs was not able to show any antidepressant effects for atomoxetine and explains improvement of depressive symptoms simply as an improvement in ADHD [30]. Yet supposing this was undoubtfully true, we should have seen a similar behavior with the data for methylphenidate, amphetamine and lisdexamfetamine, which hold a greater effect size in treating ADHD than atomoxetine.
Contrary to this, the ROR did not significantly change for these drugs, posing a risk for depressive symptoms. More evidence is required to acknowledge any antidepressant effects for atomoxetine, nevertheless, ADHD guidelines should consider recommending atomoxetine for ADHD patients with comorbid depression.
ADHD and tic disorders frequently coexist: approximately 20 percent of children with ADHD develop chronic tic disorders and approximately 50 percent of children with chronic tics or Tourette syndrome have comorbid ADHD [31,32]. Our results show that atomoxetine has significantly lowered the risk for tic disorder compared with methylphenidate, whereas lisdexamfetamine and amphetamine seem not to differ than methylphenidate. According to our study lisdexamfetamine has about a two-fold risk for tic disorders compared with other ADHD medications. No data in the literature has been documented for lisdexamfetamine and tics, thus further studies are required to translate this signal into clinical practice.
Supported by our study, the NICE and the European ADHD Guidelines Group concluded that stimulants could worsen comorbid tics, whereas atomoxetine significantly improves them [17,33]. These findings are also supported by the fact that increased dopamine activity in the basal ganglia underlies the pathogenesis of tics, however atomoxetine has negligible effects on dopamine [34]. We strongly support recommendations for atomoxetine in comorbid tic disorders.
Our study shows an increased risk of insomnia with atomoxetine and that amphetamine does not differ than methylphenidate. However, a network meta-analaysis, suggests that ADHD medications do not appear to adversely affect sleep patterns and possibly normalizes them in patients with ADHD [35]. It is well known that sleep problems may be primarily related to ADHD severity and comorbidity and not soely an adverse event of ADHD medications [36]. Hence for atomoxetine, amphetamine and methylphenidate, the comparator was narrowed to other ADHD medications resulting in an unsignificant ROR. Thus supporting the literature for that insomnia may be a symptom of the disease or a rebound effect rather than an adverse event.
Nevertheless, the results remained significant for lisdexamfetaine, supporting our hypothesis that long acting formulations possess the patient with an increased risk of insomnia. Also observed as a strong signal for lisdexamfetamine and a peak of higher frequency of the AE. Currently, no clinical trials are documented for lisdexamfetamine and insomnia.
Impulsivity is a core symptom of ADHD; moreover it is known to correlate with suicidal behavior [37]. Two-thirds of ADHD cases have at least one comorbid psychiatric disorders, which most often includes substance use or major depressive episode contributing further to this issue [37].
Stimulants were thought to increase the risk of suicide, however, a longitudinal study from the BMJ including 37 936 patients with ADHD, found no evidence for an overall increased rate of suicide related events associated with the use of stimulant or non-stimulant drug treatment for ADHD [38]. To date, stimulant medication was associated with a reduced risk of suicide attempts in patients with ADHD, and non-stimulant medication is unlikely to increase the risk of suicide attempts. However, analysis is being grouped into stimulants and non-stimulants as a whole. Further evidence is required for lisdexamfetamine individually and in head to head trials. From Our study there was an increased risk of suicidal adverse events with atomoxetine, lisdexamfetamine, amphetamine and methylphenidate when compared to other drugs in the database. However, when the comparator is narrowed to other ADHD medications, the reporting risk becomes insignificant for amphetamine and methylphenidate. Therefore, suggesting that comorbid ADHD disorders could be the main interplaying factors. However, this is not the case with atomoxetine and lisdexamfetamine, which both carry about a 1.5 fold risk of suicidal AEs compared to other ADHD medications.
In post-hoc analyses of clinical trial data, atomoxetine has been associated with an increased risk of suicidal ideation in children and adolescents [39], also supported by the FDA boxed warning and additional warning statements regarding an increased risk of suicidal thinking in children and adolescents are being treated with this drug [40]. Since no documentation and Black Box Warning was found in the literature and no regulatory information or black box warnings are documented for lisdexamfetamine, we carefully examined this signal through the EudraVigilance database, which illustrated a similar result [41]. Additionally, in comparison with methylphenidate a twofold reporting odds ratio has been demonstrated for suicidal AEs. We are aware that since 2015 lisdexamfetamine was approved for binge eating disorder (BED) [42]. Since binge eating may induce suicidal ideation and suicidal behavior, to overcome indication bias we examined the ROR before and after 2015 [43]. True the expansion of lisdexamfetamine use in BED has increased the ROR; however, the ROR was about 6 fold higher than any drug in the database prior to 2015. Thus pointing out it was likely an adverse event regardless of the drug being indicated for BED. We further compared lisdexamfetamine with topiramate, the latter approved by the Food & Drug Administration for the treatment of epilepsy and the prevention of migraine and is used as an off-label medication for BED [44]. Lisdexamfetamine illustrated a twofold reporting risk of suicidal AEs that should be looked at very carefully.
Limitations
The data for these analyses was majorly collected from spontaneous reports, prone to significant underreporting, mainly because adverse event reporting is a voluntary action. Moreover, filling report forms maybe time consuming both to the patient and health care specialist and at times the reporter may not attribute the event to the drug. The adverse events described are mostly subjective, no scores or grading scales were used in their description. It was challenging to interpratate description of psychiatric adverse events. To overcome this problem we grouped lower level terms, which may be interchangeable terms for patients, in to one acceptable term (MedDRA preferred term).
Though, medications were not adjusted for dose, the issue of dose response remains controversial in ADHD. Methylphenidate has a wide inter-individual variability in dose response illustrated in large clinical trials [45].
Since we had no access to the patient’s full medical record we could not test for comorbidities, drug interactions and compliance. Further research is required for these issues.
PRR and ROR statistics tend to overestimate adverse events rarely reported by other drugs, as seen with tics in our database. Another limitation of the PRR is that striking signals reduce the magnitude of the PRR for other signals due to denominator inflation.
REFERENCES
- Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug safety. 2013 Feb;36(2):75-81.
- Web WH. The Importance of Pharmacovigilance-Safety Monitoring of Medicinal Products 2002. Essential Medicines and Health Products Information Portal A World Health Organization resource.
- Thobeli K. A literature review on pharmacovigilance systems in off-label use of medicines.
- Khalili H, Mohebbi N, Hendoiee N, Keshtkar AA, Dashti-Khavidaki S. Improvement of knowledge, attitude and perception of healthcare workers about ADR, a pre-and post-clinical pharmacists' interventional study. BMJ open. 2012 Jan 1;2(1).
- Gupta P, Udupa A. Adverse drug reaction reporting and pharmacovigilance: Knowledge, attitudes and perceptions amongst resident doctors. Journal of pharmaceutical sciences and research. 2011 Feb 1;3(2):1064.
- Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. British journal of clinical pharmacology. 2017 Feb;83(2):227-46.
- Agu KA, Oparah AC. Adverse drug reactions to antiretroviral therapy: Results from spontaneous reporting system in Nigeria. Perspectives in clinical research. 2013 Apr;4(2):117.
- NAFDAC. NAFDAC Good Pharmacovigilance Practice Guidelines 2016. World Health Organisation. 2016.
- Olowofela A, Fourrier-Réglat A, Isah AO. Pharmacovigilance in Nigeria: an overview. Pharmaceutical Medicine. 2016 Apr 1;30(2):87-94.
- Opadeyi AO, Fourrier-Réglat A, Isah AO. Assessment of the state of pharmacovigilance in the South-South zone of Nigeria using WHO pharmacovigilance indicators. BMC Pharmacology and Toxicology. 2018 Dec;19(1):1-8.
- Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert review of clinical pharmacology. 2015 Jul 4;8(4):449-60.
- Chopra D, Wardhan N, Rehan HS. Knowledge, attitude and practices associated with adverse drug reaction reporting amongst doctors in a teaching hospital. International Journal of Risk & Safety in Medicine. 2011 Jan 1;23(4):227-32.
- Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. African health sciences. 2015;15(4):1308-17.
- Ezeuko, Amaka Y "Adverse drug reaction reporting by different categories of healthcare workers in Nnewi, Nigeria: awareness, knowledge and attitudes." Journal of Advances in Medicine and Medical Research. 2015: 932-941
Citation: Aboukaoud M (2021) Safety Comparison of Atomextine and Lisdexamfetamine: Using Pharmacovigilance Database and Tools J. Pharamacovigil. 9:315. doi-10.35248/2329-6887.21.9.315.
Copyright: © 2021 Aboukaoud M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

