Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Short Communication - (2022) Volume 13, Issue 2
Safety and Immunogenicity of Mix-Match of Vaccines- Covishield and Covaxin: A Pilot Study
Bhanu Prakash Reddy Attunuru1, Podduturi Naveenchander Reddy1, Sasikala Mitnala2*, Deepika Gujjarlapudi3, Sadhana Yelamanchili Veturi4 and Nageshwar Reddy Duvvur52Department of Internal Medicine, Asian Healthcare Foundation, AIG Hospitals, Mindspace Rd, Gachibowli, Hyderabad, India
3Department of Biochemistry, AIG Hospitals, Mindspace Rd, Gachibowli, Hyderabad, India
4Department of Microbiology, AIG Hospitals, Mindspace Rd, Gachibowli, Hyderabad, India
5Department of Hepatology and Gastroenterology, AIG Hospitals, Mindspace Rd, Gachibowli, Hyderabad, India
Received: 15-Feb-2022, Manuscript No. JVV-22-15647; Editor assigned: 17-Feb-2022, Pre QC No. JVV-22-15647 (PQ); Reviewed: 07-Mar-2022, QC No. JVV-22-15647; Revised: 14-Mar-2022, Manuscript No. JVV-22-15647 (R); Published: 21-Mar-2022, DOI: 10.35248/2157-7560.21.13.477
Abstract
This single-center prospective observational study was conducted to assess the safety and immunogenicity of combination vaccines AstraZeneca’s ChAdOx1-nCov-19 (Covishield in India) and inactivated whole virion BBV152 (Covaxin). A total of 330 unvaccinated healthy volunteers were screened for SARS-COV-2 seropositivity. RT PCR tests were conducted for seronegative volunteers (n=44). They were randomly assigned to four groups and given either same or mixed vaccines at an interval of 4 weeks between the two doses. Mix and match of vaccines did not evoke any adverse events. Combination of vaccines elicited similar immune responses in 4 groups. They were further studied dividing into homologous and heterologous vaccine groups. In Conclusion, Combination vaccines are safe and immunogenic and heterologous vaccines elicit better immunogenic response.
Keywords
Immunogenicity; Vaccine; SARS-COV-2; Heterologous vaccines; Covishield; Covaxin
Introduction
Vaccination has been shown to be protective against severe COVID-19 disease by various studies [1-4]. However, the vaccine efficacy was demonstrated to be less effective against the emerging variants of SARS-CoV-2 [5]. Recent studies by Kant R et al., Com- COV study, Combi Vacs trial, ChAdOx1 nCoV-19, and BNT162b2 have all demonstrated greater immunogenic response and increased protective efficacy with combination of vaccines [6-9]. All these studies mainly focussed on Pfizer (BNT162b2), Moderna (mRNA- 1273) and Astra Zeneneca (ChAdOx1-nCov-19). We aimed to assess the safety and immunogenicity of combination of AstraZeneca’s ChAdOx1-nCov-19 (Covishield in India) and inactivated whole virion BBV152 (Covaxin) with 4 weeks’ interval. This study was conducted to reduce various difficulties in vaccinating populations across the world specially to mitigate vaccine deficit.
Materials and Methods
This is a single-centre prospective observational study conducted at AIG Hospitals, Hyderabad, India. The study protocol was approved by the Institutional ethics committee (AIG/IEC-CT 51/08.2021-01). We recruited 330 healthy volunteers and screened for seronegativity for SARS-CoV-2 antibodies (IgM and IgG by chemiluminiscence; Roche Cobas e 601) and SARS- CoV2 negative report by RT-PCR. 3 ml of blood was drawn for screening SARS-COV2 antibodies. Nasopharyngeal swabs were collected from individuals who were seronegative (n=44) for SARS-CoV RT-PCR employing Taq path kits (Thermoscientific, USA). All the volunteers have provided written informed consent. Eligible participants (n=44) were divided randomly into four groups (Figure 1). Group1: Covishield-Covishield group (homologous), Group 2: Covaxin-Covaxin group (homologous), Group 3: Covishield- Covaxin group (heterologous), Group 4: Covaxin-Covishield group (heterologous). Healthy volunteers were given 1st dose of Covishield (0.5 ml of intramuscular in deltoid) in group 1 and group 3 while volunteers in group 2 and group 4 received Covaxin (0.5 ml of intramuscular in deltoid) as 1st dose. After 4 weeks of 1st dose, groups 1 and 4 received Covishield as 2nd dose while groups 2 and 3 received Covaxin as 2nd dose respectively. Further, the volunteers were divided into homologous (similar vaccines for two doses; Group A) and heterologous (mix-match of vaccines for two doses; Group B) vaccination groups and their antibody titers were evaluated to assess the immunogenic response. S1/S2 neutralizing antibodies titers and RBD specific antibody titers were evaluated at day 28 after the 1st dose and day 15 after the second dose (45 days of first dose) of vaccination; Sera were tested for S1/S2 antibody titers using chemiluminiscence assays (automated Diasorin Liaison XL, Italy) and electro- chemiluminescent assays for RBD specific antibody titer (Cobas e 601, Roche Diagnostics, Basel, Switzerland). They were carefully monitored for any adverse events for 60 days. The data including demographics were entered in MS-excel and analyzed using SPSS version 23. Categorical variables were expressed in percentages (frequency distribution) and continuous variables as mean and Standard Deviation (SD), median and range wherever required. Chi- square tests, student t-test and ANOVA were used appropriately.
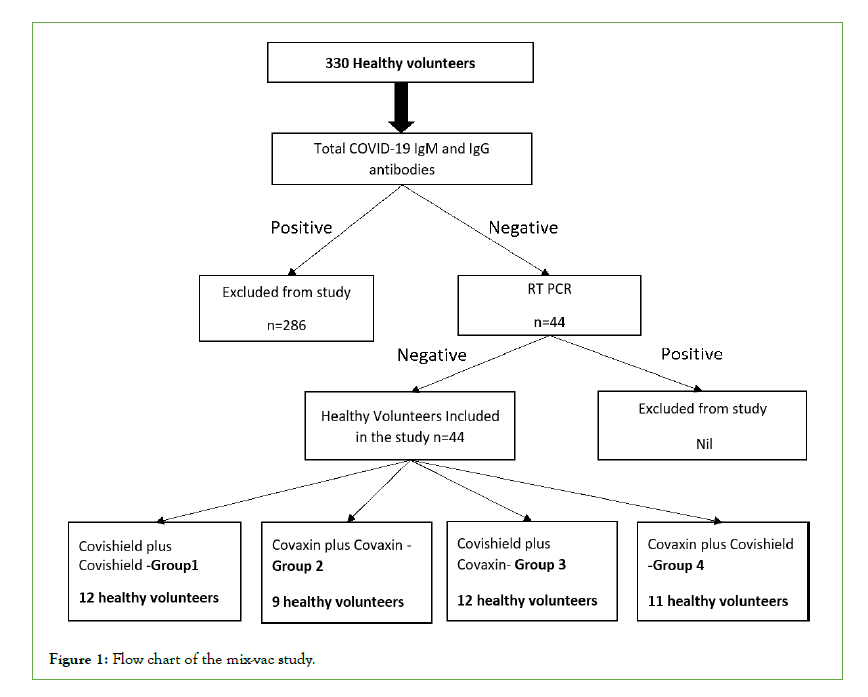
Figure 1: Flow chart of the mix-vac study.
Results
A total of 44 participants out of 330 healthy volunteers were eligible for mix and match vaccine study (mix–vac study). Among 44 volunteers, 21 received the homologous vaccines and 23 received the heterologous vaccines. The mean age in Covishield homologous vaccine group was 34.83 ± 7.58 years, and Covaxin homologous vaccine group was 31.33 ± 5.20 years, Covishield followed by Covaxin heterologous group is 31.58 ± 5.32 years and Covaxin followed by Covishield heterologous group was 32.27 ± 5.26 years with no significant difference (p=0.49). There is no significant difference between the groups with respect to comorbidities like hypertension (Table 1) and none of them had diabetes or other comorbidities. The antibody titers (median) of S1/S2-IgG and RBD specific IgG 15 days after 2nd dose of vaccination in Covishield homologous group is 841.5 (24.0-3985)AU/ml and 9823.5 (858-42800 )IU/ml, Covaxin homologous group is 68.4 (3.8-1850 )AU/ml and 951 (0.4- 13710 )IU/ml, Covishield followed by Covaxin heterologous group is 1195 (5.21-3981) AU/ml and 11554.5 (10.8- 30740) IU/ml and Covaxin followed by Covishield heterologous group is 1250 (12.8- 4225)AU/ml and 3247 (0.75-23290 )IU/ml respectively. There is no significant difference (p=0.08) in antibody titers between the groups. The volunteers experienced fever, injection site pain, and mild headache in all the groups with no significant difference between the groups (Table 1). There are no major adverse events noted in the mix-match vaccine groups.
| Both Covishield Group -1 N=12 | Both Covaxin Group -2 N=9 | First Covishield then Covaxin Group -3 N=12 |
First Covaxin then Covishield Group -4 N=11 |
P-value* | |
|---|---|---|---|---|---|
| Age | 34.83 ± 7.58 | 31.33 ± 5.20 | 31.58 ± 5.32 | 32.27 ± 5.26 | 0.49 |
| 28th Day after 1st dose S1/S2-IgG Antibodies | 605 (48.4- 3330) AU/ml |
112 (37.6- 1450) AU/ml |
633(8.68-3981) AU/ml |
109 (5.24-3970) AU/ml |
0.17 |
| 28th Day after 1st dose RBD-IgG Antibodies | 5242 (96.5- 13370) IU/ml |
843(0.4-19320) IU/ml |
. 6254.5(71.3- 23290) IU/ml |
747(0.75-41340) IU/ml |
0.43 |
| 15th Day after 2nd dose S1/S2- IgG Antibodies | 841.5 (24.0- 3985) AU/ml |
68.4 (3.8-1850) AU/ml |
1195(5.21-3981) AU/ml |
1250 (12.8-4225) AU/ml |
0.08 |
| 15th Day after 2nd dose RBD specific IgG Antibodies | 9823.5 (858- 42800) IU/ml |
951(0.4-13710) IU/ml |
11554.5(10.8- 30740) IU/ml |
3247(0.75- 23290) IU/ml |
0.08 |
| Smoking (Yes) | 2 (16.67%) | 1 (11.11%) | 1 (8.33%) | 2 (18.18%) | 0.89 |
| Alcohol intake | 2 (16.67%) | 3 (33.33%) | 2 (16.67%) | 5 (45.45%) | 0.33 |
| Comorbidities (Hypertension) | 2 (16.67%) | 4 (44.44%) | 2 (16.67%) | 6 (54.54%) | 0.12 |
| 1st Visit dose 1 | |||||
| Fever (yes) | 4 (33.33%) | 3 33.33%) | 4 (33.33%) | 4 (36.36%) | 0.99 |
| Headache (yes) | 5 (41.67%) | 4 (44.44%) | 7 (58.33%) | 5 (45.45%) | 0.85 |
| Pain at site (Yes) | 9 (75.00%) | 5 (55.55%) | 3 (25.00%) | 6 (54.54%) | 0.10 |
| 2nd Visit dose 2 | |||||
| Fever (Yes) | 2 (16.67%) | 2 (22.22%) | 1 (8.33%) | 4 (36.36%) | 0.40 |
| Headache (Yes) | 8 (66.67%) | 4 (44.44%) | 8 (66.67%) | 5 (45.45%) | 0.55 |
| Pain at site (Yes) | 5 (41.67%) | 5 (55.55%) | 6 (50.00%) | 4 (36.36%) | 0.82 |
Note: *Chi-square test and One-Way ANOVA were used.
Table 1: Demographic and clinical profile of participant’s mix-vac study.
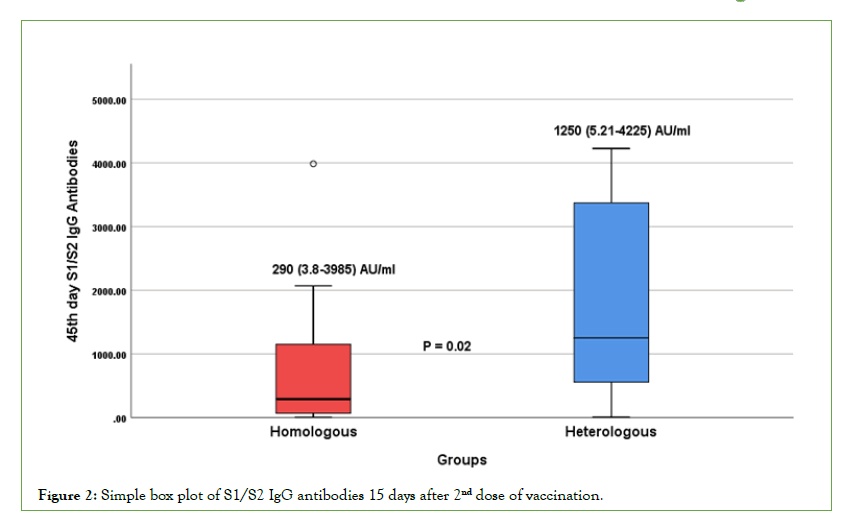
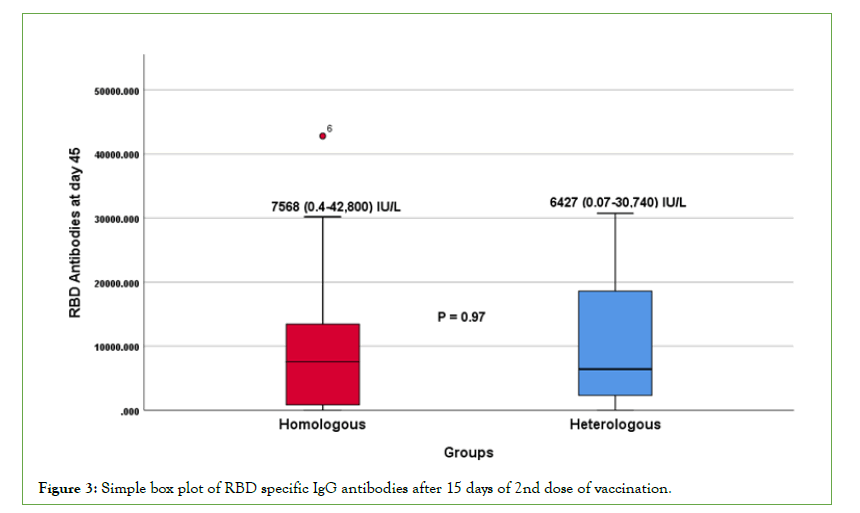
The data were then analyzed between homologous (group-A) and heterologous groups (group-B). The mean age in homologous vaccine group was 33.3+6.75 and heterologous vaccine group was 31.91+5.18 with no significant difference (p=0.44). The median antibody titers of S1/S2-IgG and RBD specific IgG at 15 days after 2nd dose of vaccine in homologous groups is 290 (3.8- 3985) AU/ml and 7568 (0.4-42800) IU/ml, and heterologous group is 1250(5.21-4225) AU/ml and 6427(0.075-30740) IU/ ml respectively. A significant difference (p=0.02) in S1/S2-IgG antibody titers was observed after 15 days of 2nd dose vaccination between homologous and heterologous groups, while there was no significant difference (p=0.97) in RBD specific-IgG antibody titers between the groups (Table 2 and Figures 2 and 3).
| Homologous vaccine group. (n=21) Group A | Heterologous vaccine group (n=23) Group B | P-value* | |
|---|---|---|---|
| Age (years) | 33.3 + 6.75 | 31.91 + 5.18 | 0.44 |
| 28th Day after 1st dose S1/S2-IgG Antibodies | 181 (37.6-3330) AU/ml |
373 (5.24-3981) AU/ml |
0.272 |
| 28th Day after 1st dose RBD –IgG Antibodies | 1769 (0.4-19320) IU/ml |
3383 (0.075-41340) IU/ml |
0.111 |
| 15th Day after 2nd dose S1/S2- IgG Antibodies | 290(3.8-3985) AU/ml |
1250 (5.21-4225) AU/ml |
0.022 |
| 15th Day after 2nd dose RBD- IgG Antibodies | 7568 (0.4-42800) IU/ml |
6427 (0.075-30740) IU/ml |
0.976 |
Note: *Student paired t-test
Table 2: Simple box plot of S1/S2 IgG antibodies 15 days after 2nd dose of vaccination.
Figure 2: Simple box plot of S1/S2 IgG antibodies 15 days after 2nd dose of vaccination.
Figure 3: Simple box plot of RBD specific IgG antibodies after 15 days of 2nd dose of vaccination.
Discussion
Our study is the first Indian prospective pilot study demonstrating the safety and immunogenicity of mix-match vaccines with Covaxin and Covishield in healthy volunteers. These results are similar to those conducted by Kant R et al. (n=18)6 which included only Covishield first dose followed by Covaxin, while our study included both Covishield followed by Covaxin and Covaxin followed by Covishield groups. The major limitation of the study was the smaller number (n=44). Despite the small number, our study included two groups with two combinations of vaccines that are in use in this part of the world. However, these results need to be assessed in larger cohorts. Since the number is small in this study, our results do not interpret the best combination but it helps to answer the apprehensions among the general public about combination vaccine and prevent vaccine hesitancy.
Conclusion
Our results show safety and immunogenicity of mix-match of COVID-19 vaccination (Covishield followed by Covaxin, and Covaxin followed by Covishield) and demonstrate enhanced antibody response with heterologous vaccines than homologous vaccines.
REFERENCES
- Frenck Jr RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239-250.
[Crossref], [Google Scholar], [PubMed]
- Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173-184.
[Crossref], [Google Scholar], [PubMed]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020.
[Crossref], [Google Scholar], [PubMed]
- Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
[Google Scholar], [PubMed]
- Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325(9):821-822.
[Crossref], [Google Scholar], [PubMed]
- Kant R, Dwivedi G, Zaman K, Sahay RR, Sapkal G, Kaushal H, et al. Serendipitous COVID-19 vaccine-mix in Uttar Pradesh, India: safety and immunogenicity assessment of a heterologous regime. MedRxiv. 2021.
[Crossref], [Google Scholar]
- Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043-2046.
- Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121-30.
[Crossref], [Google Scholar], [PubMed]
- Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255-65.
[Crossref], [Google Scholar], [PubMed]
Citation: Attunuru BPR, Reddy NP, Mitnala S, Gujjarlapudi D, Veturi SY, Duvvur NR (2022) Safety and Immunogenicity of Mix-Match of Vaccines-Covishield and Covaxin: A Pilot Study. J Vaccines Vaccin. 13:477.
Copyright: © 2022 Attunuru BPR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.