Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 0, Issue 0
Safety and Efficacy of Early Use of Iron Chelation in Infants and Toddlers with Transfusion-Dependent Thalassemia: A Multicenter Prospective Study
Mohsen Elalfy1, Amira Adly1*, Amal Elbeshlawy2, Mona Hamdy2, Tamer Hassan3, Mohamed Meabed4, Rasha El Ashry5, Khalid Elsayeh6, Seham Rageb7, Mohamed Elshanshoury8, Naglaa Shahein9, Mahmoud El-Hawy7, Gihan Lofty10, Saad Shehata11, Islam Elghamry12, Yasser Wali12,13 and Omar Elalfy142Department of Pediatrics, Cairo University, Cairo, Egypt
3Department of Pediatrics, Zagazig University, Cairo, Egypt
4Department of Pediatrics, Beni-Suef University, Cairo, Egypt
5Department of Pediatrics, Mansoura University, Cairo, Egypt
6Department of Pediatrics, Assuit University, Cairo, Egypt
7Department of Pediatrics, University Menoufia, Cairo, Egypt
8Department of Pediatrics, Tanta University, Cairo, Egypt
9Atfal Misr Hospital Health Insurance, Cairo, Egypt
10Department of Pediatrics, Elmenia University, Cairo, Egypt
11Damanhour Teaching Hospital, Hematology Unit, Cairo, Egypt
12Department of Child Health, Cairo University, Cairo, Egypt
13Department of Pediatrics, Alexandria University, Cairo, Egypt
14Department of Complementary Medicine, National Research center, Cairo, Egypt
Received: 21-Aug-2023, Manuscript No. JBDT-23-22758; Editor assigned: 23-Aug-2023, Pre QC No. JBDT-23-22758 (PQ); Reviewed: 13-Sep-2023, QC No. JBDT-23-22758; Revised: 20-Sep-2023, Manuscript No. JBDT-23-22758 (R); Published: 27-Sep-2023, DOI: 10.4172/2155-9864.23.S3.015
Abstract
Background: There are limited data on the efficacy and safety of oral iron chelators in infants and toddlers and cite the Deferiprone (DFP) and Deferasirox (DFX) label that states that those chelators were not studied in children under the age of 2 and 3 years respectively. The current study was designed to assess the long-term safety and efficacy of DFX and DFP in this young population.
Methods: A 2-year prospective multi-center, investigator-initiated non-interventional study of infants and children with TDT after at least 4 blood transfusions, naïve to chelation. The children started either on DFX at a dose of 20 mg/kg/day-25 mg/kg/day or DFP at a dose of 50 mg/kg/day-75 mg/kg/day. Throughout the study all patients were subjected to through history and clinical examination stressing on detailed transfusion data, compliance on iron chelator with reporting the Adverse Events (AEs) and anthropometric measures. Laboratory investigations including monthly complete blood count (CBC) and Serum Ferritin (SF), while measurement of liver transaminases and serum creatinine was every 3 months.
Results: Three-hundred and sixty pediatric Transfusion-Dependent Thalassemia (TDT) patients (60%males) were enrolled. Their mean age at enrolment was of 1.72 ± 0.49 months, with a mean of 8.9 ± 0.32 blood transfusions. All were naive to chelation and their initial SF ranged from 820 ng/ml-1202 ng/ml and 480 ng/ml-1070 ng/ml in DFX in a dose of 20 mg/kg/day-25 mg/kg/day and DFP in a dose 50 mg/kg/day-75 mg/kg/day groups respectively; adding during the study period a median of 16 transfusions. With a comparable transfused iron input. At 12 months of regular transfusion-chelation; SF was<1000 ng/ml in almost 62% (60% and 70% on the patients on DFX and DFF respectively) and at 24 months in almost 56% (52% and 64%) respectively, differences were statistically significant. 77% of DFX patients underwent dose increase above 25 mg⁄kg⁄day, while 12.5% of DFP group had increased their dose to above 75 mg/kg/day. Compliance was comparable.
Conclusion: Our results confirmed previous observations on safety and efficacy of both iron chelators in TDT naïve to chelation who had started it at an earlier age. AEs in this real practice study were less compared with published clinical trial data, with significant deviation from guidelines.
Keywords
Thalassemia; Transfusion; Blood; Deferiprone; Deferasirox
Introduction
Despite the new developments in treatment of Transfusion- Dependent Thalassemia (TDT) through fetal Hemoglobin (Hb) inducers, combating ineffective erythropoiesis and gene therapy or even more recently gene editing transfusion-chelation is still the standard of care for most of TDT patients especially in Middle East centers [1].
Transfusion protocols and chelation therapy in TDT are planned according to blood availability and the international guidelines [1,2]. Initiation of iron chelation after 10-20 transfusions or when SF exceeds 1000 ng/ml is still the standard of care: According to TIF guidelines [3]. Both oral chelators DFX and DFP are proved safe and effective in many trials. Recently, Maggio et al in non- inferiority Deep study; comparing patients with TDT who received either daily DFP (75 mg/kg per day-100 mg/kg per day) or daily DFX (20 mg/kg per day-40 mg/kg/day) administered as dispersible tablets showed a comparable efficacy and in common GIT adverse events, while renal toxicity and neutropenia are commoner in DFX and DFP respectively [4].
However real-life practice and guidelines might be different. In a previous large multi-center cohort study, initiation of DFP at a lower threshold of SF values and fewer transfused units was found to be safe and efficacious in preventing iron overload in most young children with no further concerns regarding AEs, it might prevent iron accumulation at an early age [5]. Moreover, there is an urgent need for improving thalassemia care due to the wide gap in current real-life practice and clinical practice guidelines [6]. This prospective multicenter investigator-initiated study; aimed to record the real-life practice in contributing centers in 2 countries.
Primary objectives were to determine a 2-year efficacy of both oral chelators DFX and DFP in infants and children with TDT on regular transfusion, naïve to chelation, by assessing % of patients at 12 and 24 M with a SF<1000 ug/L, percentage of patients with SF>1500 ng/ml and % with appropriate growth velocity over the study time as well as assessing the frequency of reporting (AEs) and serious AEs of both DFP and DFX.
Secondary objectives include reporting deviations from guidelines in management of TDT.
Methods
Study design
An investigator initiated 2-years multicenter prospective observational study enrolling over 12 months period (June 2018- June 2019); TDT infants and toddlers; on regular transfusions, naive to iron chelation; from 13 comprehensive thalassemia treating centers (each treating more than 200 thalassemia patients/ year and or more than 25 new TDT/year) from 2 countries; 12 from Egypt and one from Oman. Age at diagnosis, 1st transfusion and age at start of iron chelators were documented.
Inclusion criteria: TDT; both sex, 8-36 months who had started on regular transfusions more than 4 transfusions every 3-6 weeks to keep pre-transfusion Hb>9 g/dl; naive to iron chelation. Choice of oral iron chelator was decided according to continuous availability in each center during the study period.
Exclusion criteria: TDT<6 Monthsor>36 months, transfusion<4 packed Red Blood Cells (RBCs) units and on therapy with any iron chelation.
Parents of infants and toddlers with TDT had signed an informed consent. Patients were allocated to: Group 1 included patients started on DFX in a dose of 20 mg/kg per day-25 mg/kg/day. They received either; dispersed tablet or film-coated tablet; in an equivalent dose according to leaflet insert. Group 2 included patients on DFP solution at a starting dose of 50 mg/kg/day and were escalated to 75 mg/kg/day when SF>800 ng/ml. Doses of DFX were escalated to>25 mg/kg/day when SF reached>1500 ng/ ml. When serum creatinine was elevated>33% from basal level; re- checked in 2 weeks and action was done according to guidelines. In patients on DFP the dose was escalated to>75 mg/kg/day when SF reached>1500 ng/ml. Any patient on DFP who developed agranulocytosis (Absolute Neutrophil Count (ANC)<500/mm3), should stop the DFP and repeat CBC in 48 hours, if confirmed 48 later, repeat daily till recovery and DFP not re-challenged. In patients with moderated neutropenia (ANC 500-1000/mm3) DFP was stopped and the ANC was re-checked every other day till recovery of ANC>1500/mm3, patients resumed the DFP when neutropenia recovered, If liver transaminases were elevated>5 times the high normal; Information And Communication Technologies (ICT) was stopped for one week and rechecked, to be resumed at a lower dose and liver transaminases would be checked monthly.
Patients visited their study site monthly for clinical assessment, transfusion needs, compliance on ICT (counting DFX pills or weighing the DFP bottles), patients were considered compliant if received>90% of the prescribed dose. AEs, CBC monthly; and Serum Ferritin (SF) determination prior to transfusion were recorded. Anthropometric measures with calculation of the weight and height Sodium Dodecyl Sulfate (SDS), and plot of growth velocity, Liver transaminases and serum creatinine were checked every 3 months.
Efficacy assessment
Included SF assessment and transfusion iron intake was assessed according to the method previously described. (Cohen10) Serum ferritin, as a potential surrogate marker for total body iron was measured in the absence of infection, always pre-transfusion.
Safety assessments
Safety was evaluated every visit through the monitoring and recording of all adverse events and serious adverse events, and routine laboratory testing, including hematology, blood chemistry and renal function assessments.
Ethics approval statement
This study was conducted in accordance with the accepted version of the Declaration of Helsinki and all relevant federal regulations, and in compliance with International Council for Harmonization (ICH) E6 Good Clinical Practice (GCP) guidelines. Written Informed Consent (IC) was obtained prior to conduct of any study-related interventions. The Intracellular Fluid (ICF) form was signed and dated by the patient’s legal representative.
Statistical analysis
Incidence rates and cumulative incidences will be estimated by person-years methods and time-to-event (survival) techniques, with error margins based on Poisson and binomial distributions. The results obtained were arranged in Microsoft Excel sheet. Secondary analyses will be based on cross tabulation, survival analyses with log-rank tests and Cox regression. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) vs. 25. (Cary, NC, USA).
Results
Three-hundred and sixty infants and toddlers with TDT were enrolled in this multi-center prospective study; 60% males, age at diagnosis was 4-14 (median 9 month); age of 1st transfusion was 5-16 (median 10 months) age at enrolment (start of iron chelation) was; 8-36 months (median 20); 21 months in DFX and 15 months in DFP; median follow-up in DFX group was 23 months (20-26); in contrast to 24 months (21-27;) in DFP group. Data were presented in (Tables 1-3) (Figure 1).
| Parameter | DFX (n=296) | DFP (n=64) | p-valuea | Overall (N=360) |
|---|---|---|---|---|
| Age, months | ||||
| Mean ± SD | 1.86 ± 0.42 | 1.23 ± 0.33 | <0.01 | 1.72 ± 0.49 |
| Range/Median | (16-36) 21 | (8-24) 15 | <0.01 | (8-36) 20 |
| Patients =<2 years, n (%)b | 151 (51) | 64 (100) | 215 (59.72) | |
| Sex, n (%) | <0.01 | |||
| Male | 180 (61) | 40 (63) | 0.801 | 220 (61.1) |
| Number of transfusions | ||||
| Prior Range/Median | 8-12 (10) | 4-9 (7) | 4-12 (9) | |
| SF (µg/L) | <0.05 | |||
| Mean ± SD | 973.8 ± 336 | 741.7 ± 296 | — | 857.75 ± 316.7 |
| Range/ Median |
820-1202 960 |
480-1070 768 |
— | 480-1202 897 |
| TSAT (%) | <0.05 | |||
| Mean ± SD | 48.9 ± 19.2 | 41.4 ± 23 | — | 45.15 ± 21.1 |
| Median | 48.2 | 41.7 | — | 39.2 |
| Range | 31.5-82.3 | 28.4-71.0 | - | 28.4- 82.3 |
Note: a Fisher's exact test.; b At date of first treatment exposure.
B-TTD- B-Thalassemia Transfusion Dependent; DFP- Deferiprone; DFX- Deferasirox; SD- Standard Deviation; SF- Serum Ferritin; TSAT- Transferrin Saturation.
Table 1: B-TDT infants and toddlers naïve to iron chelation demographics and baseline characteristics at enrolment.
| Parameter, n (%) | DFP(n=64) | DFX (n=296) | p-valuea |
|---|---|---|---|
| Patients experiencing ≥ 1 AE | 8 (12.5) | 39 (13.2) | 0.885 |
| Common AEs ( ≥ 5% of patients in any group) | |||
| Neutrophil count decreased | 3 (4.7) | 9 (3.0) | 0.506 |
| Diarrhea | 4 (6.3) | 16 (5.4) | 0.788 |
| Rash | -- | 2 (0.7) | - |
| Abdominal pain | 3 (4.7) | 15 (5.1) | 0.899 |
| Vomiting | 3 (4.7) | 11 (3.7) | 0.715 |
| Patients experiencing ≥1 SAEb | 5 (7.8) | 2 (0.7) | 0.01 |
| SAEs (≥1 patient in any group) | |||
| Moderate neutropenia | 1 (1.5) | - | - |
| Liver transaminases>2-5 ULN | 3(4.0) | 13(4.4) | 1.00 |
| Serum creatinine>2 ULN | - | 2 (0.7) | - |
| Liver transaminases>10 ULN | - | 2 (0.7) | - |
| Patients experiencing ≥1 ADRc | 3 (4.7) | 11 (3.7) | <0.05 |
| Common ADRs ( ≥ 5% of patients in any group) | |||
| Elevated serum creatinine>33% | - | 12 (4.1) | - |
| Mild neutropenia | 2 (3.1) | 3 (1.0) | <0.01 |
| Vomiting | 1 (1.6) | 3 (1.0) | 0.479 |
Note: aFisher's exact test.
bADRs included AEs that were at least possibly related to the study treatment.
cADR- Adverse Drug Reaction; AE- Adverse Event; DFP- Deferiprone; DFX- Deferasirox.
Table 2: B-TDT infants and toddlers on DFX and DFP reporting AEs, SAEs, and ADRs.
| Parameter | DFX (n=296) | DFP (n=64) | p-valuea | Overall (N=360) |
|---|---|---|---|---|
| Study duration, Yearsb | ||||
| Mean ± SD | 1.92 ± 0.32 | 2.11 ± 0.23 | <0.001 | 2.02 ± 0.28 |
| Range/Median | (21-25) 23 | (22-26) 24 | 0.354 | (21-26) 23 |
| Chelator dose mg/kg | 20-40(33) | 50-100 (72) | <0.001 | |
| Hb g/dl | ||||
| Mean ± SD | 9.04 ± 1.8 | 9.09 ± 1.9 | 0.842 | 9.07 ± 1.85 |
| Number of transfusions | ||||
| During study (median or mean?) | 16-22 (19) | 17-23 (19) | 0.645 | 16-23 (19) |
| SF (<1000 µg/L) | ||||
| At 12 M N % | 178 (60.1) | 45 (70.3) | 0.128 | 223 (61.9) |
| At 24 M N % | 154 (52.0) | 41 (64.1) | 0.079 | 195 (54.2) |
| SF(>1500 µg/L) | 0.044 | |||
| 12M | 71(24.0) | 8(12.5) | 0.427 | 79(21.9) |
| 24 M | 59(19.9) | 10(15.6) | 69(19.2) | |
| TSAT (30-60%) | ||||
| At 12 M N % | 190 (64.2) | 46 (71.9) | 0.24 | 236 (65.6) |
| At 24 M N % | 164 (55.4) | 43 (67.2) | 0.083 | 207 (57.5) |
| TSAT < 20% | - | - | - |
Note: aFisher's exact test.
bAt date of first treatment exposure.
DFP- Deferiprone; DFX- Deferasirox; SD- Standard Deviation; SF- Serum Ferritin; TSAT- Transferrin Saturation.
Table 3: B-TDT infants and toddlers 12 and 24 Months on oral iron chelation characteristics during the study.
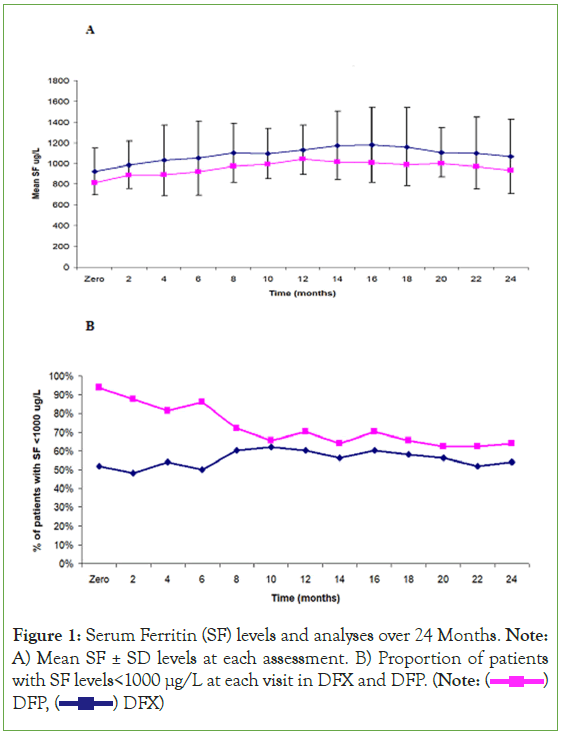
Figure 1: Serum Ferritin (SF) levels and analyses over 24 Months. Note: A) Mean SF ± SD levels at each assessment. B) Proportion of patients with SF levels<1000 μg/L at each visit in DFX and DFP. (Note:  DFP,
DFP,  DFX)
DFX)
Transfusion
(B-TDT) Transfusion-Dependent β-thalassemia had received 4-12 transfusions (median=8) prior to start of iron chelator in addition to 16-22 (median 19 transfusions) during the 2-year study period. Pre-transfusion Hb was 7.4 g/dl -12 g/dl (median 8.9, transfusion was every 3-6 weeks and 49% of patients did not achieve the target pre-transfusion (Hb=or>9 g/dl) (Table 3).
Baseline SF, Iron chelator start time and dose
At initiation of DFX, when SF ranged from 820-1202 (median 960) while those who were started on DFP; when SF was 480-1070 (median 768, ng/ml) both groups had comparable transfused iron input during the study period. Single oral chelation was initiated and maintained either on DFX; (n=296); dispersed tablet in 20% or film-coated tablet in 80%) patients were started on 20 mg- 25 mg/kg/d; escalated to be at 12 months (20 mg-44 mg/kg/d) median dose was 28 mg/kg/d;), while DFP dose range (50 mg-100 mg/kg/d), median dose after 12 months was 72 mg/kg/day.
Compliance
receiving DFX had a treatment compliance (by pill count) of 88% (film-coated tablet 89% and dispersed tablet 82%) while DFP compliance was 84%; the lunch time DFP dose is the most frequently missed dose (two-thirds of missed doses). Compliance was comparable in DFX and DFP groups.
SF follow-up
At around 12 and 24 months of regular transfusion-chelation; SF<1000 ng/ml was noticed in 62% (60% and 70% of the patients on DFX and DFP at 12 M while at 24 M, 54% (52% and 64%) respectively; difference between DFX and DFP groups was statistically insignificant, table 3. Poorly controlled patients with SF>1500 ng/ml at 12 or 24 months was not statistically different in both groups; 24%, 20% in DFX group; in contrast to 12.5%, 15.6% in DFP group respectively. None of the studied population had SF>2000 ng/ml. 90% of patients with SF>1500 ng/ml were non-compliant, with more frequent transfusion and iron input.
At enrolment, Transferrin Saturation (TSAT) was 30%-60% in most patients mean TSAT was significantly higher in DFX group, meanwhile 10% of patients had TSAT>70%. Data on TSAT follow- up at 12 M and at 24 M are shown in Table 3.
Anthropometric assessment revealed good growth velocity with both chelators; height increased>10 cm/ year till 2 years old then 4 cm/kg-6 cm/kg; weight increased 2 kg/year-3 kg/year after the 2nd year; difference was not statistically different. Three-quarter of TDT infants and toddlers had achieved proper growth velocity for age, 15% of each group was below the expected normal growth, and most of them were in the none-compliant group.
Iron chelator AEs
Patients with TDT on DFX had reported drug related AEs, vomiting and or, abdominal colic in 3.5% and 5% and rash in 0.7%. Elevated serum creatinine>33% from baseline on 2 successive visits were observed in 8.4% without modification of DFX dose. While patients on DFP had reported a vomiting and or, abdominal colic in 4.3%; but neither arthralgia, nor agranulocytosis were reported, but transient neutropenia in (6.2%). Elevated liver transaminases>2 and< 5 times high normal was reported in 4.1% and 4.7% respectively. No unexpected, serious, or severe adverse events were reported in both groups as shown in Table 3. The rate of DFP or DFX discontinuation ratio was calculated as 1.9% and 3.1% respectively; distributed equally due to intolerance, AEs and or family preference was documented.
Center-center and region variation
Thirteen centers from 3 regions in Egypt were included (Delta, Greater Cairo, and Upper Egypt) one region in Oman. DFX was available in all centers, while DFP was available mainly in one center and on request in other centers. DFX was started in most centers after more transfusions, while DFP was started early after a minimum of 4 transfusions and initial median SF less than in DFX group. At 12 M and 24 M; SF was still less in DFP group, while this difference was statistically significant from baseline till end of study. Frequency of reporting of AEs were variable in different centers.
Deviation from guidelines
DFX in a dose 30 mg/kg/d-40 mg/kg/d while SF<1500 ng/ml had been documented in 10% of patients. While 3.3% had received DFP in a dose>75 mg/kg while SF was<1500 ng/ml. Reporting AEs was documented in<10% of TDT in either group, mostly Gastro Intestinal Tract (GIT), but elevated Serum creatinine>33% from baseline to subsequent visits was reported in DFX group; however, no action was done. CBC was done monthly and or pre- transfusion in DFP group not bi-weekly in all patients.
Discussion
Two oral iron chelators; DFX and DFP have been used in Egypt over the last 12 years; however recently DFX; especially the coated tablet was prescribed more widely in most thalassemia centers in Egypt. TIF recommended start iron chelation after 10-20 transfusions [3].
There is a national guideline for management of TDT in all comprehensive thalassemia centers, yet there is a significant difference in clinical practice. An improvement of pre-transfusion Hb level in TDT was noticed over the last decade in both Egypt and Oman; (Ref) however it is still sub-optimum; due to shortage of blood donation aggravated during COVID era [5].
Follow-up of chelation therapy in infants and toddlers with TDT in 1st years after the start of iron chelators relied mainly on serial SF measurement, transfusion burden, compliance and tolerability of the iron chelators during the study period.
Deferasirox had been initiated around 2 years, after 8-12 transfusions or SF around 1000 ng/ml in most Egyptian centers. Deferiprone had been started earlier after 4-10 transfusions and SF>400 ng/ ml and<1000 ng/ml in 95%, most of the patients were from one center and when available in other centers based on studies which had shown that early start of DFP (before 2nd year of life) was safe and that it delayed the time to reach SF>1000 μg/L [5].
Compliance with long term ICT
Compliance with long-term ICT is challenging, however compliance was better compared with previous published Egyptian experience; (ref). Compliance was comparable with the 2 chelators; non-significantly higher with coated DFX tablet then DFP and was least with dispersed tablet.
Efficacy
Both DFX and DFP offer an important treatment option for people with TDT to prevent or ameliorate iron overload. Our data confirmed that both iron chelators in compliant TDT were effective to maintain SF below 1000 ng/ml in most patients when used earlier. Reduction in SF level was almost consistent across post-baseline time points. Based on the published data, DFX dose at the usually recommended half the dose of deferoxamine [7]. Results from the ECLIPSE study had evaluated the safety of DFX dispersible tablet and Film-Coated Tablet (FCT) formulations in patients with TDT [8]. ESCALATOR study was concerned primarily heavily iron-overloaded patients had confirmed the importance of timely DFX dose adjustments based on serial SF levels and transfusion iron intake. Ali Taher. In another study median SF was decreased from 1,702 ng/ml at baseline to 1,127 ng/ml at 5 years [9].
Liquid DFP was 1st used a decade ago and was effective and safe in a prospective multi-center study [5]. Recently, Maggio et al in non- inferiority Deep study; comparing patients with TDT who received either daily DFP (75 mg/kg per day-100 mg/kg per day) or daily DFX (20 mg/kg/day-40mg/kg/day) administered as dispersible tablets showed a comparable efficacy and in common GIT adverse events, while renal toxicity and neutropenia were commoner in DFX and DFP respectively [4].
As early as less than 10 transfusions and 2 years or less, TSAT was critical (>70%) in almost 10%, TSAT was 30%-60% in most patients at enrolment, non-had SF< 20% in both groups, which is considered essential before start of iron chelation. Mean TSAT was significantly higher in DFX group. At 12 M more than 60% of patients had TSAT 30%-60% while at 24 M less patients kept good TSAT level compared with 12 M.
AEs reporting and action taken
Iron chelation therapy is generally safe in young patients. Each iron chelation regimen has its specific safety risks Taher et al reported that DFX doses of>30 mg/kg/d in pediatric patients, with B-TDT, had adverse event profile as previously published data. Frequency of common AEs and drug discontinuation was much lower compared with recently published data, mainly regarding gastric irritation symptoms (19.4%). The total DFX discontinuation ratio was calculated as 9.7% [10].
Reporting of AEs in both DFP and DFX groups was clear in this real practice study was less compared with published clinical trial data. That is possible as these are young children do not express what they are feeling. Another option is that young children manifest AEs less frequently than older children. However, it is known that reporting of AEs in clinical trials is always more frequent than in clinical practice because in clinical trials the participants are specifically asked about AEs. In a recently published study, 89.9% of patients reported ≥ 1 Adverse Event (AE), of which 20.3% experienced ≥ 1 serious AE [11]. Elevated serum creatinine>33% from baseline on 2 successive visits was uncommon in DFX group without an attempt for reducing DFX dose or even re-check of serum creatinine higher than age-adjusted Upper Limit of Normal (ULN).
Asymptomatic renal dysfunctions are prevalent in young Beta- Thalassemia (β-TM) [12,13]. Kidney disease may develop through progressive renal tubular and glomerular damage; thus, its early recognition is important in order to prevent and/or reverse deterioration [14]. Acute Kidney Injury (AKI) risk is a dose- dependent manner; with high-dose DFX and lower serum ferritin concentration is consistent with over-chelation as a causative factor was not reported in this study. Physicians should closely monitor renal function and serum ferritin, use the lowest effective dose to maintain acceptable body iron burden [15,16].
Agranulocytosis was not reported in the DFP cohort, while neutropenia was reported infrequently, and no action was taken in this study. In a community with ethnic neutropenia, mild to moderate neutropenia is common (Ref). Higher incidence of severe neutropenia and agranulocytosis mandates careful monitoring and rational modification of iron-chelating agents to avoid life- threatening complications. Neutropenia and agranulocytosis are rare side effects of Deferiprone (DFP) [17]. Less frequent ANC monitoring and continuation of DFP therapy during neutropenia was not associated with prolonged neutropenia or with progression to agranulocytosis [5].
Elevated Liver Transaminases (ELT) was infrequently reported in this study, however ALT more than two times the ULN was 4.4% and 4.0%, respectively. There were no new safety signals in another study [9]. Discontinuation rate is low compared with clinical trials which were up to 48% in EPIC study.
Therapy discontinuations either due to AEs or non-preference were around 1.5%; in contrast; severe gastrointestinal disorders and increased transaminases with DFP and DFX were comparable [18].
Center to center variations
Center to center variation was greater in Upper-Egypt centers; compared to centers in Cairo as regards both nadir pre-transfusion Hb and deviation from guidelines of iron chelation dose as well as under-reporting of AEs. However unnecessary high dose DFX>40 mg/kg was not uncommon, while Under-reporting of AEs in both groups was observed. Corrective actions are planned to be implemented to minimize deviations.
Real-world, pragmatic trials in different clinic settings are needed that compare the two oral iron chelators; when to start, dose, combination, compliance, reported adverse events and efficacy. However early oral iron chelation might be the standard of care in the future.
Limitations
Data was collected prospectively; however, deviations from guidelines were not uncommon and action taken was not clear in some situations in this study. Comparing efficacy of DFP and DFX was difficult due to differences in basal transfused iron burden or SF being less in DFP group compared with DFX which continued till the end of the study. DFX had not enough data to its used below 2 years; on the other hand, presence of published data on early use of DFP, so at end of 2 years SF was significantly less in DFP group. Serum ferritin is not the best marker for assessment of iron overload control however due to young age of the study population with difficulty to do MRI, SF was the accepted marker.
Conclusion
B-TM infants and toddlers naïve to chelation; half of them were under transfused, had been started on oral iron chelation monotherapy at younger age than reported before. Both oral chelators were effective, well tolerated and with no serious safety concerns. However, deviation from guidelines was observed with unnecessary over-dosing more mainly in DFX group and under- reporting of AEs in both groups in the real-life practice.
What is New Aspect of the Work
We believe this manuscript is appropriate for publication in the European Journal of Hematology because it describes a novel therapeutic approach to treating Transfusion-Dependent Thalassemia (TDT) in young children and infants. Current treatment delays iron chelator treatment until reaching iron overload criteria to minimize iron depletion toxicities that were observed during "early-start" studies with some iron chelators. However, the delayed start may increase iron overload risks later in life. Deferiprone and Deferasirox are more recent iron chelators with distinct pharmacological properties that may enable "early- start" treatment with minimal risk of iron depletion and reduced long-term toxicities associated with iron overload.
What is the Central Finding
This manuscript studied prospectively the idea that if "early- start" deferiprone vs. deferasirox can be safely initiated before recommended by current guidelines in young children with TDT. The primary objective was to measure iron load via serum ferritin levels in patients treated with deferiprone vs. deferasirox for up to 24 months. The serum ferritin results show deferiprone was safe, not associated with iron depletion, and efficacious in minimizing iron overload in infants/children with TDT. This is significant because initiating treatment earlier may help reduce iron overload in organs associated with toxicities later in life.
Clinical Relevance
It will improve the care of young TDT and change the current protocols of chelation.
Running Head
Transfusion dependent thalassemia, early chelation, Safety, DFP, DFX
All authors contribute to the manuscript writing and approval and working in the clinical and follow up of patients
Funding
No fund
Conflict of interest
No conflict of interest for all authors
References
- Shah FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019; 37:100588.
[Crossref] [Google Scholar] [PubMed]
- Kaur M, Kaur R, Sood T, Jindal G, Kaur P, Mittal K. Efficacy of packed red blood cell transfusions based on weight versus formula in thalassemia children: An open-label randomized control trial. Transfusion. 2022; 62(4):791-796.
[Crossref] [Google Scholar] [PubMed]
- Farmakis D, Giakoumis A, Cannon L, Angastiniotis M, Eleftheriou A. COVID-19 and thalassaemia: A position statement of the Thalassaemia International Federation. Eur J Haematol. 2020; 105(4):378-386.
[Crossref] [Google Scholar] [PubMed]
- Maggio A, Kattamis A, Felisi M, Reggiardo G, El-Beshlawy A, Bejaoui M, et al. Evaluation of the efficacy and safety of deferiprone compared with deferasirox in paediatric patients with transfusion-dependent haemoglobinopathies (DEEP-2): a multicentre, randomised, open-label, non-inferiority, phase 3 trial. Lancet Haematol. 2020; 7(6):469-478.
[Crossref] [Google Scholar] [PubMed]
- El Alfy M, Sari TT, Lee CL, Tricta F, El-Beshlawy A. The safety, tolerability, and efficacy of a liquid formulation of deferiprone in young children with transfusional iron overload. J Pediatr Hematol Oncol. 2010; 32(8):601-605.
[Crossref] [Google Scholar] [PubMed]
- Musallam KM, Bou-Fakhredin R, Cappellini MD, Taher AT. 2021 update on clinical trials in β-thalassemia. Am J Hematol. 2021; 96(11):1518-1531.
[Crossref] [Google Scholar] [PubMed]
- Bollig C, Schell LK, Ruecker G, Allert R, Motschall E, Niemeyer CM, et al. Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database Syst Rev. 2017; 2017(8).
[Crossref] [Google Scholar] [PubMed]
- Tartaglione I, Origa R, Kattamis A, Pfeilstocker M, Gunes S, Crowe S, et al. Two-year long safety and efficacy of deferasirox film-coated tablets in patients with thalassemia or lower/intermediate risk MDS: Phase 3 results from a subset of patients previously treated with deferasirox in the ECLIPSE study. Exp Hematol Oncol. 2020; 9:1-1.
[Crossref] [Google Scholar] [PubMed]
- Vichinsky E, El-Beshlawy A, Al Zoebie A, Kamdem A, Koussa S, Chotsampancharoen T, et al. Long-term safety and efficacy of deferasirox in young pediatric patients with transfusional hemosiderosis: Results from a 5 year observational study (ENTRUST). Pediatr Blood Cancer. 2017; 64(9):26507.
[Crossref] [Google Scholar] [PubMed]
- Zengin Ersoy G, Aycicek A, Odaman Al I, Bayram C, Arslantas E, Ozdemir GN, et al. Safety and efficacy of deferasirox in patients with transfusion-dependent thalassemia: A 4-year single-center experience. Pediatr Hematol Oncol. 2021; 38(6):555-563.
[Crossref] [Google Scholar] [PubMed]
- Viprakasit V, Hamdy MM, Hassab HMA, Sherief LM, Al-Bagshi M, Khattab M, et al. Patient preference for deferasirox film-coated versus dispersible tablet formulation: A sequential-design phase 2 study in patients with thalassemia. Ann Hematol. 2023; 25:1-11.
[Crossref] [Google Scholar] [PubMed]
- Tantawy AA, El Bablawy N, Adly AA, Ebeid FS. Early predictors of renal dysfunction in Egyptian patients with β-thalassemia major and intermedia. Mediterr J Hematol Infect Dis. 2014; 6(1).
[Crossref] [Google Scholar] [PubMed]
- Nafea OE, Zakaria M, Hassan T, El Gebaly SM, Salah HE. Subclinical nephrotoxicity in patients with beta-thalassemia: Role of urinary kidney injury molecule. Drug Chem Toxicol. 2019; 45(1):93-102.
[Crossref] [Google Scholar] [PubMed]
- Demosthenous C, Vlachaki E, Apostolou C, Eleftheriou P, Kotsiafti A, Vetsiou E, et al. Beta-thalassemia: Renal complications and mechanisms: a narrative review. Hematol. 2019; 24(1):426-438.
[Crossref] [Google Scholar] [PubMed]
- Bird ST, Swain RS, Tian F, Okusanya OO, Waldron P, Khurana M, et al. Effects of deferasirox dose and decreasing serum ferritin concentrations on kidney function in paediatric patients: an analysis of clinical laboratory data from pooled clinical studies. Lancet Child Adolescent Health. 2019; 3(1):15-22.
[Crossref] [Google Scholar] [PubMed]
- Yui JC, Geara A, Sayani F. Deferasirox-associated Fanconi syndrome in adult patients with transfusional iron overload. Vox Sang. 2021; 116(7):793-797.
[Crossref] [Google Scholar] [PubMed]
- Nazir HF, Alshizawi M. Neutropenia and life-threatening agranulocytosis among children with β-Thalassemia treated with oral iron chelators in a community with background of ethnic neutropenia. J Pediatr Hematol Oncol. 2020; 42(8):750-755.
[Crossref] [Google Scholar] [PubMed]
- Botzenhardt S, Li N, Chan EW, Sing CW, Wong IC, Neubert A. Safety profiles of iron chelators in young patients with haemoglobinopathies. Eur J Haematol. 2017; 98(3):198-217.
[Crossref] [Google Scholar] [PubMed]
Citation: Elalfy M, Adly A, Elbeshlawy A, Hamdy M, HassanT, Meabed M, et al (2023) Safety and Efficacy of Early Use of Iron Chelation in Infants and Toddlers with Transfusion-Dependent Thalassemia: A Multicenter Prospective Study. J Blood Disord Transfus. S3.015.
Copyright: © 2023 Elalfy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.