PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 2
Safety and Efficacy of Convalescent Plasma Treatment in COVID-19 Patients: Collate Trial
Muhammad Hasan1, Mohammad Usman Shaikh2, Muhammad Jehangir Malik3, Bushra Jamil4, Nosheen Nasir4, Kiren Habib4, Adil Aziz5, Iffat Khanum4, Aisha Ilyas4, Ramla Ghafoor4, Natasha Ali2* and Faisal Mahmood42Department of Pathology and Laboratory Medicine/Oncology, Aga Khan University, Karachi, Pakistan
3Department of Pathology, Aga Khan University, Karachi, Pakistan
4Department of Internal Medicine, Section of Infectious Diseases, Aga Khan University, Karachi, Pakistan
5Department of Internal Medicine, Aga Khan University, Karachi, Pakistan
Received: 05-Feb-2021 Published: 26-Feb-2021, DOI: 10.35248/2157-7560.21.12.448
Abstract
Introduction: The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred initially in December 2019 in the city of Wuhan, Hubei province, China where patients mainly presented with respiratory symptoms. In Pakistan the first case was identified on February 26, 2020 and since then Aga Khan University Karachi is at the forefront of the fight against COVID-19. After receiving all required approvals, this trial was undertaken to determine safety and efficacy of transfusing Convalescent Plasma (CP) in patients admitted with COVID-19.
Methods: This was a non-randomized, open label, phase II clinical trial with 110 cases and 34 controls recruited during April 2020 till July 2020. Convalescent plasma donors and patients who received it were recruited using donor eligibility criteria issued by U.S. Department of Health and Human Services Food and Drug Administration. All donors were screened for transfusion transmitted diseases and tested for SARS-CoV-2 infection by rRT-PCR. Documentation of IgG antibody in donors was done through Novel Coronavirus COVID-19 IgG ELISA Kits. Patients in the intervention group received 500 ml of CP along with concomitant therapies. Patients in the control group received concomitant therapies only. Outcome measures included assessment of safety, decreased length of stay and decrease in values of inflammatory makers (CRP, D-Dimer, procalcitonin, serum ferritin).
Results: We recruited 96 males and 48 females during the study period. The median age was 60.2 years. Age was found to be a significant prognostic marker in both groups as patients less than 60 years had increased overall survival (hazard ratio: 0.33, p-value: 0.001). Presence of two or more co-morbidities provided disadvantage to the overall outcome. Survival was increased by 10 days in patients who received plasma as compared to controls. However, it was not significant. The overall survival in cases was 68% while in controls it was 62%. There was an improvement seen in all inflammatory markers after transfusion of convalescent plasma in cases. Use of concomitant therapies e.g. tocilizumab (hazard ratio: 1.09, 95% CI: 0.54-2.23) and methylprednisolone (hazard ratio: 1.3, 95% CI: 0.6-2.88) did not affect overall survival. There was no serious adverse event reported after transfusion of convalescent plasma.
Conclusion: Transfusion of CP was found to be safe as no adverse event was reported. There was a significant decrease in the inflammatory marker levels in cases. There was no significant difference in length of stay and overall survival in both groups.
Keywords
COVID-19; Vaccine; Convalescent plasma; Diagnosis
Introduction
The outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) occurred initially in December 2019 in the city of Wuhan, Hubei province, China [1]. Patients mainly presented with respiratory symptoms and this novel pathogen was identified. SARS-CoV-2 is the seventh virus of the coronavirus family to infect humans. The outbreaks before this have been caused by SARS-CoV-1, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Human Coronavirus (HCoV) HKU1, HCoV NL63, HCoV OC43 and HCoV 229E [2]. Since the outbreak in China, the virus has catastrophically spread all over world and eventually World Health Organization (WHO) declared coronavirus disease of 2019 (COVID-19), a pandemic on March 11, 2020. COVID-19 primarily spreads through the respiratory tract by droplets, respiratory secretions and by direct contact [3]. Therefore, it is highly transmissible in humans, especially in the elderly and people with underlying comorbidities.
As of October 23 2020, 41.7 million people have been infected and 1.14 million have succumbed to the disease. In Pakistan the figures stand at 326,000 patients being infected while the virus has proved to be fatal in 6,715 cases [4]. Despite the exorbitant number of cases, till date, no cure has been found for this disease. Given the lack of effective antiviral therapy, the current treatment options include symptomatic and respiratory support according to the Diagnosis and Treatment of Pneumonia Caused by COVID-19 (updated to version 6) issued by National Health Commission of the People’s Republic of China and WHO [5].
Apart from antivirals and supportive care, one investigational treatment option that is readily available is immune or convalescent plasma, which refers to plasma being collected from individuals following resolution of infection and development of antibodies [6]. Though short lived, this form of passive antibody confers immediate immunity to susceptible individuals. There are numerous examples in which Convalescent Plasma (CP) has been used successfully as post exposure prophylaxis and/or treatment of infectious diseases, including other outbreaks of coronaviruses e.g. SARS-1, MERS-CoV and very recently in 2014, the Ebola virus outbreak [7]. In SARS-CoV-2, Shen [8] published a case series of 5 critically ill patients with COVID-19 and acute respiratory distress syndrome showing improvement in clinical status after transfusion of CP. Following this, published studies on CP till date have proved that administration of CP is safe as negligible serious adverse events have been reported in literature.
Aga Khan University Karachi is at the forefront of the fight against COVID-19 in Pakistan. As the first case of COVID-19 was reported in Karachi on February 26 2020, a 105 bedded COVID hospital was set up by the University. Along with providing the best possible supportive care and therapeutic management, the infectious disease and hematology team proposed a clinical trial protocol where admitted patients with moderate/severe/critical disease were to be enrolled to receive convalescent plasma based on the eligibility criteria of Food and Drug Administration (FDA). Therefore, the objective of our study was to determine safety and efficacy of transfusing convalescent plasma in patients admitted with COVID-19 at Aga Khan University Karachi, Pakistan. We hypothesized that CP will be safe to transfuse, will decrease length of stay and will improve overall outcome.
Methods
This open label, non-randomized trial was conducted at the Section of Hematology and Transfusion Medicine, Department of Pathology & Laboratory Medicine and the Department of Medicine at the Aga Khan University Karachi, Pakistan from April 2020 till July 2020.
The trial was conducted in compliance with the principles of the declaration of helsinki (version 2013), and principles of good clinical practice. Inclusion in the trial was voluntary and subject to provision of written informed consent. Each participant was informed of their right to withdraw from the study at any time without penalty or loss of benefits including standard of care. Confidentiality of all subjects was maintained throughout the trial.
Ethical approval was obtained from the institutional Ethics Review Committee (ERC) and the National Bioethics Committee (NBC) of Pakistan, before trial commencement. Approval was also taken from the Drug Regulatory Authority of Pakistan (DRAP) and the Blood Transfusion Authority of the provincial government of Sindh (SBTA). This study was registered at clinicaltrials.gov with identifier number: NCT04476888.
The Clinical Trial Unit of the hospital provided the perpetual oversight and technical support to the investigators throughout the study.
Study population: CP recipients (treatment arm)
Inclusion criteria
I. Inpatients at AKUH with positive SARS-CoV-2 infection by rRT-PCR and who have provided written informed consent for inclusion in the trial;
II. Age ≥ 18 years;
III. Severe or immediately life-threatening COVID-19 defined by any of:
• Respiratory rate ≥ 30/min;
• Blood oxygen saturation ≤ 93% at room air;
• Lung infiltrates >50% within 24 to 48 hours on radiology (X-ray or CT scan);
• Need for mechanical ventilation.
• Septic shock
• Multiple organ dysfunction or failure
Exclusion criteria
I. Negative rRT-PCR from respiratory secretions or blood within 48 h prior to assessment of eligibility.
II. History of allergic reaction to blood or plasma products (as judged by the investigator).
III. Medical conditions in which receipt of 500 mL intravascular volume may be detrimental to the patient (e.g., actively decompensated congestive heart failure).
IV. Enrolment in any other clinical trial for an investigational therapy.
Control arm
Patients with severe or immediately life threatening COVID-19 who were admitted during the period before CP was available or for whom no compatible CP was available were recruited in control arm. They were not transfused with convalescent plasma but were given standard treatment (see concomitant therapies below).
Procurement of convalescent plasma: CP donors
Inclusion criteria
I. Outpatients or discharged inpatients diagnosed with SARS-CoV- 2 infection by real time Reverse Transcriptase-Polymerase Chain Reaction (rRT-PCR) and who have provided written informed consent for inclusion in the trial;
II. Evidence of viral clearance by negative rRT-PCR at (1) clinical recovery and (2) 24 hours before the intended time of CP collection. The interval between these two tests should was at least 14 days.
III. Complete resolution of symptoms at least 14 days before the donation.
IV. Age between 18-60 years.
Exclusion criteria
Ineligible to donate plasma according to blood donor selection criteria followed in the section of transfusion medicine.
Convalescent plasma collection and storage
Potential donors, who met the inclusion criteria and provided informed consent, underwent pre-donation testing to assess final suitability for donation, according to the routine policy and procedures. Pre-donation testing included:
• ABO and RhD grouping
• Blood screening tests for Transfusion Transmissible Infections (TTIs) according to the blood bank routine policy and procedures including serology of HBV, HCV, HIV, malaria and syphilis and Nucleic Acid Testing (NAT) for HBV, HCV and HIV.
• IgG antibody testing through Novel Coronavirus COVID-19 IgG ELISA Kits
The investigators used to review the results of pre-donation testing. Potential donors who tested negative for all TTIs and had positive COVID IgG antibody results, were selected for CP donations.
CP was collected from all donors through plasma apheresis procedure as it enables collection and storage of large volumes of CP that may be used for more than one patient. In apheresis procedure, approximately one-liter plasma was obtained from each donor and replacement fluid (1000 ml normal saline) was infused. This 1000 ml plasma was divided into 4 portions of 250 ml each, using sterile tube connection.
The collected Convalescent Plasma was frozen within 8 hours of collection at -40 degree centigrade in a controlled plasma freezer. Upon request from the ward, it was thawed at 37 degree centigrade and dispensed for the transfusion.
Convalescent plasma transfusion
Patients had their blood type determined. CP was ABO compatible with the recipient’s blood type. Patients received two consecutive transfusions of 250 ml ABO-compatible convalescent plasma (i.e. 500 ml of convalescent plasma in total). Each transfusion was administered over a 30-minute period, with a 15-minute interval between the two transfusions. Transfusions were done in accordance with the standard policy routinely used at our hospital for administration of blood products.
Concomitant therapies
The clinical team had complete independent control of patient management and as such, therapies other than CP therapy were not influenced by the intervention or study team. Co-interventions included corticosteroids, antiviral drugs, hydroxychloroquine and IL-6 inhibitor e.g. Tocilizumab. These treatment modalities were also given to patients in control group. Convalescent plasma was given to subjects in whom tocilizumab and remdesivir were contraindicated due to derangement of liver enzymes. It was also given to subjects who did not show adequate improvement response after receiving other therapies.
Outcome measures
The primary clinical outcomes were safety, which was assessed by the frequency of adverse or serious adverse events and decrease in length of hospital stay.
The secondary clinical outcome was improvement on ordinal scale modified from WHO ordinal scale (Table 1). The secondary laboratory outcomes include improvement in inflammatory markers i.e. D-Dimer, procalcitonin, C-reactive protein, ferritin, CBC parameters i.e. hemoglobin, total leucocyte count, platelet count, lymphocyte count percentage and improvement in chest X-Ray findings.
| Score | Description |
|---|---|
| 1 | Discharge with no oxygen therapy |
| 2 | Oxygen by face mask or nasal prongs |
| 3 | High flow oxygen |
| 4 | BiPAP or CPAP |
| 5 | Intubated/mechanical ventilation |
| 6 | Death |
Abbreviations: BiPAP: Bilevel Positive Airway Pressure; CPAP: Continuous Positive Airway Pressure
Table 1: Modified WHO ordinal scale.
Statistical analysis
Continuous variables were summarized by using median ± Standard Deviation (SD) and range for the total number of patients who contributed to the value. Paired t-test was used to determine the mean difference in inflammatory markers (CRP, D-Dimer, procalcitonin and serum ferritin) between cases and controls. Treatment effects for secondary endpoints were assessed using Hazard Ratio (HR) with 95% Confidence Interval (CI) through covariates adjusted cox regression model. Overall survival was analyzed using Kaplan Meier method and the median time to event with corresponding 95% CI was calculated. Statistical analysis was performed using software for statistics and data science (STATA) version and Statistical Package for Social Sciences (SPSS) version 22.
Results
Cases
We enrolled 110 cases during the study period. There were 75 male and 35 female patients with moderate/severe/critical COVID-19. The median age ± SD (range) was 59.3 ± 12.6 (27 – 91 years). In 60% of the patients at least two co-morbid conditions were documented which included diabetes mellitus and hypertension. Forty-one patients were admitted in the Intensive Care Unit (ICU) requiring mechanical ventilation, 63 patients were treated in Special Care Unit (SCU) with noninvasive ventilation (high flow oxygen, BIPAP, CPAP) while 6 were isolated in COVID ward. In concomitant therapies, 29 patients received tocilizumab alone while 25 patients received a combination of tocilizumab and methylprednisolone. Methylprednisolone alone was given to 31 patients (other concomitant therapies are given in Figure 1. All patients received 500 ml blood group specific or O group COVID Convalescent Plasma (CP) based on FDA criteria of therapy. None of the patients developed any adverse or serious adverse events after transfusion. The mean ± SD (range) length of stay for all patients was 13 ± 7 (3-39) days. The mean ± SD (range) of days taken to receive CP transfusion was 3.1 ± 3.5 days (range: 0 to 18 days).
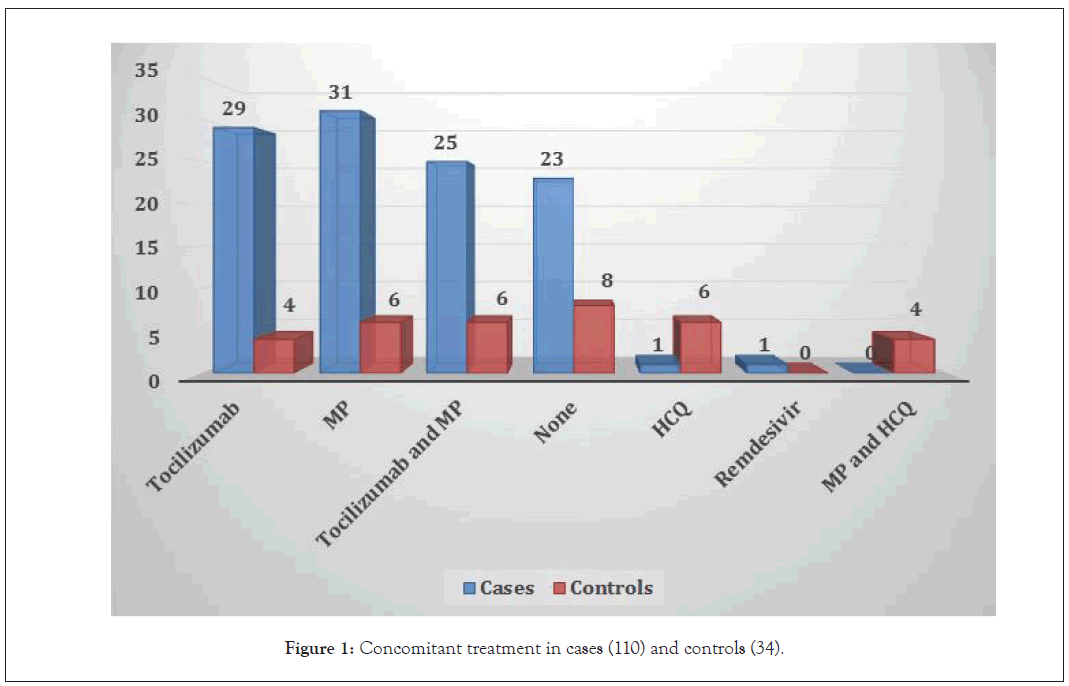
Figure 1: Concomitant treatment in cases (110) and controls (34).
Cases–laboratory parameters: Prior to CP transfusion, in Complete Blood Count (CBC) values, the mean lymphocyte percentage as indicated by paired sample t-test, was 8.7 (range: 0.6%-33.1%) that changed to 7.5% (range: 0.5%-40%) at last day of admission. The mean platelet count before CP transfusion was 268 × 109/L (range: 28–767 × 109/L) and at discharge the platelet count ranged from 50–856 × 109/L (mean 290 × 109/L). Both parameters were not significant. The significant parameters were hemoglobin with p-value of 0.002 (mean value: 12.1 gm/dl pre transfusion and 11.5 gm/dl post transfusion) and total leucocyte count with p-value of 0.001 (mean value: 16.3 × 109/L and 12.5 × 109/L, pre and post transfusion respectively (Table 2). Other significant laboratory parameters were C-Reactive Protein (CRP), the mean value of which was 90.7 mg/dL (range: 0.02–292 mg/ dL) pre-transfusion and 24.5 mg/dL (range: 0.1–235 mg/dL) post transfusion, procalcitonin (4.3 ng/ml pre-transfusion and 1.12 ng/ ml post transfusion), D-Dimer with a mean value of 6.7 mg/L FEU (range: 0.5–40 mg/L FEU) pre-transfusion and 3.5 mg/L FEU (range: 0.1–30 mg/L FEU) post-transfusion, (p-value: .001) and serum ferritin with mean values of 2219 ng.ml and 1268 ng/ml pre and post transfusion respectively (p-value 0.003), also showed improvement. Complete laboratory profile of inflammatory markers is provided in Table 3. The chest X ray findings prior to infusion of convalescent plasma were found to be progressive in 52 patients (46%) and improved in 30/52 (58%) patients at discharge (p-value 0.001). According to WHO ordinal scale (Table 3) which we modified with respect to our study parameters, at the time of admission there were 31 (28%) patients which required oxygen therapy through face mask/nasal prongs (score 3), 67 patients (61%) required non-invasive ventilation or high flow oxygen (score 4) while 12 patients (11%) were intubated (score 5). At the time of discharge, 45 patients (41%) went home on room air (score 1), 25 patients (23%) required low flow oxygen (score 2) and 5 patients (4.5%) needed BiPAP.
| Cases, 110 | Controls, 110 | |||||
|---|---|---|---|---|---|---|
| (Mean) | (Mean) | |||||
| Pre transfusion | Post transfusion | p value | On admission | At discharge | p value | |
| Hemoglobin (g/dL) | 12.1 | 11.5 | 0.002 | 12.5 | 11.9 | 0.118 |
| Total leucocyte count (x 109/L) | 16.3 | 12.5 | 0.001 | 15.9 | 10.5 | 0.032 |
| Lymphocyte count (%) | 8.7 | 7.5 | 0.941 | 18.1 | 16.1 | 0.547 |
| Platelet count (x 109/L) |
268 | 290 | 0.119 | 232 | 323 | 0.428 |
Table 2: Comparison of complete blood count parameters.
| Cases, 110 | Controls, 34 | |||||
|---|---|---|---|---|---|---|
| Laboratory parameters | (Mean) | (Mean) | ||||
| Pre transfusion | At discharge | p-value | On admission | At discharge | p-value | |
| C reactive protein (mg/dl) | 90.7 | 24.5 | 0.001 | 88.7 | 45.9 | 0.001 |
| D-Dimer (mg/L FEU) |
6.7 | 3.5 | 0.001 | 4.9 | 4.2 | 0.542 |
| S. Ferritin (ng/ml) |
2219 | 1268 | 0.003 | 1456.9 | 1324.3 | 0.83 |
| Procalcitonin (ng/ml) | 4.3 | 1.12 | 0.029 | 4.32 | 5.1 | 0.151 |
Table 3: Comparison of inflammatory makers in cases and controls.
Outcome of cases: Overall survival at day 7 of CP transfusion was 70% (77/110) and at day 30 was 68% (75/110). In the 75 (68%) cases, which were alive till study endpoint, the median duration of hospital stay was 12 days (range 3–21 days). The median day to CP transfusion from the time of admission was 3 (range: 1–7 days). The overall mortality was 31%. The main causes of mortality were acute respiratory distress syndrome due to COVID-19 (9 patients), multiorgan failure due to sepsis (8 patients), thromboembolism (5 patients), hospital acquired/fungal pneumonia (6 patients), bleeding diathesis (3 patients) and exacerbation of underlying comorbid conditions (4 patients).
Controls
We enrolled 34 controls in our study. Ten controls were enrolled during the study period while 27 were historical controls. Data for controls was recorded at day of admission and on the day of discharge/death. There were 26 males and 8 females. The median age ± SD (range) was 61 ± 13.3 (range: 27–84 years). In 51% of controls, at least two or more co-morbid conditions were documented which included chronic kidney disease, diabetes mellitus and hypertension. Fourteen patients were admitted in the ICU and required mechanical ventilation at admission, 16 were admitted in SCU while 4 patients were managed in COVID ward. The median ± SD (range) length of stay in this group was 10 ± 8.5 (range: 1–34 days). In CBC values, the only significant parameter was total leucocyte count with a p-value of 0.032 (Table 2). Four patients received tocilizumab as part of treatment while 6 patients received methylprednisolone. In 6 patients a combination of tocilizumab and methylprednisolone was used while 6 received hydroxychloroquine (other treatment options are provided in Figure 1. Except for CRP none of the other inflammatory marker was found to be significant (complete inflammatory markers profile is provided in Table 3. The overall survival in the control group at study endpoint was 62%. The causes of mortality in this group were ARDS due to COVID-19, sepsis, pneumonia and thromboembolism.
Comparison of cases and controls
Survival was increased by 10 days (Figure 2) in patients who received plasma as compared to controls. However, it was not significant. The overall survival in cases was 68% while in controls it was 62%. There was an improvement seen in all inflammatory markers after transfusion of convalescent plasma in cases. Covariates adjusted cox regression model stipulated that use of concomitant therapies e.g. tocilizumab (hazard ratio: 1.09, 95% CI: 0.54-2.23) and methylprednisolone (hazard ratio: 1.3, 95% CI: 0.61–2.88) did not affect overall survival. Age was found to be a significant prognostic marker in both groups as patients less than 60 years had increased overall survival (hazard ratio: 0.33, p-value: 0.001). Presence of two or more co-morbidities provided disadvantage to the overall outcome.
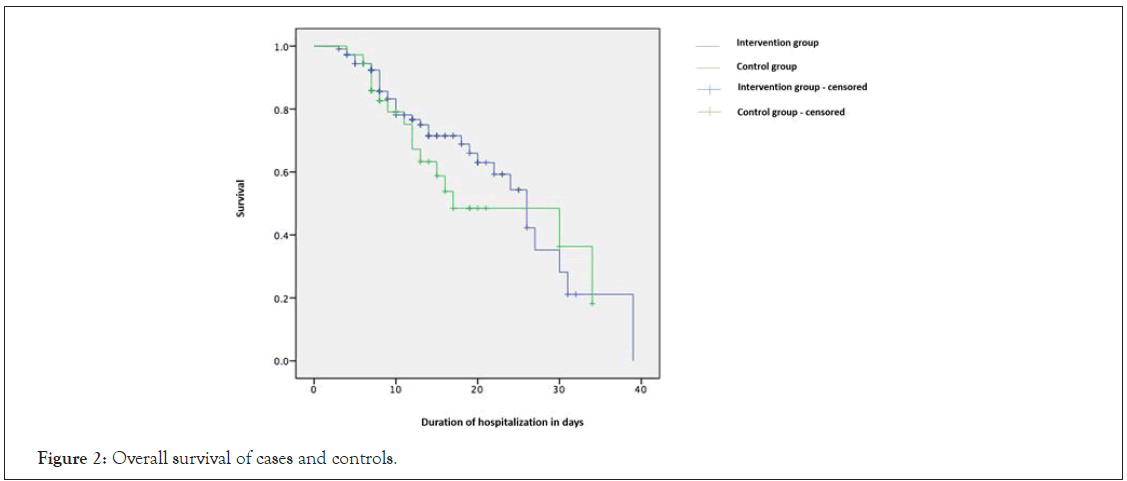
Figure 2: Overall survival of cases and controls.
Discussion
The coronavirus disease 2019 has developed into an unprecedented global health crisis with crucial humanitarian consequences [9]. The current treatment revolves around supportive and critical care, as till date there are no approved vaccines or therapies. It has been shown previously that convalescent plasma transfusion from recovered patients to critically ill patients can prove to beneficial. Shen [8] and Duan [10] have proved that convalescent plasma can be used in the treatment of COVID-19 while large scale clinical trials are being conducted in multiple countries. We carried out a similar non-randomized clinical trial in patients who presented at our center with novel COVID-19 infection. Approximately twothirds of the cohort was males and one-third females, similar to proportion of men and women seen in previous studies [11]. In our cohort, age and presence of co-morbidities (e.g. diabetes mellitus, hypertension) was a significant prognostic indicator since patients and controls more than 60 years of age with one or two underlying chronic disease had a poor outcome. This is similar to study conducted by Salazar et al where a univariate analysis indicated that factors associated with higher mortality were advanced age, hypertension, diabetes, chronic kidney disease and other co-factors [12].
In both the groups of our trial, Tocilizumab and Methylprednisolone were the most common concomitant therapies used which did not affect the overall survival. A confounding variable in many convalescent plasma trials for COVID-19 is the addition of other treatment regimens. These regimens mainly include anti-inflammatory compounds like IL-6 inhibitor tocilizumab, methylprednisolone as seen in our study along with antivirals (e.g. remdesivir) and hydroxychloroquine (which were less commonly used in our cohort). Though tocilizumab and steroids have recently shown to reduce mortality, since convalescent plasma is administered in moderate to severe COVID-19 patients, it is difficult to assess its benefits as a stand-alone treatment due to its use in emergency situations [13]. A blinded, randomized controlled trial will give us the answer.
Various studies have indicated multiple parameters in CBC to be of prognostic value. In our study total leucocyte count was found to significant in both groups. Case series by Ahn [14] and Duan [10] have established that there is improvement in TLC and lymphocyte count after transfusion of convalescent plasma. However, this contrasts with the results reported by us. Interestingly, hemoglobin was found to be a significant parameter in our cases. In the control group, the hemoglobin remained unchanged, however the cases showed a decreased hemoglobin value at discharge, but it was not profound. The current data on hemoglobin values shows heterogeneity but results from one meta-analysis [15] indicate that hemoglobin values are essentially reduced in COVID-19 patients with severe disease as compared to those with milder forms. Our cohort of cases contained all patients with disease severity ranging from moderate to critical. It can be hypothesized that transfusion of convalescent plasma prevented sick patients from developing profound anemia due to severe disease subsequently preventing increased morbidity and mortality.
Since we did not have the facility of Interleukin-6 (IL-6) testing at the time of recruitment of patients in the trial, we looked at four inflammatory markers to monitor the response of convalescent plasma transfusion. All four markers (CRP, D-Dimer, serum Ferritin and procalcitonin) showed significant reduction from baseline. CRP has been widely used to gauge prognosis and decreasing levels of CRP after the use of therapeutics indicates good outcomes in patients with severe disease [16] as seen in our patients as well. A retrospective study from Wuhan, China including 191 patients showed that non-survivors as compared to survivors presented with increased serum ferritin levels [17]. A study done on 25 patients who received CP for COVID-19 showed a trend towards increasing pattern of ferritin by day 3 which gradually settled down to decreased levels by day 7 (12). However, another study done by Omrani [18] showed conflicting results in two groups of patients who received standard of care and convalescent plasma, as ferritin was not found to be a significant prognostic marker (p-value: 0.61). In our cohort the p-value of CP group was 0.003 and controls was 0.830 suggesting that CP does play a role in downgrading the cytokine storm activity in these patients. Shen [8] showed that levels of CRP and procalcitonin decreased considerably after transfusing convalescent plasma in five critically ill patients with COVID-19. Procalcitonin levels are normal in patients with mild disease and elevated in severe/critical disease. Elevated levels are correlated to a nearly 5-fold higher risk for severe SARS-CoV-2 infection [19]. In our cases the procalcitonin decreased to almost normal levels after CP transfusion and was also found to be significant while in the controls the value did not show significant correlation between admission and discharge values.
A high D-dimer at admission was an independent predictor for mortality in COVID-19 patients from Wuhan. Patients with a D-dimer ≥ 2.0 mg/ml had a much higher mortality incidence than those with levels ≤ 2.0 mg/ml (HR 51.5), where the HR was 18.4 in D-dimers ≥ 1.0 mg/ml [20]. Our cases showed a noteworthy reduction in D-Dimer levels in pre and post transfusion CP values (p-value: 0.029). Similar trend was not observed in controls indicating that CP had a role in this phenomenon. CP that is obtained from COVID-19 recovered patients with documented humoral immunity contains large amounts of neutralizing antibodies that are responsible for alleviation of inflammation and overreaction of the immune system caused by coronavirus known as cytokine storm. Cytokine storm, a hyper inflammatory state mediated by the release of cytokines, is the main cause of Acute Respiratory Distress Syndrome (ARDS) [21]. In this regard, disrupting cytokine storm is an important potential therapeutic approach and significant reduction in all inflammatory markers as seen in our cases can be hypothesized to be secondary to neutralizing antibodies present in convalescent plasma.
The oxygen requirements of all alive cases improved considerably as at the time of discharge as approximately 90% of patients did not require non-mechanical ventilation for oxygen support. Previous studies e.g. Abolghasemi [22] suggested that addition of 500 ml of CP to standard of care therapy of hospitalized patients may reduce the need of mechanical ventilation as seen in our study where 11% of the patients with score 5 at admission converted to score 1 or 2 at the time of discharge.
There was no statistically significant overall survival difference in our cases and control group, comparative to various convalescent plasma trials where CP has made a difference in clinical characteristics, inflammatory markers and mechanical ventilation without eliciting a consequential difference in overall survival. The open label phase II PLACID trial [23] also reported no association with reduction in mortality or progression to severe COVID-19 after infusion of convalescent plasma. Our study consolidated these results as well by significantly reducing the levels of cytokine storm markers and ventilator requirements in patients who received CP as compared to the control group.
Plasma transfusion can cause acute and delayed transfusion reactions including febrile and allergic transfusion reactions, transfusion associated dyspnea, transfusion associated acute lung injury and transfusion transmitted infections [24]. The study done on 20,000 hospitalized patients who received CP showed a <1% risk of transfusion reactions [25]. In our cohort, none of the 110 cases reported any adverse or serious adverse events. It can therefore be inferred that transfusion of convalescent plasma is safe and does not result in grave reactions associated with transfusion of blood ± blood products.
This study has several limitations. First, the sample size especially for the controls was small. It is possible that the study was underpowered to investigate the benefit of convalescent plasma transfusion. Second, this study was not a randomized, controlled trial. Although we made every effort to control for important covariates, it was not possible to match for all potentially relevant variables. Third, we did not repeat COVID PCR in all patients to assess for viral clearance since we looked at clinical parameters to document improvement. Fourth, the use of concomitant therapies was allowed in both groups, which could have potentially influenced outcomes. Fifth, we could not measure the antibody titers in CP before transfusion, since validated; reliable, commercial tests for qualitative or quantitative antibody measurement were not available at the time of commencement of this trial. The study has several strengths as well. This is one the few studies from a developing country to report use of convalescent plasma and it was undertaken following all regulatory protocols as per international trial guidelines. We used a standard dose of convalescent plasma (i.e. 500 ml) in all cases deeming the trial to be standardized for CP transfusion. We also followed all donor eligibility criteria as outlined by our institution, American Association of Blood Banks and College of American Pathologists. The transfusion of convalescent plasma did not result in any serious adverse event.
The COVID-19 infection continues to progress with an intense inflammatory response that subsequently leads to serious lung damage causing increased mortality. In the absence of a definitive curative treatment options or vaccine, many therapeutic algorithms have been explored. The use of plasma collected from recovered patients is also being explored; however published results of randomized clinical trials are needed before we make definitive conclusions on efficacy of this passive antibody therapy.
Conclusion
The transfusion of convalescent plasma did not result in any serious adverse event and it was very safe. There was no significant effect in length of hospital stay in both groups. Considerable improvement in all inflammatory markers was noted in cases that received CP. Age and presence of co-morbid conditions were significant factors affecting overall outcome. There was no statistically significant difference in overall survival in both groups.
Funding Disclosure
This study was supported by intramural grant of PKR 2.5 million
Acknowledgements
MH – designed study protocol and submitted for approvals, recruited donors
MUS – conceptualized the idea, contributed in writing of study protocol, critical review of manuscript
MJM – collected data, critical review of manuscript
BJ, NN, KH, AA, IK, AI, RJ, FM – recruited patients, collected data, critical review of manuscript
NA – recruited donors, analyzed data, drafted manuscript
1. Clinical Trial Unit at Aga Khan University – for complete trial oversight and monitoring of practices.
2. Ethical review committee, Aga Khan University – for approval of trial.
3. Sindh Blood Transfusion Authority – for approval of trial.
4. Drug Regulatory Authority of Pakistan – for approval of trial.
5. National Bioethics Committee – for approval of trial.
6. PHC global – for analysis of data.
REFERENCES
- Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054.
- Van der Hoek L. Human coronaviruses: What do they cause? Antivir Ther. 2007;12(4):651-658.
- Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ Res. 2020;109819.
- www.worldometers.info
- Peng F, Tu L, Yang Y, Hu P, Wang R, Hu Q, et al. Management and treatment of COVID-19: The Chinese experience. Can J Cardiol. 2020.
- Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757-2765.
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152.
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582-1589.
- Abdi M. Coronavirus disease 2019 (COVID-19) outbreak in Iran: Actions and problems. Infect Control Hosp Epidemiol. 2020;41(6):754-755.
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490-9496.
- Li S, Zhao H, Sun Y, Wang P, Li H, Duan M. Application of convalescent plasma for the treatment of adult patients with coronavirus disease 2019. 2020;32(6):646-651.
- Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290-2303
- Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: A multicentre cohort study (SAM-COVID-19). Clin Microbiol Infect. 2020.
- Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35.
- Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116-117.
- Rodriguez Z, Shane AL, Verkerke H, Lough C, Zimmerman MG, Suthar M, et al. COVID-19 convalescent plasma clears SARS-CoV-2 refractory to remdesivir in an infant with congenital heart disease. Blood Adv. 2020;4(18):4278.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
- Omrani AS, Zaqout A, Baiou A, Daghfal J, Elkum N, Alattar RA, et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J Med Virol. 2020.
- Wu CP, Adhi F, Highland K. Recognition and management of respiratory coinfection and secondary bacterial pneumonia in patients with COVID-19. Cleve Clin J Med. 2020;87(5).
- Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324-1329.
- Xi Y. Convalescent plasma therapy for COVID-19: A tried-and-true old strategy? Signal Transduct Target Ther. 2020;5(1):1-4.
- Abolghasemi H, Eshghi P, Cheraghali AM, Fooladi AA, Moghaddam FB, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci. 2020;59(5):102875.
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;324(5):460-470.
- Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888-1897.
Citation: Hasan M, Shaikh MU, Malik MJ, Jamil B, Nasir N, Habib K, et al. (2021) Safety and Efficacy of Convalescent Plasma Treatment in COVID-19 Patients: Collate Trial. J Vaccines Vaccin. 12:448.
Copyright: © 2021 Hasan M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

