Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 11, Issue 4
Retrospective Microbiological Study of Atypical Recurrent Pharyngitis in Patients Presenting the White-line Clinical Sign
Neri G1*, Pennelli A2, Del Boccio M2, Neri L1, Toniato E2, Tenaglia R2, Gallenga CE3, Gallenga PE4 and Del Boccio G22Medical, Oral and Biotechnological Sciences Department, University G.d’Annunzio, Chieti, Italy
3Sperimental and Clinical Sciences Department, University G.d’Annunzio, Chieti, Italy
4Biomedical/Specialist Surgical Sciences Department, University of Ferrara, Italy
Received: 13-Apr-2019 Published: 15-May-2019
Abstract
Background: Chronic atypical oropharyngeal disease in adults, accompanied with chronic cough, can occur at any age manifesting itself with different grade evolutive diseases. Often their pathogenesis is attributed to gastroesophageal reflux, to virosis or to unspecified immune deficiencies but some clinical aspects, such as the simultaneous presence of urinary disorders, the temporal scanning of the recurrence and the reduced response to antibiotic therapy, suggests a different or a superimposed pathology.
Methods: The present study was carried out to assess retrospectively biopsy and biological materials from a population afflicted by atypical recurrent pharyngitis, presenting a âwhite lineâ clinical sign into the context of respiratory difficulties, manifesting chronic choking cough (CCC), laryngopharyngeal (LPR) and gastroesophageal (GERD) reflux diseases. This population, already clinically, endoscopically and histologically characterized, was newly studied following the microbial approach by cultural and molecular procedures.
Results: We analyzed 14 biopsy, 60 biological pooled materials from lingual, pharyngeal, post nasal drip mucoid secretions and sputum (here initialled: LPNS) and 60 lingual cell and salivary secretions (LCSS) resulted positive to Chlamydiaceae [(Chlamydia pneumoniae (Cp) and Chlamydia trachomatis (Ct)], to urogenital Mycoplasmas [Mycoplasma hominis (Mh) and Ureaplasma urealyticum (Uu)], to Helicobacter pylori (Hp) into the context of a changeable overlapping with other typical bacteria, belonging to Corynebacteria, Enterobacteria, Streptococci and Staphylococci groups.
Conclusions: Our data indicated that atypical infections [C. trachomatis and urogenital Mycoplasmas (Mh and Uu)], together with Cp, were the underhand pathogens of an initial chronic oropharyngeal scenery until now unrecognized, triggering, after decades, the respiratory problems in middle and old subjects genetically susceptible. The presence of white line clinical sign, endoscopically observed, together with an altered pH salivary secretion, into the scenario of CCC, LPR and GERD reflux manifestations, refractory to non-specific medical therapy, represents a pathognomonic triad to include routinely these valuations into the diagnostic protocol of an atypical recrudescent pharyngitis.
Keywords
Whiteline reflux; Chronic choking cough; Laryngopharyngeal and gastroesophageal reflux diseases; Chlamydiaceae; Mycoplasmataceae; Helycobacter Pilory; Molecular age
Introduction
Oral cavity, rhinopharynx and larynx, are anatomical structures of access to respiratory and digestive tracts [1] and their mucosae are continuously stressed by the chemical and microorganism agents. The healthy human microbiome is the consequence of a durable complex interaction developing between microbe distributions and different human modulating mechanisms, regulated (and/ or harmonized) by the coexistence of several factors, beginning from conception to death [2,3]. Physiologically, the laryngeal reflex (LR) represents an involuntary and necessary protective response to stimuli in the larynx to regulate the three principle functions: airway protection, respiration and voice production. The LR entity is valued by ENT specialists to predict dysphagia or to portend clinical phenotypes of chronic cough, vocal cord dysfunction or paediatric apnoeas [4]. In adult, the LR has been observed frequently in population showing gastroesophageal reflux, obstructive sleep apnoea, chronic obstructive pulmonary disease, neurodegenerative disorders and advanced age [4,5]. An important defence mechanism against external noxious stimuli is operated by mucociliary transport which carrying out the mucous released by goblet cells, protect their mucosae from chemical agent and microorganism [6]. In spite of different typological aetiology, the evaluation and the management of chronic cough is still a much debated question [7]. In neonatal age, a cough is considered chronic if present for more than four weeks, while in most childhood cases it is caused by chronic bronchitis, asthma and/or upper airway cough syndrome. Initially, the evaluation of these exacerbating scenarios should be focused on bacterial aetiologies, targeting at treatment and monitoring for resolution, but unfortunately still nowadays we are witnessing a persistence of an old vision of these infective diseases [8,9], without the inclusion of atypical sexual bacteria, like Chlamydia trachomatis and urogenital Mycoplasmas. On the contrary, in adults, several clinical chronic oropharyngeal scenarios, accompanied with chronic cough, can occur at any age manifesting itself with different grade evolutive diseases [10-12]. Further, chronic choking cough may be produced by a number of different disorders in distinct anatomic sites and still represent a frustrating and common problematic condition resulting in significant psychological and physical sequelae as well as enormous financial costs in terms of expensive health care and loss of working days [13]. Further, chronic choking cough (CCC) can be caused by other worsening symptomatologies as gastroesophageal reflux disease (GERD), laryngopharyngeal reflux disease (LPR) and no asthmatic eosinophilic bronchitis overlapping themselves with other bacterial aetiologies assignable to different principal conditions. So, recently it is also reported a possible H. pylori association with chronic tonsillitis and laryngopharyngeal reflux in the tonsillar biopsy samples [14]. Although from human proteomic studies on the salivary protein characterizations and them genetic polymorphisms [15] emerged the important role of saliva as a non-invasive approach for diagnostic and prognostic purposes, only recently has been clarified the different role of atypical pathogen [M. pneumoniae (Mp) or C. pneumoniae (Cp)] in terms of ethnogenesis [16], in terms of polymorphic genetic susceptibility [17] and that of common pathogens [M. catarrhalis (Mc), H. influenza (Hi), or S. pneumoniae (Sp)] in terms of recrudescent childhood asthmatic episodes [18]. At this purpose, the oral and nasal cavity, rhinopharyngeal, laryngeal and upper airway mucosae represent perfect niches for common and atypical microbial adhesion and biofilm formation [19]. From long time, Chlamydia trachomatis, an endocellular obligate parasite prevalent in genital tract, had been defined “Trojan horse” [20], presenting different sequelae and representing a significant source of morbidity. At the same time, urogenital Mycoplasmas, esoparasites of mucosae membrane surfaces, living their inside, were frequently found together with Ct, when they were researched in any human chronically inflamed source investigated by appropriate cultural procedures [10-12,21-23]. On the basis of these considerations, we have investigated the biopsy, LPNS and LCSS sources to evaluate the presence of atypical and common bacteria in patients affected by chronic pharyngeal inflammatory process presenting CCC, GERD and LPR reflux diseases, showing the white-line clinical sign [24]. Virus and micete were excluded for their well typified clinical manifestations and resolutive medical therapy.
Materials and Methods
Cohort patients
Biopsy (14/60), LPNS and LCSS (60/60) samples, from outpatients of an initial cohort of 200 (30%) of Caucasian subjects, examined from June 2011 to September 2011 and already clinically selected for rhino-pharyngeal globe (RPG) sensation, manifesting CCC, LPR and GERD reflux symptomatologies into the chronic pharyngitis scenery, presenting a white line sign, were reassessed hypothesizing a causative microbial involvement.
Ethics statement
The informed consent was already obtained from each outpatient during the enrolment in the prior study [24], while attending at the first visit in the ambulatory of the ENT Clinic Unit, SS. Annunziata Hospital (Chieti). A waiver of informed consent was not necessary included, because this study excludes a direct contact with a population already studied and all patient identifiers were removed from the dataset on initial collection. All patients already had adhered to the Declaration of Helsinki and to the ICH-GCP, GU 184/2003. The methodologies of this study were commercial products and others were conformed to conventional procedure standard of literature.
Sample preparations
Biopsy material from rhinopharynx wall, below the white line and from the reticular tissue, above the white line, was chopped finely by little bistoury and immediately placed into an Eppendorf tube, stored at -30°C. This material was thawed before DNA extraction and recovered immediately with 1,0 mL of sterile saline solution, vigorously stirred to disperse cellular material and immediately processed to detect the Cp-, Ct-, Hp- DNA by PCR and to culture for common and atypical urogenital bacteria.
LPNS sample of the pooled biological materials, from epithelial lingual and pharyngeal living cells, added to mucoid post nasal drip and sputum, collected by scraping and expectorant modalities respectively, were pooled for each patient with 2.0 mL sterile saline solution and stored at -30°C. Before use, this suspension was thawed, vigorously stirred to disperse cellular material and treated with 8.0 mL of 2.0 mM cysteine saline solution, incubated at 37°C to solubilize mucoid material; afterwards it was centrifuged at 5000 rpm at 4°C for 10 min. The pellet was collected with 1.0 mL of sterile saline solution and used for molecular and cultural researches.
LCSS material from lingual surface was sampled by sterile wood spatula scraping, dispersed with 1.0 mL sterile saline solution and stored at -30°C. An aliquot of each of three sources was used to measure the pH value immediately before of freezing. It was thawed and immediately used as above reported for molecular and cultural analyses.
Molecular section
Each of biopsy, LPNS and LCSS aliquots (300 μL) for Ct-DNA analysis were centrifuged at 12,000 rpm for 5‘at 4°C; each pellet was immediately scattered with 180 μL of 0.025 M buffer phosphate pH=7.0 plus 1.0 mM EDTA, added with 25 μL of proteinase K, incubated at 56°C overnight; 210 μL of absolute ethanol was added to precipitate the DNA extracted, filtered and eluted from column with 60 μL of elution buffer. 50 μL of this solution was amplified into a Thermocycler of Applied Biosystem (Clemens GmbH), following the manufacturer’s instructions [BioAesis srl, Jesi (AN), Italy (Line-20 Chlamydia trachomatis code 04LI20]. Cp- DNA sample (300 μL) was carried out following the procedure already reported [25,26] and used for our previously studies [8-10,24]. Hp-DNA sample (300 μL) was processed for cagA and ureC templates, using QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s procedure [27].
Cultural section
Aliquot of 75 μL from biopsy, LPNS and LCSS was dispersed separately and cultured by Mycoplasma IST 2, following the BioMerieux Manual procedures. 1.0 μL of this remaining suspension was plated onto chromID CPS agar and Columbia CAN agar. Total Microbial Charge (TMC), expressed as the total number of colony-forming units per milliliter (CFU/mL), was calculated and the relative presence of each species was reported as percentage of TMC (considered as 100%), and as previously reported [10]. Monoculture was considered significant at ≥106 CFU/mL. API Coryne galleries (code 20 900) were identified by an API automated system, the confidence ranged between 94.7% and 99.9%, indicating a high level of identification. Cultural section and relative colony characterization were carried out using products purchased from BioMerieux Italia. Streptococcus pneumoniae (Spn), grown on Columbia CNA, underwent PCR analysis to confirm the eventual misidentification of Streptococcus pseudopneumoniae as true S. pneumoniae [26].
Results
All biopsies, LPNS and LCSS sources were positive for atypical pathogens (Table 1). In detail, C. pneumoniae (Cp-DNA) was positive into 3/14 biopsies (21.43%), while it was found also into 13/60 LPNS (21.67%), but only 6/60 into LCSS (10.00%). C. trachomatis (Ct-DNA) was positive into 12/14 biopsies (85.71%), while it was present into 53/60 (88.33%) of both LPNS and LCSS sources, overlapping itself between them (Cp and Ct) with twelve cases. The mycoplasmal positiveness (Mh and Uu) into the biopsy was of the 100%. Particularly, the Mh positivity was of the 64.28% (n=9/14), while the Uu positivity was of the 78.57% (n=11/14), spreading themselves completely in all samples and overlapping into 42.86% (n=6/14) with a very low infectant charge (102 UCC/ mL). The Mh positiveness for LPNS and LCSS sources was higher than biopsy, resulting in 80.00% (48/60) and 85.00% (51/60) with a UCC/mL of 103 and 104 respectively, while the Uu positiveness for the same sources were both of the 88.33% (53/60), but with a major infectant charge 104 and 105 UCC/mL. At the same time, each of two different patients resulted positive respectively for H. pylori-DNA and for S. pneumoniae (Sp-DNA) in all sources together with atypical sexual pathogens. Corynebacteria, Enterobacteria, Streptococci and Staphylococci species were random distributed in the same samples together atypical pathogens at a variable percentage, ranging from 5.0 x 104 to 5.0 x 105 UFC/mL of the TMC. Median pH value of the three sources were pH=6.7 ± 0.2, pH=7.7 ± 0.4 and pH=8.4 ± 1.4 for biopsy, LPNS and LCSS respectively.
| Biopsy (n=14) | LPNS (n=60) | LCSS (n=60) |
|---|---|---|
| Cp-DNA: positive n=3/14 (21.43%) | Cp-DNA: positive n=13/60 (21.67%) | Cp-DNA: positive n=6/60 (10.00%) |
| Ct-DNA: positive n=12/14 (85.71%) | Ct-DNA: positive n=53/60 (88.33%) | Ct-DNA: positive n=53/60 (88.33%) |
| Mh:102 UCC/mL n=9/14 (64.28%) | Mh:103 UCC/mL n=48/60 (80.00%) | Mh:104 UCC/mL n=51/60 (85.00%) |
| Uu:102 UCC/mL n=12/14 (85.71%) | Uu:104 UCC/mL n=53/60 (88.33%) | Uu:105 UCC/mL n=53/60 (88.33%) |
| Hp-DNA: positive n=1/14 | Hp-DNA: positive n=1/60 | Hp-DNA: positive n=1/60 |
| Sp-DNA(*) n=1/14 | Sp-DNA(°) n=1/60 | Sp-DNA (°) n=1/60 |
| pH = 6.7 ± 0.2 | pH = 7.7 ± 0.4 | pH = 8.4 ± 1.4 |
(°) culturally isolated and molecularly confirmed.
Table 1: Atypical bacteria results from biopsy, LPNS and LCSS sources from oropharyngeal cavity and upper respiratory tract.
Discussion and Conclusion
It is universally accepted that these sexual atypical bacteria are asymptotically and symptotically present into 70-80% and into 20-30% respectively of the whole population with different grade and evolutive sequelae, depending on the individual host genetic susceptibility [28]. Recent immunological advances demonstrated the pivotal roles of different individual host genetics in directing the innate immune response to Cp infection [29] and in modulating Ct pathogenesis [30]. Considering the initial Chlamydia trachomatis and urogenital Mycoplasmas contaminations an event always possible during sexual activity, these atypical infections, impairing the trophoblast function [31], could inflict serious injuries on different cellular structures of foetal tissue in active accretion, colonizing the humankind from early stage of conception [32]. At this purpose, it has been noted from several times, but recently updated [33], that chlamydial and mycoplasmal infections in early asymptomatic pregnant women [34] are linked to increased risk of spontaneous abortions, preterm birth and/or stillbirth [35]. Although the atypical bacteria, like C. pneumoniae and M. pneumoniae, were frequently considered in the acute and/or chronic manifestations of the different oropharyngeal and upper respiratory diseases of the pediatric age [8,9], only very poor studies had been carried out on neonates including both those atypical sexual-derived and evaluating them into mother’s cervical-vaginal ecosystem [23]. Actually, although recent acquisitions have clarified the human microbiome compositions and its development during three crucial developmental stages, like pregnancy, birth, and infancy [2], and new research strategies have been established [3], new approaches on the maintenance of human health and microbial perturbations into the contest of human different pathophysiology of autoimmune disorders will be necessary. In spite of these recent acquisitions on human microbiome and on mechanisms of chlamydial and mycoplasma diseases, the clinical studies on ill hospitalized population are carried out still on the knowledge of old microbiological protocols following outdated laboratory procedures, excluding them from routine investigations. Thus, in this retrospective preliminary remark, we found a full dispersion and overlapping of sexual atypical bacteria into the biopsy, LPNS and LCSS of patients affected by chronic pharyngitis evolving, decades later, in CCC, LPR and GERD manifestations. These results constitute a new indication in directing further the methodological approaches for studying a population of patients presenting a white line clinical sign into the contest of CCC, LPR and GERD reflux diseases, as recently detailed in a new clinical case [36]. Further, the lack of a careful clinical evaluation of these atypical unrecognized scenarios (Figures 1 and 2), of a careful timing choice of the molecular procedures [Ct-DNA stability [36-38] against Ct-RNA increased clearance in spontaneous pharyngeal chronic scenery [39], and of a sampling procedural adequacy (swabbing in “acute” [23]) against (scraping in “chronic” moment of the inflammatory state [12-12,21,23,36-38]) make the choice of molecular procedure a technique yet to be well defined. The results for chlamydial and mycoplasmal presences into biopsy, LPNS and LCSS demonstrate once again the temporal and procedural correctness of our molecular choice in detecting the nucleic acids presence for these atypical bacteria. No additional oropharyngeal alterations were observed for H. pylori (Figures 1 and 2, panel B and B’) and for S. pneumoniae (Figures 1 and 2, panel C and C’) positive patients into the contest of to Chlamydiaceae and urogenital Mycoplasmas. An exhaustive description of the biopsy histological pictures, performed above and below the white line, previously reported [24], revealed a changeable morphology of the inflammatory state, varying from chronic flogistic infiltration to intraepithelial apoptosis, changing through the different evolutive phases of chronic inflammatory setting, probably determined by genetic susceptibility and by a temporary spontaneous pharyngeal chlamydia trachomatis RNA clearance [39], but decisively assignable to sexual atypical pathogens. To confirm this, there were the progressive quantification of atypical bacteria culturally detected into LPNS and LCSS sources respect to biopsy. Further, we can affirm that LPNS and LCSS biological materials from oral cavity represent the best sources to evaluate these pathogens during a precocious contamination. Thus, the highest mean value of pH of LCSS (pH=8.4 ± 1.4) compared to LPNS (pH=7.7 ± 0.4), directly confirmed the high significance of this parameter in signaling in advance a silent mycoplasmal infection in two different, but contiguous localizations of the oral cavity. At this purpose, it had already been reported that the high pH value represent the first step towards the unbalance of hypothiocyanite ion (OSCN-) human saliva formation [39], one of the physiological antimicrobial products of the salivary peroxidase system of human microbiome [41,42]. The physical salivary valuation (“serous” against “mucous” change), together with the high pH value, would represent an additional precocious marker of a complex protein mixture, indicative of an oral altered microbiota [10] and of a source material easily, quickly and noninvasively obtainable to evaluate human microbiome in different phenotype diseases, as already reported for ocular microbiota [23]. Thus, an increase of pH value from physiological range would represent the first parameter accurately measurable and easily obtainable to orient their researches. In conclusion, identification of sexual atypical infections in oral cavity, collecting an accurate anamnesis on the clinical signs and symptomatology of oral cavity sexually involved, introducing two simple chemical and physic parameters, paying utmost attention to the sampling modality, swabbing vs scraping, for chronic chlamydial infections [23,36,38], introducing routinely their researches, we ascertained their involvement in oropharyngeal and respiratory diseases, that sometimes could trigger reactive arthritis also in younger patient genetically susceptible [12,21] and/or in other clinical case still to reveal. Nowadays, it remains hard for us to comprise the motivation of their persistent exclusion in clinical studies on pathogens causing upper respiratory tract infections in outpatients, considering the chronicity of these infections and relative diseases, the necessity of health economy reduction and of loss working hours, providing a better medical assistance, attending the development of effective and durable vaccine [43]. Lastly, considering their dangerous effects on embryonal development during any phase of foetal growth [28], depending on their capacity to escape from mother’s innate immune system, manifesting their pathogenicity at the birth [23] and/or on their capacity to modify the estrogen-progesterone hormonal levels [31], they could prompt a major tissue alterations causing a variety of other embryonal and genetic anomalies. Further studies will be necessary to comprehend truly complexity of the human microbiome considering their asymptomatic presence into the major part of human population.
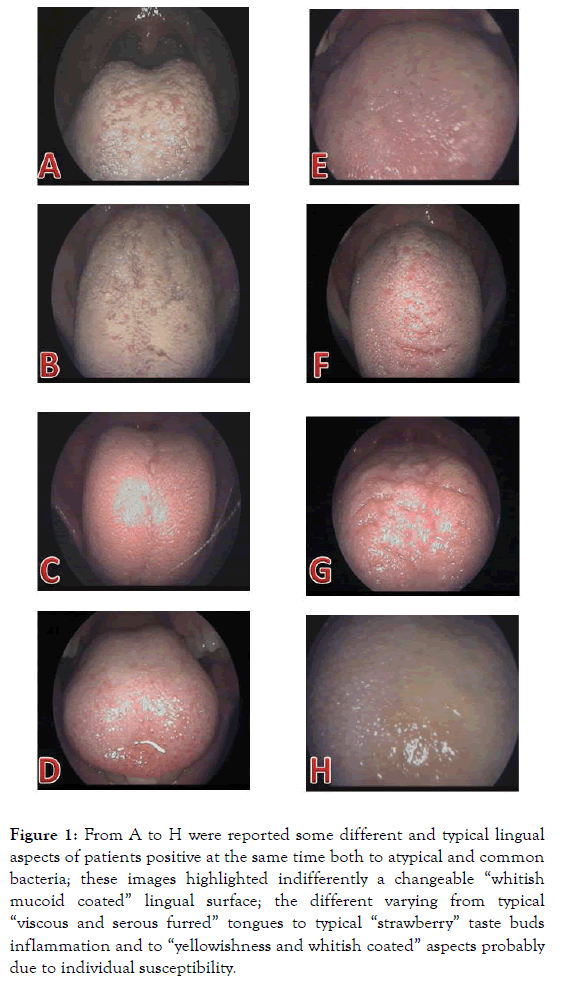
Figure 1. From A to H were reported some different and typical lingual aspects of patients positive at the same time both to atypical and common bacteria; these images highlighted indifferently a changeable “whitish mucoid coated” lingual surface; the different varying from typical “viscous and serous furred” tongues to typical “strawberry” taste buds inflammation and to “yellowishness and whitish coated” aspects probably due to individual susceptibility.
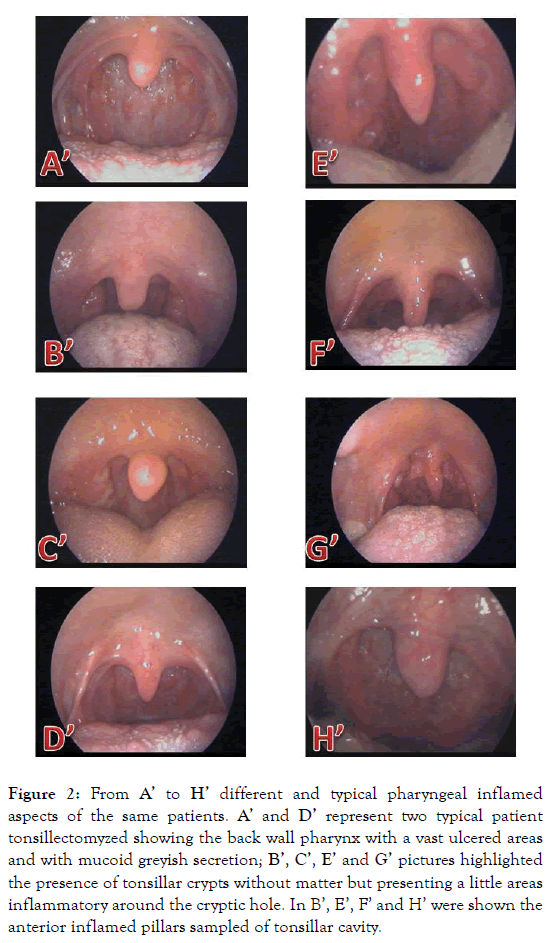
Figure 2. From A’ to H’ different and typical pharyngeal inflamed aspects of the same patients. A’ and D’ represent two typical patient tonsillectomyzed showing the back wall pharynx with a vast ulcered areas and with mucoid greyish secretion; B’, C’, E’ and G’ pictures highlighted the presence of tonsillar crypts without matter but presenting a little areas inflammatory around the cryptic hole. In B’, E’, F’ and H’ were shown the anterior inflamed pillars sampled of tonsillar cavity.
REFERENCES
- Domer AS, Kuhn MA, Belafsky PC. Neurophysiology and clinical implications of the laryngeal adductor reflex. Curr Otorhinolaryngol Rep. 2014;1(3):178-182.
- Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 2016;7:1031.
- https://doi.org/10.17226/24960
- Phua SY, McGarvey LP, Ngu MC, Ing AJ. Patients with gastro-oesophageal reflux disease and cough have impaired laryngopharyngeal mechanosensitivity. Thorax. 2005;60(6):488-491.
- Clayton NA, Carnaby-Mann GD, Peters MJ, Ing AJ. The effect of chronic obstructive pulmonary disease on laryngopharyngeal sensitivity. Ear Nose Throat J.2012;91(9):372-374.
- Allaire JM, Morampudi V, Crowley SM, Stahl M, Yu H, Bhullar K, et al. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am J Physiol Gastrointest Liver Physiol.2017;314:360-377.
- Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183(6):708-715.
- Jama-Kmiecik A, Frej-Mądrzak M, Sarowska J, Choroszy-Król I. Pathogens causing upper respiratory tract infections in outpatients. Adv Exp Med Biol.2016;934:89-93.
- Mantero M, Aliberti S, Azzari C, Moriondo M, Nieddu F, Blasi F, et al. Role of Streptococcus pneumoniae infection in chronic obstructive pulmonary disease patients in Italy. Ther Adv RespirDis.2017;11(10):403-07.
- Di Bonaventura G, Del Boccio M, Pennelli A. Unusual oropharyngeal asymptomatic manifestations caused by atypical pathogens detected by PCR into altered ecosystems of an infertile couple. BMRJ. 2015;5:447-458.
- Neri G, Del Boccio M, Pennelli A, Martinotti S, Tenaglia R, Pugliese M, et al. Jugulodigastric lymph node inflammation derived from chronic atypical oropharyngeal phlogosis recurrent annually after the flu virus vaccination: a holistic vision of clinical case solved after chlamydicidal antibiotic therapy. Int J Immunopathol Pharmacol. 2012;25(4):835-847.
- Del Boccio M, Pennelli A, Toniato E, Martinotti S, Tenaglia R, et al. Enigmatic question of early reactive arthritis disclosed after researches of Mycoplasmas, Chlamydia trachomatis and enteropathogens following the holistic vision of human being. J Biol Regul Homeost Agents. 2013;27(4):933-946.
- Palombini BC, Villanova CA, Araújo E, Gastal OL, Alt DC, Stolz DP, et al. A pathogenic triad in chronic cough: asthma, postnasal drip syndrome, and gastroesophageal reflux disÂÂease. Chest. 1999;116(2):279-284.
- Siupsinskiene N, Katutiene I, Jonikiene V, Janciauskas D, Vaitkus S. Helicobacter pylori in the tonsillar tissue: A possible association with chronic tonsillitis and laryngopharyngeal reflux. J Laryngol Otol. 2017;131(6):549-556.
- Cabras T, Iavarone F, Manconi B, Olianas A, Sanna MT, Castagnola M, et al. Topdown analytical platforms for the characterization of the human salivary proteome. Bioanalysis. 2014;6:563-581.
- Sharavin AO, Smirnova SV. Mycoplasma and Chlamydia as ethiological factors of bronchial asthma in terms of ethnogenesis. Vestn Ross Akad Med Nauk. 2013;7:57-60.
- March ME, Sleiman PMA, Hakonarson H. Genetic polymorphisms and associated susceptibility to asthma. Int J Gen Med. 2013;6:253-265.
- Ver Heul A, Planer J, Kau AL. The Human Microbiota and Asthma. Clin Rev Allergy Immunol. 2018.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol.2010;8:623-633.
- Diebel ND Jr, Williams JK. Chlamydia trachomatis. A trojan horse.J Fla Med Assoc. 1995;82(6):411-414.
- Del Boccio M, Lobefalo L, Pennelli A, Toniato E, Martinotti S, Tenaglia R, et al. Can latent synergism of intestinal pathogens be responsible for inflammaging process causing Reiter’s syndrome in a young patient HLA-B27 infected by atypical pathogens? A holistic view and clinical biochemistry reinterpretation.J Biol Regul Homeost Agents. 2012;26(4):741-755.
- Astrauskiene D, Griskevicius A, Luksiene R, Panaviene V, Venaliene J. Chlamydia trachomatis, Ureaplasma urealyticum, and Mycoplasma hominis in sexually intact girls with arthritides. Scand J Rheumatol.2012;41:275-279.
- Gallenga PE, Del Boccio M, Gallenga CE, Neri G, Pennelli A, Toniato E, et al. Diagnosis of a neonatal ophthalmic discharge, Ophthalmia Neonatorum, in the “molecular ageâ€Â: Investigation for a correct therapy. J Biol Regul Homeost Agents. 2018;32(1):91-98.
- Neri G, Pugliese M, Castriotta A, Mastronardi V, Pasqualini P, Colasante A, et al. White-line: A new finding in laryngopharyngeal reflux objective evaluation. Med Hypotheses. 2013;80(6):769-772.
- Gnarpe J, Eriksson K. Sample preparation for Chlamydia pneumonia PCR. APMIS. 1995;103(4):307-308.
- Stralin K, Backman A, Holmberg H, Fredlund H, Olcén P. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples. APMIS. 2005;113(2):99-111.
- Pandya HB, Agravat HH, Patel JS. Prevalence of Specific Helicobacter Pylori cagA, vacA, iceA, ureC genotypes and its clinical relevance in the patients with acid-peptic diseases. J Clin Diagn Res.2017;11(8):23-26.
- Asner SA, Morré SA, Bochud PY, Greub G. Host factors and genetic susceptibility to infections due to intracellular bacteria and fastidious organism. Clin Microbiol Infect. 2014;20(12):1246-1253.
- Shimada K, Crother TR, Arditi M. Innate immune responses to Chlamydia pneumoniae infection: Role of TLRs, NLRs, and the inflammasome. Microbes Infect.2012;14(14):1301-1307.
- Al-Kuhlani M, Rothchild J, Pal S, de la Maza LM, Ouburg S, Morré SA, et al. TRAIL-R1 Is a negative regulator of pro-inflammatory responses and modulates long-term sequelae resulting from Chlamydia trachomatis infections in humans. PLoS ONE. 2014;9(4):e93939.
- Azenabor AA, Kennedy P, Balistreri S. Chlamydia trachomatis infection of human trophoblast alters estrogen and progesterone biosynthesis: An insight into role of infection in pregnancy sequelae. Int J Med Sci. 2007;4(4):223-231.
- Heerema-McKenney A. Defense and infection of the human placenta. APMIS. 2018;126(7):570-588.
- Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW et al. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116-133.
- Cao CJ, Wang YF, Fang DM, Hu Y. Relation between mycoplasma infection and recurrent spontaneous abortion. Eur Rev Med Pharmacol Sci.2018;22(8):2207-2211.
- Bagheri S, Roghanian R, Golbang N, Golbang P, Nasr Esfahani MH. Molecular evidence of Chlamydia trachomatis infection and its relation to miscarriage. Int J Fertil Steril. 2018;12(2):152-56.
- Neri G, Pennelli A, Del Boccio M, et al. Molecular Evidences of Chlamydia trachomatis and Urogenital Mycoplasmas Infections from Birth Evolving into Multisite Infections. EC Microbiology 15.5 (2019).
- Gallenga PE, Del Boccio M, Rapinese M, Di Iorio A, Toniato E, Martinotti S. Molecular approach by PCR is the best method to detect the presence of Chlamydia trachomatis and to define the true agent of ocular bacterial inflammation. Int J Immunopathol Pharmacol. 2011;24(2):285-296.
- Gallenga PE, Del Boccio M, Lobefalo L, Rapinese M, Pennelli A, Martinotti S. Ocular clinical pictures disclosed by PCR molecular diagnosis of Chlamydia trachomatis infection performed following the appropriate sampling modality in ocular ecosystem. Int J Immunopathol Pharmacol. 2012;25:1099-1105.
- van Rooijen MS, van der Loeff MF, Morré SA, van Dam AP, Speksnijder AG, de Vries HJ. Spontaneous pharyngeal Chlamydia trachomatis RNA clearance. A cross-sectional study followed by a cohort study of untreated STI clinic patients in Amsterdam, The Netherlands. Sex Transm Infect. 2015;91(3):157-164.
- Xulu BA, Ashby MT. Small molecular, macromolecular and cellular chloramines react with thiocyanate to give the human defense factor hypothiocyanite. Biochemistry. 2010;49(9):2068-2074.
- Kalmár J, Woldegiorgis KL, Biri B, Ashby MT. Mechanism of decomposition of the human defense factor hypothiocyanite near physiological pH. J Am Chem Soc. 2011;133(49):19911-19921.
- Amado FM, Ferreira RP, Vitorino R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin Biochem. 2013;46(6):506-517.
- Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348(6241):aaa8205.
Citation: Neri G, Pennelli A, Del Boccio M, Neri L, Toniato E, Tenaglia R, et al. (2019) Retrospective Microbiological Study of Atypical Recurrent Pharyngitis in Patients Presenting the White-line Clinical Sign. J Microb Biochem Technol 11:420.
Copyright: © 2019 Neri G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

