Indexed In
- Academic Keys
- ResearchBible
- CiteFactor
- Access to Global Online Research in Agriculture (AGORA)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 9, Issue 2
Researching Novel Variants in Endometriosis using Next Generation Sequencing Variant Analysis
Shambo Datta1 and Manisha Rana2*2Department of Forensic Science, Amity University, Uttar Pradesh, India
Received: 17-Aug-2020 Published: 07-Aug-2020, DOI: 10.35248/2329-6682.20.9.153
Abstract
Endometriosis is characterized as the presence of ectopic endometrial tissue outside of the uterine, most generally in the ovaries and peritoneum. It is an illness that is impacted by various elements. It is additionally a typical gynaecological confusion and influences roughly 10-15% of all women of regenerative age. Later molecular and pathological examinations demonstrate that endometriosis may fill in as an antecedent of ovarian malignant growth (endometriosis associated ovarian disease, EAOC), especially endometrioid furthermore, clear cell ovarian malignant growths. Albeit histological and epidemiological investigations have shown that endometriosis has a malignant potential, the molecular component that underlies the harmful change of endometriosis is as yet questionable, and the exact component of carcinogenesis must be completely illustrated. At present, the advancement and improvement of another sequencing innovation, next-generation sequencing (NGS), has been progressively significant in malignant growth genomics examine. Lately, NGS has likewise been used in clinical oncology to propel the customized treatment of malignancy. Also, the affectability, speed, and cost make NGS a profoundly alluring stage contrasted with other sequencing modalities. Thus, NGS may lead to the recognizable proof of driver mutations and fundamental pathways related with EAOC. Our sole motivation behind the study was to decipher new variants if any and report any unreported variants identified with genes. We have performed a variant analysis investigation with the assistance of Next Generation Sequencing GALAXY device accessible on the web.
Keywords
Endometriosis; Next Generation Sequencing; Variant analysis; Novel variants
Introduction
Endometriosis (E) is benign gynaecological condition, debilitating, estrogens-subordinate, progesterone-safe, inflammatory issue related with pelvic pain and infertility, with endometrial (uterine covering)-like tissue present outside the uterus (Giudice). By retrograde menstruation, endometrial tissue cells are transplanted to the pelvis (Sampson) where they set up a blood supply, react to cyclic hormones, develop, attack encompassing structures, progress toward becoming innervated (Berkley, et al.; Tokushige, et al.), and inspire a nearby inflammatory reaction and scarring (Giudice LC) [1,2].
Endometriosis influences 5%–10% of regenerative age women (Eskenazi and Warner) [3] and half of women with pelvic pain as well as infertility (>100 million women around the world) (Meuleman et al.) [4] and is a noteworthy reason for inability and bargained personal satisfaction (Sasson and Taylor; Anglesio, et al.) [5-7]. Pelvic, lower stomach and back pain, and urinary and gastrointestinal indications make diagnosis challenging, on the grounds that numerous indications are nonspecific or are related with different disorders (Giudice) [8]. Pelvic inflammation and nerve invasion result in pain (Berkley, et al.; Tokushige, et al.) [9,10], and infertility is expected to ovulatory dysfunction, poor egg quality, unusual (progesterone-safe) uterine endometrium, and bargained embryo implantation (Giudice; Bulun SE) [1,-11]. The meaning of endometriosis is histological and requires the distinguishing proof of the presence of endometrial organ and stroma-like tissue outside the uterus (Sourial) [12]. A few hypotheses have shown that the histogenesis of endometriosis is that emanating streams retrograde through the lumen of the fallopian tubes into the pelvic-peritoneal depressions at feminine cycle (Robboy and Bean; Robboy, et al.) [13,14].
Moreover, it can create distant foci through expansion, connection, and intrusion of endometrial glandular epithelial tissue to distant organs (Somigliana, et al.) [15]. The most normally influenced parts of the body incorporate the ovaries, fallopian tubes, bladder, rectosigmoid colon, and myometrium (Giudice; Pavone and Lyttle) [1,16]. Another hypothesis, the coelomic metaplasia hypothesis, recommends that endometriosis emerges from the metaplasia of cells that line the instinctive and stomach peritoneum following hormonal, ecological, or irresistible incitement (Overton, et al.) [17]. A later hypothesis underpins stem/ progenitor cells and bone marrow-determined immature microorganisms in the pathogenesis of endometriosis (Sasson and Taylor) [5]. Notwithstanding, Anglesio. recognized substantial malignant growth driver mutations in the glandular epithelium of deep infiltrating endometriosis sores, and the authors recommended that the undifferentiated cell related hypothesis requires extra investigations to affirm the rational of a speculation (Anglesio, et al.) [7]. Additionally, (Noe, et al.) recognized 19 mutations enhanced in epithelial however not in stromal sores utilizing bead advanced PCR innovation [18]. The authors proposed another theory that epithelial and stromal segments in creating endometriotic sores co-create from independent ancestors. As right on time as 1925, Sampson proposed a potential relationship among's endometriosis and malignant change (Sampson) [19]. Czernobilsky and Morris portrayed a "middle stage" in the harmful change alluded to as "atypical endometriosis"; it is as of now characterized by the level of dysplastic histologic atypia (Czernobilsky and Morris) [20]. Quite, endometriosis is viewed as a potential pre-intrusive sore and is as of now named a tumor-like sore under the World Health Organization (WHO) histologic arrangement of ovarian tumors. Lately, Tsai et al. (Tai et al.) [21] demonstrated that patients with pelvic incendiary ailment had a three-fold increment in the danger of creating endometriosis dependent on the National Health Insurance Research Database (NHIRD) of Taiwan. The hidden mechanism of endometrisis might be related with three unique procedures:
Endometriosis pieces move from the uterus through the fallopian tubes amid retrograde feminine cycle, spreading these endometriosis sections to the peritoneal depression and embedding on the serosal surface. Metaplasia of the coelom and Vascular and lymphatic metastatic spread (Sasson and Taylor; Anglesio; Bulun; Sampson; Sampson; Figueira) [5,6,11,19,22].
Risk factors and Etiology of endometriosis
Huge hazard factors for the development of endometriosis incorporate conditions that increase the odds of retrograde menstruation and hereditary/genetic factors. Hazard factors for endometriosis incorporate early menarche, nulliparity, broken uterine bleeding, variant estrogen levels (Darrow; Signorello, et al.; Cramer, et al.; Candiani, et al.), and low weight record (Signorello, et al.) [23-26]. Factors, for example, sufficient exercise might be precaution against development of endometriosis (Kvaskoff) [27]. It is realized that the occurrence of endometriosis in women with first-degree relatives who likewise have the ailment might be up to multiple times higher than that of the all inclusive population (Matalliotakis; Treloar) [28,29]. There is probably going to be a multifactorial hereditary inclination for endometriosis, and genome-wide association studies (GWAS) have shown singlenucleotide polymorphism (SNP) profiles which may expand the danger of endometriosis in people (Rahmioglu) [30]. In 2012, Nyholt et al (Nyholt, et al. 2012] [31] distinguished 18 genomic areas harboring 38 putative endometriosis-related SNPs in a GWAS including 4,604 instances of endometriosis.
Among the huge aberrations distinguished were SNPs related with the WNT4 gene, known to be critical in reproductive tract differentiation and advancement in mammalian females (Jaaskelainen; Vainio et al.) [32,33] just as steroidigenesis (Boyer A et al.) [34], VEZT, appeared to be down regulated in gastric diseases (Guo X et al.) [35], and GREB1, an estrogen-managed gene not yet been elucidated. The various elements detailed in the pathogenesis of endometriosis-related ovarian malignancy are outlined in (Figure 1).
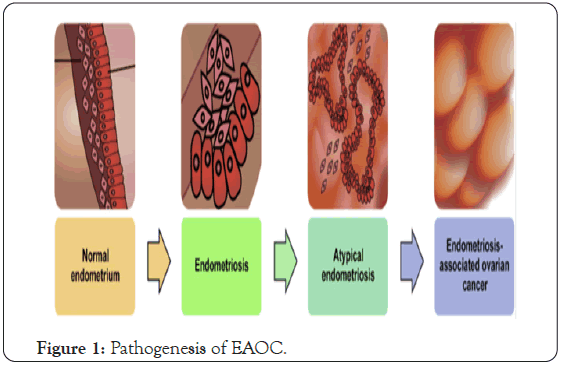
Figure 1: Pathogenesis of EAOC.
Development of EAOC from endometriosis
The idea that endometriosis is the forerunner lesion of some ovarian malignant growth subtypes has been upheld by various lines of examination. The affiliation was noted by pathological techniques, however epidemiological, and hereditary examinations have been important (Sampson, et al.; Sampson, et al.; Vercellini.; LaGrenade and Silverberg, et al.; Fukunaga, et al.; Pearce, et al.; Jiang, et al.; Scott; Lu, et al.; Prowse, et al.; McMeekin, et al.; Sainz de la Cuesta, et al.) [73,79,80-88]. Jiang et al depicted a portion of the principal contemplates recommending a molecular basis connecting endometriosis with cancer development in 1998. They exhibited a similar loss of heterozygosity (LOH) occasions in endometriosis lesion and contiguous endometrioid ovarian malignant growths in 82% of cases inspected (n=11) (Jiang, et al.) [83]. Comparable proof was accounted for by Prowse et al in 2006, who exhibited normal LOH occasions in both endometrioid and clear cell OCs and their related endometriosis lesion, including both nearby and contralateral endometriosis (Prowse, et al.) [86]. Moreover, LOH bringing about PTEN loss might be an early driver occasion in the beginning of in EAOC from endometriosis (Worley, et al.; Sato, et al.) [89,90]. Throughout the most recent 7 years, sequencing and immunohistochemical research have given corroborative proof that changes found in endometriosis-related malignant growths are found in adjoining endometriosis. These sequencing examines unmistakably exhibit a clonal connection among benign and malignant partners affirming that the malignant growths have actuality emerged from the endometriotic lesions (Stamp, et al.; Anglesio, et al.; Wiegand, et al.; Chene, et al.) [9194]. Somatic mutations and other genomic deviations are found in endometriosis that have been embroiled in the advancement of cancer. Mutations in TP53 (Bischoff, et al.; Sainz de la Cuesta, et al.) [95,96] KRAS (Anglesio, et al.; Vestergaard, et al.) [7,97], PTEN (Sato, et al.), PIK3CA (Laudanski, et al.; Yamamoto, et al.) [98,99], and ARID1A gene locales (Anglesio, et al.) have been portrayed. Loss of expression of mismatch repair proteins (Grassi, et al.) [100], microsatellite precariousness (Fuseya, et al.) [101], and tissue-explicit gene copy number changes (Yang, et al. 2013; Mafra, et al.) [102,103], may likewise be found in endometriosis sores. LOH in endometriosis at known oncogenic loci is additionally habitually observed (Sato, et al.; Ali-Fehmi, et al.; Xu, et al.; Obata and Hoshiai, et al.; Thomas and Campbell, et al.; Jiang, et al.; Silveira, et al.) [83,90,104-108]. SNPs that are related with oncogenic change (seen in GWAS datasets) have been recognized in instances of endometriosis (Nyholt, et al.; Albertsen, et al.; Uno, et al.; Painter, et al.) [31,38-40]. In 2015, a meta-investigation detailed by Lee et al including more than 15,000 ovarian disease patients, assessed the 38 putative endometriosis- related SNPs distinguished by Nyholt in 2012 (Nyholt, et al.). Eight of these were related with critical hazard for ovarian malignancy (rs7515106, rs7521902, rs742356, rs4858692, rs1603995, rs4241991, rs6907340, and rs10777670) (Lee, et al.) [109]. Likewise in 2015, Lu et al exhibited shared hereditary hazard among endometriosis and epithelial ovarian malignancy, especially clear- cell and endometrioid histotypes utilizing genome wide affiliation (GWAS) datasets (Lu, et al.) [85].
ARID1A is a tumor silencer gene that was observed to be transformed in an extensive number of EAOC (Wiegand, et al.) [93]. Examiners were initially eager to find that up to 42–61% of CCC and 21–33% EnOC show loss of the comparing ARID1A gene protein articulation (BAF250a) on IHC (Stamp, et al.; Wiegand, et al.; Yamamoto, et al.) [91,93,110]. ARID1A manages essential celularl capacities (expansion and genomic stability) as a tumor silencer gene; along these lines, it was believed that it may play a role in the change of endometriosis to malignancy (Wu, et al.) [111]. In 2015, Anglesio et al showed that clear cell ovarian carcinomas imparted numerous transformations to related simultaneous endometriosis sores, incorporating mutations in ARID1A. Shared transformations in PIK3CA were additionally distinguished among endometriosis and clear-cell sores, an occasion happening in early movement components in other malignant growth types (Anglesio, et al.). This investigation unmistakably exhibited depicted transformations in coterminous endometriosis shared by EAOC, and even some distant sores contained the equivalent (PIK3CA and ARID1A) transformations. Studies looking at BAF250a expression by IHC demonstrate that in simply over half of the announced instances of EAOC, loss of BAF250a expression is seen most of the time (67–80%) in regions of coterminous endometriosis or atypical endometriosis, and that lost Baf250a protein expression appeared to be an early molecular occasion in the advancement of Baf250a-negative EAOC (Stamp, et al.; Chene, et al.; Nishikimi, et al.) [91,94,112]. Strangely, ARID1A transformations are not adequate all alone to cause malignancy (Guan, et al.) [113]. In help of this perception, Borrelli et al portrayed halfway loss of BAF250a in ordinary endometrium without disease (Borrelli, et al.) [114]. An imperative examination lately announced that that 65% of malignancy causing genomic variations are arbitrary DNA repair anomalies (Tomasetti and Vogelstein, et al.) [115]. Bringing this data into context, one can infer that BAF250a loss in endometriosis could speak to an EAOC antecedent sore; nonetheless, ARID1A transformations are neither a fundamental driver transformation nor a critical determinant of the malignant phenotype. The presence of transformations in endometriosis is an indication of more extensive genomic interruption prompting the advancement of EAOC. Figure 2 demonstrates a schematic of the foundation and development of endometriosis sores to EAOC. Investigations have been finished looking at patient results in EAOC dependent on the presence or absence of BAF250a expression. In view of the accessible proof, it still can't seem to be resolved concerning whether there are contrasts in visualization or treatment results identified with BAF250a loss in EAOC (Katagiri, et al.; Lowery, et al.) [116,117]. There are couple of recognizable proteomic changes in a board of proteins assessed by reverse phase protein array (RPPA) recommending that BAF250a loss does not characterize a particular proteomic signature (Wiegand, et al.) [118]. Moreover, the presence or absence of an endometriosis antecedent sore in EAOC has not been related with change in overall disease result (Minlikeeva, et al.) [119].
Figure 2: Erythema in heliotrope.
Endometriosis as neoplasm
Deep infiltrating endometriosis is an intriguing uncommon subtype of endometriosis which was lately exposed to genomic assessment. Deep endometriosis has a penchant to locally attack encompassing structures (entrail, bladder, ureter) yet seldom metastasises. Anglesio et al showed the presence of somatic mutation occasions in 79% of 24 cases, with 26% of all cases screened harbouring measurably somatic mutation in known malignant growth driver genes, for example, KRAS, PIK3CA, ARID1A, and PPP2R1A. In the investigation of a littler subset of tests, mutations in KRAS observed to be available in the epithelial part of endometriosis sores were missing in the stroma. Moreover, one patient was found to have the equivalent KRAS transformation in three spatially unmistakable endometriosis sores. While these molecular occasions are usually found in EAOCs, this investigation showed their essence in deep infiltrating endometriosis. While customarily oncogenic driver transformations (like KRAS) were available in a quarter of tests, they didn't seem to demonstrate the probability of the sore to advance into a gynaecologic malignant growth nor have all the earmarks of being required for the improvement of the deep-infiltrating sores.
This recommends extra or distinctive molecular components might be having an effect on everything in the improvement of endometriosis, and future research utilizing an expansive cluster of molecular advances (epigenetic, grafting deviations, complex chromosomal adjustments, transcriptome, proteome and post- translational changes) to examine the functional science of endometriosis is justified. Novel molecular innovations may likewise help clarify the biology of clonally indistinguishable sores in a similar patient. At long last, the bizarre presence of endometriosis in lymph hubs has been portrayed, with a few cases indicating BAF250a loss (Borrelli, et al.) [114]. Consequently, one may expect that these extremely irregular cases are molecularly particular as they copy locally metastatic malignant growths. Maybe even the deep-infiltrating subtype of endometriosis, which shows unequivocal intrusion of encompassing tissues, might be more fittingly considered a neoplasm than a benign condition. Better comprehension of the molecular pathology of this disease may give helpful procedures to analyze and treat complex cases, with the objective of decreasing morbidity and ailment inconveniences like infertility.
Advanced technologies are revolutionizing the aspects of the athogenesis of endometriosis
The next-generation sequencing (NGS) stage will significantly affect disease diagnosis, management and treatment and anticipating result and reaction (Meldrum, et al.) [120]. NGS innovation is a plausible and solid strategy with that might be utilized to identify novel and uncommon somatic mutations. Also, NGS has been effectively utilized to distinguish germline and somatic mutations in a different of malignancies, including gynecological cancer (Evans, and Matuloni) [121], and it can go about as a diagnostic technique and aiding the customized treatment of malignant growth (Valtcheva, et al.) [122]. What's more, NGS innovation substantially affects precision medication and hazard assessment, including early diagnosis, prognosis, and optimization of treatment choice (Morash, et al.; Fountzilas and Tsimberidou) [123,124]. By performing genomic screening by means of NGS innovation, it is conceivable to distinguish whether a patient has previous hereditary conditions that would make them progressively susceptible to creating malignancy in their lifetime (Meldrum, et al.) [120]. In the ongoing years, NGS has been used to describe genomic alterations in EAOC. A few investigations had shown the utility of NGS in recognizing driver mutations in EAOC patients utilizing whole genome sequencing and target sequencing (Wiegand, et al.; Er, et al.) [93,125]. In our past investigation, ultra-deep (>1000×) target sequencing was performed on 409 cancer related genes to distinguish pathogenic changes related with EAOC, and hopeful genes prescient of threatening change were recognized (Zondervan, et al.) [78]. In light of these discoveries, the recognized driver mutations for benign to premalignant sores could be focuses to control the early diagnosis and avoidance of EAOC. As recently examined, endometriosis is a confusion in which the endometriotic tissue is outside the uterus, andit is commonly thought to be a benign sickness. Also, we realized that NGS or ultra-deep sequencing empowers the revelation of novel sequence variants. (Li, et al.) [126] recently demonstrated that hereditary changes in cyto-skeletal and chromatin re-modelling proteins assume a critical job in the pathogenesis of endometriosis utilizing whole-exome sequencing. Lately, exome sequencing likewise yielded promising discoveries that sores in deep infiltrating endometriosis, which are related with for all intents and purposes no danger of malignant transformation, harbor substantial malignant growth driver mutations (Anglesio, et al.). In spite of the fact that endometriosis is viewed as a benign issue, the consequences of NGS innovation recommend another point of view, that the glandular epithelium of deep infiltrating endometriosis injuries harbor understood malignant related somatic transformations. Suda K et al. (Suda, et al.) [127] distinguished numerous malignant related somatic transformations in epithelial cells from ovarian endometriosis and ordinary endometrium utilizing whole exome sequencing. They affirmed that KRAS and PIK3CA were the most oftentimes transformed genes in endometriotic and ordinary uterine endometrial epithelium tests utilizing target-gene sequencing. They additionally showed that clonal extension of epithelial cells with malignant related somatic transformations prompts the advancement of endometriosis. These discoveries reinforce the past hypothesis that the root of endometriosis happens at the genomic level. Lately, Lac, et al.) [128] distinguished physical somatic driver transformations in incisional endometriosis and profound invading endometriosis utilizing an overly sensitive malignant growth hotspot sequencing board, incorporating hotspot changes in KRAS, ERBB2, PIK3CA and CTNNB1. Taken together, NGS innovation may enable us to grow our insight into the pathogenesis of endometriosis and subvert the traditional hypothesis. These examinations have involved endometriosis as a potential premalignant issue and have demonstrated it might give chances to diagnostics and treatments sooner rather than later. In any case, the impact and job of malignant related transformations in the pathogenesis of endometriosis must be completely clarified.
Materials
The variant analysis was performed on study accession PRJNA326570 where sample SRR3711510 and SRR3711512 were considered as a control sample for rest all 8 samples (SRR3711641, SRR3711642, SRR3711644, SRR3711645, SRR3711646, SRR3711647, SRR3711648 and SRR3711649). For NGS data analysis the library layout was Illumina sequenced. In the Illumina platform, the raw reads produced by the sequencing machine are shown in FASTQ, viewed as the standard design configuration of sequencing reads. The prepared library for the sample is a singleend library.
Methods
Next-generation sequencing is an incredible asset for recognizing uncommon and de novo variations, disease mapping, and evaluating expression levels. For the investigation, NGS reads are first adjusted to a reference genome, and afterward exposed to variant calling after fundamental quality control strategies. The alignment is pivotal for variant calling precision, and BWA is a broadly utilized aligner with great execution. Galaxy system is a web open application for high-throughput genomics, uncovering well known third-party data sources and standard bioinformatics investigation bundles in an incorporated and steady structure, intended to help scholar clients performing reproducible examinations. There is a free open site (http://usegalaxy.org). To import information, we utilized the ENA (European nucleotide document) governs by EMBL (website https://www.ebi.ac.uk/ena). When the file is uploaded from the ENA FASTQ Groomer (Galaxy Tool Version 1.1.1) is performed. It changes over between different FASTQ quality organizations. FASTQ Groomer is open-source toolset was executed in Python and has been coordinated into the online data examination platform Galaxy (Goss, et al.; Nichols et al.) [129,130]. After grooming of the data quality check is done using FASTQC tool. FastQC Read Quality reports (Galaxy Tool Version 0.72) gives quality control keeps an eye on raw sequence data originating from high throughput sequencing pipelines. The report incorporates synopsis charts and tables in an H. T. M. L based configuration. These outcomes got from QC investigations give us adequate data concerning whether the data has any issues or not before continuing forward. For above samples the quality was not good enough to perform mapping, therefore before mapping trimming is performed using TRIMMOMATIC (Galaxy Version 0.36.5) methods. BOWTIE2 (Galaxy Tool Version 2.3.4.2) is utilized to list reference genome which works at rapid and memory proficient way. Bowtie2 is utilized for short read alignment. What makes bowtie2 fascinating is the utilization of almost no RAM with precision and unobtrusive execution in ordering the alignment (Langmead and Salzberg) [131]. The alignment results yield in SAM format (Li, et al.) [132] after mapping to remove PCR duplicates RmDup tool (Galaxy Tool Version 2.0.1) is used and after removing duplicates quality is checked before proceeding. Mpileup (Galaxy Tool Version 2.1.4) reports variants for one or various B.A.M documents. Alignments records are gathered giving one log document (content organization) and other Variant Calling record (V.C.F format) which will give data like probability genotype, position on reads, mapping quality (Blankenber, et al.; Blankenberg, Daniel, et al.; Giardine, Belinda, et al.; Goecks, et al.; Sherry, et al.; Team The Galaxy] [133-136]. Varscan for variants (Galaxy Tool Version 2.4.2) performs variant location for enormously parallel sequencing data, for example, exome, W.G.S, and transcriptome information. It calls variants from M Pileup dataset and produces a Variant Calling File (V.C.F) (Andrew) [137]. Finally, we used wANNOVAR to perform regional and functional annotations. Variant calls are then clarified utilizing Annovar (Wang, et al.) [138]. The comment incorporates the utilization of databases, for example, ClinVar, Exac, dbSNP, and dbNSFP.
Results and Discussion
The novel variants acquired from the outcomes as appeared in the Table 1 which were not recently observed associated with Endometriosis. We found an aggregate of 24 new variations from Shenzhen Second Hospital (Shenzhen, Guangdong, China) endometrium sample. Among which, the majority of the variants got were non-synonymous SNVs, aside from them just a single of the variant (ADRA1B) indicated stop-gain SNP. This could be then additionally considered upon for their jobs in different disease or can be contrasted with different samples for same disease (Table 2).
| Sample No. | Novel Variants Obtained | Type Mutations in Exonic Functions | Chromosome Location |
|---|---|---|---|
| SRR3711641 | ATP6V0D2 | Nonsynonymous SNV | Chr8: 86150277 |
| VPS13B | Nonsynonymous SNV | Chr8: 99859386 | |
| GLG1 | Nonsynonymous SNV | Chr16: 74493027 | |
| SRR3711642 | LTBP3 | Nonsynonymous SNV | Chr11: 65540877 |
| CLCN7 | Nonsynonymous SNV | Chr16: 1474949 | |
| MNX1 | Nonsynonymous SNV | Chr7: 1.57E+08 | |
| ADRA1B | Stopgain Mutation | Chr5: 1.6E+08 | |
| SRR3711644 | UROD | Nonsynonymous SNV | Chr1: 45015374 |
| CLCN7 | Nonsynonymous SNV | Chr16: 1474949 | |
| RABGEF1 | Nonsynonymous SNV | Chr7: 66805250 | |
| SRR3711645 | ASCL2 | Nonsynonymous SNV | Chr11: 2269837 |
| DSCAML1 | Nonsynonymous SNV | Chr11: 1.17E+08 | |
| ZNF274 | Nonsynonymous SNV | Chr19: 58211630 | |
| PLXNA1 | Nonsynonymous SNV | Chr3: 1.27E+08 | |
| SEMA6A | Nonsynonymous SNV | Chr5: 1.16E+08 | |
| RABGEF1 | Nonsynonymous SNV | Chr7: 66805250 | |
| SRR3711646 | LPR5 | Nonsynonymous SNV | Chr11: 68410020 |
| MFAP3L | Nonsynonymous SNV | Chr4: 1.7E+08 | |
| GPR22 | Nonsynonymous SNV | Chr7: 1.07E+08 | |
| ATP6V0D2 | Nonsynonymous SNV | Chr8: 86150277 | |
| SRR3711647 | DENND3 | Nonsynonymous SNV | Chr8: 1.41E+08 |
| SRR3711647 | USP7 | Nonsynonymous SNV | Chr16: 8904506 |
| L3MBTL1 | Nonsynonymous SNV | Chr20: 43534909 | |
| MFAP3L | Nonsynonymous SNV | Chr4: 1.7E+08 | |
| ADRA1B | Stopgain Mutation | Chr5: 1.6E+08 | |
| PRICKLE4 | Nonsynonymous SNV | Chr6: 41786956 | |
| SRR3711649 | DSCAML1 | Nonsynonymous SNV | Chr11: 1.17E+08 |
| ARHGAP40 | Nonsynonymous SNV | Chr20: 38637795 | |
| CEBPB | Nonsynonymous SNV | Chr20: 50191512 | |
| SEMA3A | Nonsynonymous SNV | Chr7: 83961468 | |
| VPS13B | Nonsynonymous SNV | Chr8: 99859386 |
Table 1: Novel Variants with chromosome location and SNP.
| Genes | Mutation Frequencies (From intogen) |
|---|---|
| VPS13B | 5.65% |
| PLXNA1 | 3.91% |
| DSCAML1 | 2.61% |
| GLG1, LRP5 | 1.74% |
| RABGEF1, DENND3, PRICKLE4, SEMA3A, SEMA6A, ADRA1B | 1.30% |
| CLCN7, USP7, MFAP3L | 0.87% |
| L3MBTL1, UROD, GPR22, ATP6V0D2, ZNF274, ARHGAP40 | 0.43% |
| LTBP3, MNX1, CEBPB | 0% |
| ASCL2 | No Data |
Table 2: The frequency for mutation of the novel variants for entometriosis was discovered utilizing Intogen Database (https://www.intogen.org/seek).
The variants involvement in endometriosis was affirmed utilizing Driver: A database for malignancy driver gene (driverdb.tms. cmu.edu.tw/ddbv2/index.php). Four of the variants acquired ARHGAP40, UROD, MNX1 and MFAP3L were appeared to have some association in endometriosis as saw on Driver. The remaining genes are novel and once in a while connected with endometriosis. Majority of the variants obtained showed relativeness in other diseases, apart from endometriosis. The molecular genetics of some of the novel variants is discussed:
LRP5: Gong et al. (2001) demonstrated that LRP5 influences bone mass gathering during development and recognized changes in the LRP5 gene (e.g., 603506.0001) that develop autosomal recessive osteoporosis-pseudoglioma disorder (OPPG; 259770) [139]. They found that obligate bearers of mutant LRP5 gene had decreased bone mass when contrasted with age and sexual orientation coordinated controls. Little et al. (2002) recognized a gly171-to-val transformation in the LRP5 gene (G171V; 603506.0013) that outcomes in an autosomal prevailing high bone mass attribute (see 601884) [140]. Boyden, et al. (2002) found the equivalent LRP5 transformation in a family with autosomal dominant [141], high bone density related with square jaw and torus palatinus. Guo, et al. (2006) genotyped 1,873 Caucasian people from 405 family units for SNPs and haplotypes of the LRP5 gene and found that the regular allele A for SNP4 (rs4988300) and the minor allele G for SNP6 (rs634008) were essentially connected with obesity and body mass index (BMI) [142]. Critical affiliations were additionally seen between the regular haplotype A-G-G-G in block 2 (intron 1) with obesity, BMI, and fat mass (p under 0.001, p under 0.001, and p=0.003, individually). Guo et al. (2006) inferred that intronic variations of the LRP5 gene are particularly connected with weight. In affected people from 4 irrelevant families with polycystic liver disease-4 with or without kidney cysts (PCLD4; 617875), Cnossen et al. (2014) recognized 4 diverse heterozygous missense transformations in the in the LRP5 gene (603506.0035- 603506.0038) [143]. Two transformations influenced the intracellular domain, and 2 influenced the extracellular domian. The transformation in the main family was found by whole exome sequencing and affirmed by Sanger sequencing; the 3 different transformations were found by direct sequencing of the LRP5 gene in a cohort of 150 probands with cystic liver disease. The transformations isolated with the turmoil in the families, with some proof for age-subordinate deficient penetrance. None of the patients conveying transformations had proof of clinical highlights of other LRP5-related disease, including bone density or ocular abnormalities.
CLCN7: In light of the closeness between the phenotype of patients with childish harmful osteopetrosis (see OPTB4; 611490) which create serious osteopetrosis and retinal degeneration, Kornak et al. (2001) hunt down transformations in the human CLCN7 gene in 12 patients with juvenile osteopetrosis [144]. They recognized compound heterozygosity for a nonsense (Q555X; 602727.0001) and a missense (R762Q; 602727.0002) change in the CLCN7 quality in 1 persistent with the illness who had early visual hindrance. No retinal histology was accessible. Blair et al. (2004) developed CD14 cells from control and 4 osteopetrotic human subjects within the sight of bone and analysed their osteoclastic separation in vitro [145]. The osteopetrotic cells indicated absconds in acid transport, natural framework evacuation, and cell fusion with inadequate connection compared with the ordinary cells. Genotype investigation demonstrated that cells from 2 patients compound heterozygous for TCIRG1 (604592) transformations had acid transport defects, though cells from 1 patient compound heterozygous for CLCN7 transformation had natural framework evacuation defects. The cells with a connection defect were from a patient who needed TCIRG1 and CLCN7 transformations. In affected people from 12 disconnected families with autosomal prevailing osteopetrosis-2 (OPTA2; 166600), Cleiren et al. (2001) distinguished heterozygosity for 7 unique transformations in the CLCN7 gene (see, e.g., 602727.0004 and 602727.0005) [146]. Examination of microsatellite markers showed that the changes emerged autonomously in every family. Among these families was the Danish family that Van Hul et al. (1997) at first connected to chromosome 1p21. Also, Cleiren et al. (2001) distinguished 1 patient with the extreme autosomal recessive puerile type of osteopetrosis (OPTB4) who was homozygous for a CLCN7 missense transformation (L766P; 602727.0003), for which her asymptomatic guardians were heterozygous [146].
UROD: In the UROD cDNA from a patient with familial porphyria cutanea tarda (PCT; 176100), Garey et al. (1989) showed a heterozygous gly281-to-val substitution (G281V; 613521.0001). The change was not distinguished in affected people from 7 other PCT families with an autosomal dominant pattern of legacy. In a Tunisian family with hepatoerythropoietic porphyria (HEP; see 176100), de Verneuil et al. identified homozygosity for a G281E change (613521.0002) in the UROD gene product [147].
SEMA3A: In 2 sibs and their dad with Kallmann disorder (HH16; 614897), Young et al. distinguished heterozygosity for a 213-kb cancellation in the SEMA3A gene (603961.0001). Sequencing of the nondeleted SEMA3A allele and of 12 known HH-related gene in affected individuals from the family did not reveal some other transformations. Youthful et al. reasoned that SEMA3A play a job in anosmic hypogonadotropic hypogonadism.
USP7: In a 13-year-old young lady with formative deferral, hypotonia, and seizures, Hao et al. distinguished a once more heterozygous c.429C-G transversion in the USP7 gene, bringing about a tyr143-to-ter (Y143X) substitution and anticipated to result in haplo insufficiency. Direct utilitarian investigations of the variation and investigations of patient cells were not performed [148]. Be that as it may, in vitro knockdown of USP7 in cells brought about a diminishing in TRIM27 (602165) protein levels and impeded endosomal protein reusing with diminished F-actin collection. Hao et al. announced 6 random kids with variable neuro developmental issue related with de novo heterozygous micro deletions of chromosome 16p13.2 and 1 patient with a new heterozygous truncating variation in the USP7 gene (602519) on chromosome 16p13.2. All had formative postponement and scholarly incapacity, and 5 were determined to have chemical imbalance range issue. Extra regular highlights included seizures (5 patients), cryptorchidism or micro penis (in 4 of 5 guys), hypotonia (4 patients), and aggressive conduct (4 patients). Different highlights included gentle nonspecific dysmorphic highlights and poor or missing speech with speech apraxia. Practically all patients were in a specialized curriculum.
LTBP3: In affected individuals from a consanguineous Pakistani family with specific tooth agenesis and short stature (DASS; 601216), Noor et al. distinguished a homozygous nonsense transformation in the LTBP3 gene (Y744X; 602090.0001). Two affected guys were analyzed in detail [149]. The phenotype was described by absence of a large number of the perpetual teeth, just as obvious expanded bone density in the spine and skull base. The discoveries proposed an essential job for LTBP3-intervened transcription being developed of the axial skeleton. In a mother and her 2 children who indicated highlights reliable with mellow geleophysic dysplasia (GPHYSD3; 617809), McInerney-Leo et al. recognized heterozygosity for a missense transformation in the LTBP3 gene (S696C; 602090.0008) [150]. In 2 inconsequential young men determined to have geleophysic dysplasia, who kicked the bucket in early youth from respiratory failure, McInerney- Leo et al. recognized heterozygosity for a stop-loss transformation (602090.0009) and a splice site transformation (602090.0010) in LTBP3, respectively.
VPS13B: In a 33-year-elderly person who showed the typical facial gestalt of Cohen disorder and had neutropenia and retinopathy, yet who did not show truncal stoutness or mental impediment, Gueneau et al. distinguished compound heterozygosity for 2 splice site transformations in the VPS13Bgene (607817.0014; 607817.0015) [151]. The authors proposed that a dose impact of remaining typical VPS13B protein may clarify the deficient phenotype in this patient. In 2 Lebanese siblings with Cohen disorder and the extra highlights of cutis verticis gyrata and sensorineural deafness, initially announced by Megarbane et al. as an unmistakable disorder, Megarbane et al. recognized a homozygous grafting transformation in the VPS13B gene (607817.0016) [152].
MNX1: In 2 predominantly acquired sacral agenesis families, Lynch et al. discovered linkage to 7q36 markers. Ross et al. refined the sub chromosomal confinement in a few extra inherited sacral agenesis families and recognized causative transformations in the MNX1 gene (142994.0001-142994.0006) [153]. In affected individuals from a 3-age family isolating Currarino disorder, Urioste et al. identified a frameshift transformation in the MNX1 gene (142994.0009). Malignant mutation of a presacral teratoma was seen in the 22-year-old proband, and presacral teratomas were found in 6 other relatives, including the 3 asymptomatic people. Of 9 influenced individuals, just 2 showed the total set of three. In affected individuals from a 4-age family with Currarino disorder, Wang et al. (2006) recognized heterozygosity for a nonsense transformation in the MNX1 gene (142994.0010) [154].
ADRA1B: The distal end of 5q, 5q31.1-qter, contains the genes for 2 adrenergic receptors, ADRB2 (109690) and ADRA1B, and the dopamine receptor type 1A gene (DRD1A; 126449). Krushkal et al. utilized an effective conflicting sib-pair ascertainment plan to examine the effect of this area of the genome on variety in systolic blood pressure in youthful Caucasians [155]. They quantified 8 exceedingly polymorphic markers crossing this positional applicant gene rich district in 427 people from 55 3-age families containing 69 conflicting sib-pair, and determined multipoint character by plunge probabilities. The after effects of hereditary linkage and affiliation tests showed that the district between markers D5S2093 and D5S462 was altogether connected to at least 1 polymorphic genes influencing inter individual variety in systolic blood pressure. Since the ADRA1B and DRD1A genes are found near these markers, the information recommended that hereditary variety in 1 or both of these G protein-coupled receptors, which partake in the control of vascular tone, assumes an essential job in affecting inter individual variety in systolic blood pressure levels (Table 3).
| Gene Name | Expression | Associated Cancer (Mutation Frequency) |
|---|---|---|
| VPS13B | Ubiquitous expression in endometrium (RPKM 3.0) | Cutaneous melanoma (12.47%) |
| PLXNA1 | Ubiquitous expression in lung (RPKM 7.9) | Lung squamous cell carcinoma (5.71%) |
| DSCAML1 | Biased expression in brain (RPKM 3.3) | Cutaneous melanoma (9.76%) |
| CDK11A | Ubiquitous expression in bone marrow (RPKM 22.4) | Cutaneous Melanoma (1.08%) |
| GLG1 | Ubiquitous expression in ovary (RPKM 26.0) | Small cell lung carcinoma (8.07%) |
| LRP5 | Ubiquitous expression in fat (RPKM 20.9) | Cutaneous melanoma (6.78%) |
| RABGEF1 | Ubiquitous expression in bone marrow (RPKM 17.2) | Bladder carcinoma(2.04%) |
| DENND3 | Broad expression in bone marrow (RPKM 17.0) | Cutaneous melanoma (8.40%) |
| PRICKLE4 | Ubiquitous expression in spleen (RPKM 16.5) | Stomach adenocarcinoma (1.86%) |
| SEMA3A | Broad expression in placenta (RPKM 2.5) | Bladder carcinoma (4.08%) |
| SEMA6A | Broad expression in adrenal (RPKM 13.5) | Lung adenocarcinoma (2.56%) |
| ADRA1B | Biased expression in spleen (RPKM 2.0) | Stomach adenocarcinoma (1.86%) |
| CLCN7 | Ubiquitous expression in spleen (RPKM 16.9) | Non-small cell lung carcinoma (3.23%) |
| USP7 | Ubiquitous expression in testis (RPKM 31.0) | Stomach adenocarcinoma (3.73%) |
| MFAP3L | Broad expression in kidney (RPKM 7.1) | Stomach adenocarcinoma (1.86%) |
| L3MBTL1 | Broad expression in testis (RPKM 4.3) | Cutaneous melanoma (2.17%) |
| UROD | Ubiquitous expression in bone marrow (RPKM 73.9) | Bladder carcinoma (1.08%) |
| GPR22 | Biased expression in heart (RPKM 6.9) | Lung adenocarcinoma (1.08%) |
| ATP6V0D2 | Biased expression in kidney (RPKM 22.7) | Small cell lung carcinoma (2.90%) |
| ZNF274 | Ubiquitous expression in thyroid (RPKM 9.7) | Cutaneous melanoma (2.44%) |
| ARHGAP40 | Biased expression in skin (RPKM 12.2) | Acute myeloid leukemia (0.51%) |
| LTBP3 | Ubiquitous expression in ovary (RPKM 27.2) | Cutaneous melanoma(3.25%) |
| MNX1 | Biased expression in colon (RPKM 3.3) | Lung squamous cell carcinoma(1.15%) |
| CEBPB | No Data | Bladder carcinoma(1.02%) |
| ASCL2 | Broad expression in colon (RPKM 4.3) | No Data |
Table 3: Novel variants with their expression (taken from gene database of NCBI- https://www.ncbi.nlm.nih.gov/gene) and their highest cancer associated mutation frequency (taken from IntOgen- https://www.intogen.org/seek).
Conclusion
The investigation can additionally expand in discovering the job of such novel genes in interaction and metabolic pathways and can additionally be contemplated for DNA-protein interaction investigation to help novel research particularly towards its molecular relationship or interaction of the powerful gene products.
REFERENCES
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Giudice LC. Clinical practice Endometriosis. N Engl J Med. 2010;362(25):2389-98.
- Eskenazi B, Warner ML. Epidemiology of endometriosis. ObstetGynecol Clin North Am. 1997;24(2):235-258.
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. FertilSteril. 2009;92(1):68-74.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-15.
- Anglesio MS and Yong PJ. Endometriosis-associated Ovarian Cancers. Clin Obstet Gynecol. 2017;60(4):711-27.
- Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376(19):1835-1848.
- Giudice LC. Clinical practice Endometriosis. N Engl J Med. 2010;362 25:2389-98.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21(11):3001-3007.
- Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268-79.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Robboy SJ, Bean SM. Pathogenesis of endometriosis. Reprod Biomed Online. 2010;21(1):4-5.
- Robboy SJ, Haney AF, Russell P. Endometriosis. In: Robboy, SJ, Mutter, GL, Prat, J, Bentley, RC, Russell, P, Anderson, MC (Eds), Pathology of the Female Reproductive Tract, second ed. London: Churchill Livingstone. 2009.
- Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. GynecolOncol. 2006;101(2):331-41.
- Pavone ME, Lyttle BM. Endometriosis and ovarian cancer: links, risks, and challenges faced. Int J Womens Health. 2015;7:663-72.
- Overton C, Shaw RW, McMillan L, Davis C. Atlas of Endometriosis, 3rd Edition. CRC Press; 2007.
- Noe M, Ayhan A, Wang TL, Shih IM. Independent development of endometrial epithelium and stroma within the same endometriosis. J Pathol. 2018;245 3:265-9.
- Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Archives of Surgery. 1925;10:1-72.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53:250-253.
- Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J Clin Med. 2018;7(11):35-39.
- Figueira PG, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10-7.
- Darrow SL, Vena JE, Batt RE, Michalek AM, Selman S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4(2):135–142.
- Signorello LB, Harlow BL, Cramer DW. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7(4):267-741.
- Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11-22.
- Candiani GB, Danesino V, Gastaldi A, Ferraroni M. Reproductive and menstrual factors and risk of peritoneal and ovarian endometriosis. FertilSteril. 1991;56(2):230-234.
- Kvaskoff M, Bijon A, Clavel-Chapelon F. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology. 2013;24(2):261-269.
- Matalliotakis IM, Arici A, Cakmak H. Familial aggregation of endometriosis in the yale series. Arch Gynecol Obstet. 2008;278(6):507-511.
- Treloar SA, O’Connor DT. Genetic influences on endometriosis in an Australian twin sample. FertilSteril. 1999;71(4):701-710.
- Rahmioglu N, Nyholt DR, Morris AP. Genetic variants underlying risk of endomStriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20(5):702-716.
- Nyholt DR, Low SK, Anderson CA. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355-1359.
- Jaaskelainen M, Prunskaite-Hyyrylainen R, Naillat F, et al. WNT4 is expressed in human fetal and adult ovaries and its signaling contributes to ovarian cell survival. Mol Cell Endocrinol. 2010;317(1-2):106-111.
- Vainio S, Heikkila M, Kispert A. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405-409.
- Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010-3025.
- Guo X, Jing C, Li L. Down-regulation of VEZT gene expression in human gastric cancer involves promoter methylation and miR-43c. Biochem Biophys Res Commun. 2011;404(2):622-627.
- Rae JM, Johnson MD, Scheys JO. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92(2):141-149.
- Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60(22):6367-6375.
- Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PLoS One. 2013;8(3):e58257.
- Uno S, Zembutsu H, Hirasawa A. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 2010;42(8):707-710.
- Painter JN, Anderson CA, Nyholt DR. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43(1):51-54.
- Uimari O, Rahmioglu N, Nyholt DR. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod. 2017;32(4):780-793.
- Sapkota Y, Steinthorsdottir V, Morris AP. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539.
- Fung JN, Rogers PA, Montgomery GW. Identifying the biological basis of GWAS hits for endometriosis. Biol Reprod. 2015;92(4):87.
- Berker B, Seval M. Problems with the diagnosis of endometriosis. Womens Health (Lond). 2015;11(5):597-601.
- Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005;58(3):308-312.
- Huhtinen K, Suvitie P, Hiissa J. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100(8):1315-1319.
- Leyland N, Casper R, Laberge P. Endometriosis: diagnosis and management. J ObstetGynaecol Can. 2010;32(7):S1–32.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204-1212.
- Hori Y, Committee SG. Diagnostic laparoscopy guidelines:this guideline was prepared by the SAGES guidelines committee and reviewed and approved by the Board of Governors of the society of American gastrointestinal and endoscopic surgeons (SAGES), November 2007. Surg Endosc. 2008;22(5):1353-1383.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. FertilSteril. 2012;98(3):511-519.
- Rogers PA, D’Hooghe TM, Fazleabas A. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis in Montpellier, France. Reprod Sci. 2013;20(5):483-499.
- Burghaus S, Haberle L, Schrauder MG. Endometriosis as a risk factor for ovarian or endometrial cancer-results of a hospital-based case-control study. BMC Cancer. 2015;15:751.
- Wykes CB, Clark TJ, Chakravati S. Efficacy of laparoscopic excision of visually diagnosed peritoneal endometriosis in the treatment of chronic pelvic pain. Eur J ObstetGynecol Reprod Biol. 2006;125(1):129-133.
- Bedaiwy MA, Alfaraj S, Yong P, Casper R, et al. New developments in the medical treatment of endometriosis. FertilSteril. 2017;107(3):555-565.
- Streuli I, de Ziegler D, Borghese B. New treatment strategies and emerging drugs in endometriosis. Expert Opin Emerg Drugs. 2012;17(1):83-104.
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149(3):1190–1204.
- Han SJ, O’Malley BW. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum Reprod Update. 2014;20(4):467-484.
- Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587-607.
- Kobayashi H, Yamada Y, Kanayama S. The role of iron in the pathogenesis of endometriosis. Gynecol Endocrinol. 2009;25(1):39-52.
- Vercellini P, Crosignani P, Somigliana E. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26(9):2262-2273.
- Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100(1):9-16.
- Ota H, Igarashi S, Sasaki M. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561-566.
- Lin YJ, Lai MD, Lei HY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147(3):1278-1286.
- Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol. 2015;195(6):2591-2600.
- Zhang X, Xu H, Lin J. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112(8):1153-1155.
- McKinnon BD, Bertschi D, Bersinger NA. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26(1):1-10.
- Worley MJ, Welch WR, Berkowitz RS. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14(3):5367-5379.
- Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer SK, Graubard BI, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev. 2005;1412:2929-35.
- Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control. 2008;19(10):1357-64.
- Forte A, Cipollaro M, Galderisi U. Genetic, epigenetic and stem cell alterations in endometriosis: new insights and potential therapeutic perspectives. Clin Sci (Lond). 2014;126 2:123-38.
- Nezhat FR, Apostol R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262-7.
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21 4:500-16.
- Pearce CL, Templeman C, Rossing MA. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385-394.
- Matalliotakis M, Matalliotaki C, Goulielmos GN, Patelarou E, Tzardi M, Spandidos DA, et al. Association between ovarian cancer and advanced endometriosis. OncolLett. 2018;15(5):7689-92.
- Kok VC, Tsai HJ, Su CF, Lee CK. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int J Gynecol Cancer. 2015;25 6:968-76.
- Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience. 2018;12:803.
- Oda K, Hamanishi J, Matsuo K , Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. GynecolOncol. 2018;151(2):381-9.
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9.
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93-110.
- Vercellini P, Crosignani P, Somigliana E. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26(9):2262-2273.
- LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19(9):1080-1084.
- Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30(3):249-255.
- Jiang X, Morland SJ, Hitchcock A. Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res. 1998;58(8):1707-1712.
- Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2(3):283-289.
- Lu Y, Cuellar-Partida G, Painter JN. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum Mol Genet. 2015;24(20):5955–5964.
- Prowse AH, Manek S, Varma R. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119(3):556-562.
- McMeekin DS, Burger RA, Manetta A. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. GynecolOncol. 1995;59(1):81-86.
- Sainz de la Cuesta R, Eichhorn JH, Rice LW, et al. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. GynecolOncol. 1996;60(2):238–244.
- Worley MJ Jr, Liu S, Hua Y. Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur J Cancer. 2015;51(13):1831-1842.
- Sato N, Tsunoda H, Nishida M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60(24):7052-7056.
- Stamp JP, Gilks CB, Wesseling M. BAF250a expression in atypical endometriosis and endometriosis-associated ovarian cancer. Int J Gynecol Cancer. 2016;26(5):825-832.
- Anglesio MS, Bashashati A, Wang YK, Senz J, Ha G, Yang W, et al. Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J Pathol. 2015;236(2):201-209.
- Wiegand KC, Shah SP, Al-Agha OM. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532-1543.
- Chene G, Ouellet V, Rahimi K, Barres V, Provencher D, Mes-Masson AM. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynaecol Obstet. 2015;130(1):27-30.
- Bischoff FZ, Heard M, Simpson JL. Somatic DNA alterations in endometriosis: high frequency of chromosome 17 and p53 loss in late-stage endometriosis. J Reprod Immunol. 2002;55(1-2):49-64.
- Sainz de la Cuesta R, Izquierdo M, Canamero M. Increased prevalence of p53 overexpression from typical endometriosis to atypical endometriosis and ovarian cancer associated with endometriosis. Eur J ObstetGynecol Reprod Biol. 2004;113(1):87-93.
- Vestergaard AL, Thorup K, Knudsen UB. Oncogenic events associated with endometrial and ovarian cancers are rare in endometriosis. Mol Hum Reprod. 2011;17(12):758-761.
- Laudanski P, Szamatowicz J, Kowalczuk O. Expression of selected tumor suppressor and oncogenes in endometrium of women with endometriosis. Hum Reprod. 2009;24(8):1880-1890.
- Yamamoto S, Tsuda H, Takano M. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J Pathol. 2011;225(2):189-194.
- Grassi T, Calcagno A, Marzinotto S. Mismatch repair system in endometriotic tissue and eutopic endometrium of unaffected women. Int J Clin Exp Pathol. 2015;8(2):1867-1877.
- Fuseya C, Horiuchi A, Hayashi A, et al. Involvement of pelvic inflammation-related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis. Hum Pathol. 2012;43(11):1964-1972.
- Yang W, Zhang Y, Fu F. High-resolution array-comparative genomic hybridization profiling reveals 20q13.33 alterations associated with ovarian endometriosis. Gynecol Endocrinol. 2013;29(6):603-607.
- Mafra F, Mazzotti D, Pellegrino R. Copy number variation analysis reveals additional variants contributing to endometriosis development. J Assist Reprod Genet. 2016;34(1):117-124.
- Ali-Fehmi R, Khalifeh I, Bandyopadhyay S, Lawrence WD, Silva E, Liao D, et al. Patterns of loss of heterozygosity at 10q23.3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int J Gynecol Pathol. 2006;25(3):223-229.
- Xu B, Hamada S, Kusuki I. Possible involvement of loss of heterozygosity in malignant transformation of ovarian endometriosis. GynecolOncol. 2011;120(2):239-246.
- Obata K, Hoshiai H. Common genetic changes between endometriosis and ovarian cancer. GynecolObstet Invest. 2000;50(S1):39-43.
- Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. GynecolObstet Invest. 2000;50(S1):44-50.
- Silveira CG, Abrao MS, Dias JA. Common chromosomal imbalances and stemness-related protein expression markers in endometriotic lesions from different anatomical sites: the potential role of stem cells. Hum Reprod. 2012;27(11):3187-3197.
- Lee AW, Templeman C, Stram DA. Evidence of a genetic link between endometriosis and ovarian cancer. FertilSteril. 2015;105(1):35-43.
- Yamamoto S, Tsuda H, Takano M. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25(4):615-624.
- Wu RC, Wang TL, Shih Ie M. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15(6):655-664.
- Nishikimi K, Kiyokawa T, Tate S. ARID1A expression in ovarian clear cell carcinoma with an adenofibromatous component. Histopathology. 2015;67(6):866-871.
- Guan B, Rahmanto YS, Wu RC, et al. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J Natl Cancer Inst. 2014;106(7):146.
- Borrelli GM, Abrao MS, Taube ET, Darb-Esfahani S, Köhler C, Chiantera V, et al. (Partial) Loss of BAF250a (ARID1A) in rectovaginal deep-infiltrating endometriosis, endometriomas and involved pelvic sentinel lymph nodes. Mol Hum Reprod. 2016;22(5):329-337.
- Tomasetti C, Vogelstein B. Cancer etiology. variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78-81.
- Katagiri A, Nakayama K, Rahman MT. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol. 2012;25(2):282–288.
- Lowery WJ, Schildkraut JM, Akushevich L. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2012;22(1):9-14.
- Wiegand KC, Hennessy BT, Leung S. A functional proteogenomic analysis of endometrioid and clear cell carcinomas using reverse phase protein array and mutation analysis: protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation. BMC Cancer. 2014;14:120.
- Minlikeeva AN, Freudenheim JL, Eng KH. History of comorbidities and survival of ovarian cancer patients, results from the ovarian cancer association consortium. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1470-1473.
- Meldrum C, Doyle MA, Tothill RW. Next-generation sequencing for cancer diagnostics: a practical perspective. Clin Biochem Rev. 2011;32(4):177-95.
- Evans T, Matulonis U. Next-Generation Sequencing: Role in Gynecologic Cancers. J Natl ComprCanc Netw. 2016;14(9):1165-73.
- Valtcheva N, Lang FM, Noske A, Samartzis EP, Schmidt AM, Bellini E, et al. Tracking the origin of simultaneous endometrial and ovarian cancer by next-generation sequencing-a case report. BMC Cancer. 2017;17:25-27.
- Morash M, Mitchell H, Beltran H, Elemento O, Pathak J. The Role of Next-Generation Sequencing in Precision Medicine: A Review of Outcomes in Oncology. J Pers Med. 2018;8(3):24-29.
- Fountzilas E, Tsimberidou AM. Overview of precision oncology trials: challenges and opportunities. Expert Rev Clin Pharmacol. 2018;11 8:797-804.
- Er TK, Su YF, Wu CC, Chen CC, Wang J, Hsieh TH, et al. Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. J Mol Med (Berl). 2016;94(7):835-47.
- Li X, Zhang Y, Zhao L, Wang L, Wu Z, Mei Q, et al. Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum Mol Genet. 2014;23(22):6008-21.
- Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018;24(7):1777-89.
- Lac V, Verhoef L, Aguirre-Hernandez R, Nazeran TM, Tessier-Cloutier B, Praetorius T, et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum Reprod. 2018.
- Goss M, James R, Caleb E Finch, Morgan DG. Age-Related Changes in Glial Fibrillary Acidic Protein Mrna in the Mouse Brain. Neurobiology of aging 1991;12(2):165-170.
- Nichols A, Nancy R, Day JR, Laping NJ, Johnson SA, Finch CE. GfapMrna Increases with Age in Rat and Human Brain. Neurobiology of aging. 1993;14(5):421-429.
- Langmead B, Salzberg, SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357-359.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. Genome Project Data Processing S. 2009.
- Blankenberg D, Gordon A, Von Kuster G, Coraor N, Taylor J, Nekrutenko A, et al. Manipulation of Fastq Data with Galaxy. Bioinformatics. 2010.
- Blankenberg, D, Kuster GV, Coraor N, Ananda G, Lazarus R, Mangan M, et al. Galaxy: A Web-Based Genome Analysis Tool for Experimentalists. In Current Protocols in Molecular Biology: John Wiley & Sons, Inc., 2001.
- Goecks JA. Nekrutenko, J. Taylor. Galaxy: A Comprehensive Approach for Supporting Accessible, Reproducible, and Transparent Computational Research in the Life Sciences. Genome Biol. 2010;11(8):R86.
- Sherry A, Stephen T, Ward M-H, M Kholodov, J Baker, Lon Phan, et al. Dbsnp: The Ncbi Database of Genetic Variation. Nucl acid res. 2001;29(1):308-311.
- Andrews S. Fastqc a Quality Control Tool for High Throughput Sequence Data.
- Wang K, M Li, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nuc Acid Res, 2010;38(16):e164.
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513-523.
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11-19.
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. New Eng J Med. 2002;346:1513-1521.
- Guo X, Jing C, Li L. Down-regulation of VEZT gene expression in human gastric cancer involves promoter methylation and miR-43c. Biochem Biophys Res Commun. 2011;404(2):622-627.
- Cnossen WR, teMorsche RHM, Hoischen A, Gilissen C, Chrispijn M, Venselaar H, et al. Whole-exome sequencing reveals LRP5 mutations and canonical Wntsignaling associated with hepatic cystogenesis. Proc. Nat. Acad. Sci. 2014;111:5343-5348.
- Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205-215.
- Blair HC, Borysenko CW, Villa A, Schlesinger PH, Kalla SE, Yaroslavsky BB, et al. In vitro differentiation of CD14 cells from osteopetrotic subjects: contrasting phenotypes with TCIRG1, CLCN7, and attachment defects. J Bone Miner Res. 2004;19:1329-1338.
- Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslav J, Singer FR, et al. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Molec Genet. 2001;10:2861-2867.
- de Verneuil H, Hansen J, Picat C, Grandchamp B, Kushner J, Roberts A, et al. Prevalence of the 281 (gly-to-glu) mutation in hepatoerythropoietic porphyria and porphyria cutaneatarda. Hum Genet. 1988;78:101-102.
- HaoY-H, Fountain MD, Tacer F, Xia K, Bi F, Kang W, et al. USP7 acts as a molecular rheostat to promote WASH-dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Molec Cell. 2015;59:956-969.
- Noor A, Windpassinger C, Vitcu I, Orlic M, Rafiq MA, Khalid M, et al. Oligodontia is caused by mutation in LTBP3, the gene encoding latent TGF-beta binding protein 3. Am J Hum Genet. 2009;84:519-523.
- McInerney-Leo AM, Le Goff C, Leo PJ, Kenna TJ, Keith P, Harris JE, et al. Mutations in LTBP3 cause acromicric dysplasia and geleophysic dysplasia. J Med Genet. 2012.
- Gueneau L, Duplomb L, Sarda P, Hamel C, Aral B, El Chehadeh S, et al. Congenital neutropenia with retinopathy, a new phenotype without intellectual deficiency or obesity secondary to VPS13B mutations. Am J Med Genet. 2014;164A:522-527.
- Megarbane A, Waked N, Chouery E, Moglabey YB, Saliba N, Mornet E, et al. Microcephaly, cutis verticisgyrata of the scalp, retinitis pigmentosa, cataracts, sensorineural deafness, and mental retardation in two brothers. Am J Med Genet. 2001;98: 244-249.
- Ross AJ, Ruiz-Perez V, Wang Y, Hagan D-M, Scherer S, Lynch SA, et al. Homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nature Genet. 1998;20:358-361.
- Wang RY, Jones JR, Chen S, Rogers RC, Friez MJ, Schwartz CE, et al. A previously unreported mutation in a Currarino syndrome kindred. Am J Med Genet. 2006;140A:1923-1930.
- Krushkal J, Xiong M, Ferrell R, Sing CF, Turne, ST, Boerwinkle E. Linkage and association of adrenergic and dopamine receptor genes in the distal portion of the long arm of chromosome 5 with systolic blood pressure variation. Hum Molec Genet. 1998;7:1379-1383.
Citation: Datta S, Rana M (2020) Researching Novel Variants in Endometriosis Using Next Generation Sequencing Variant Analysis. 9:153. DOI: 10.35248/2329-6682.20.9.153.
Copyright: © 2020 Datta S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

