Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Commentary - (2020) Volume 11, Issue 2
Regulation of Glucagon Secretion and Insulin Resistance in Pancreatic A-Cells
Norikiyo Honzawa1* and Kei Fujimoto22Department of Internal Medicine, Jikei University Kashiwa Hospital, 163-1 Kashiwashita, Kashiwa-shi,, Japan
Received: 15-Jun-2020 Published: 06-Jul-2020
Abbreviations
cAMP:Cyclic Adenosine Monophosphate; GABAR: Gamma- Aminobutyric Acid Receptor; Gi: Inhibitory G Protein; R: Insulin Receptor; IRS:Insulin Receptor Substrate; PI3K:Phosphatidylinositol-3 Kinase; PKA:Protein Kinase A; SSTR: Somatostatin Receptor
Introduction
Type 2 diabetes is caused by reduced insulin secretion and/or insulin resistance, “insulinocentric hypothesis”. Recently, it has been reported that blood glucose levels do not increase in glucagon-deficient mice, in which β-cells are destroyed [1]. In addition, blood glucose levels were found to be normal in glucagon receptor-deficient mice as well [2]. These reports suggest that glucagon contributes to maintaining the blood glucose level more than insulin in diabetes, “glucagonocentric hypothesis ” [3]. Accordingly, the importance of glucagon has been increasing in diabetes field. Thus, understanding the regulation of glucagon secretion from pancreatic α-cells can help to elucidate the pathophysiology of diabetes and the development of novel antidiabetic drugs. Moreover, it has been suggested that insulin resistance might exist in pancreatic α-cells [4-6], thus it is important to understand the regulation of glucagon secretion by pancreatic islet hormones such as insulin, somatostatin and so on. Here, we have outlined the recently proposed regulatory mechanism(s) of glucagon secretion and insulin resistance especially in pancreatic α-cells.
Regulation Of Glucagon Secretion By Islet Hormones
The pancreatic islets are comprised from α, β, δ, ε, and pancreatic polypeptide (PP)-cells. Glucagon secretion from pancreatic α-cells is regulated not only by insulin but somatostatin which is secreted from pancreatic β- and δ-cells, respectively.
Insulin suppresses glucagon secretion by reducing the sensitivity of KATP channel which is regulated by insulin receptor (IR) followed by phosphatidylinositol-3 kinase (PI3K)-pathway [7,8]. In addition, binding of insulin to α-cell IR induces gammaaminobutyric acid (GABA) receptor translocation from cytosol to cell surface, which results in suppression of glucagon secretion [9]. In addition to this, insulin inhibits cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) pathway in pancreatic α-cells, resulting in suppression of glucagon secretion [10].
Somatostatin also suppresses glucagon secretion from pancreatic α-cells [11-14]. Somatostatin receptor (SSTR) 2 and SSTR3 expressed in pancreatic α-cells [15,16]. In somatostatin KO mice and SSTR2KO mice, the glucagon secretion was enhanced in response to glucagon secretagogues stimuli, such as arginine and high K+ [17,18]. Somatostatin suppresses glucagon secretion via inhibitory G protein (Gi) coupled to SSTRs, which suppress the cAMP/PKA pathway in pancreatic α-cells [16]. Somatostatin also suppresses glucagon secretion by directly inhibiting exocytosis by activating the inward rectifying K⁺ (GIRK) current followed by suppression of membrane hyperpolarization [19]. Collectively, glucagon secretion is subjected to paracrine regulation by insulin and somatostatin from adjacent pancreatic β- and δ-cells, respectively (Figure 1).
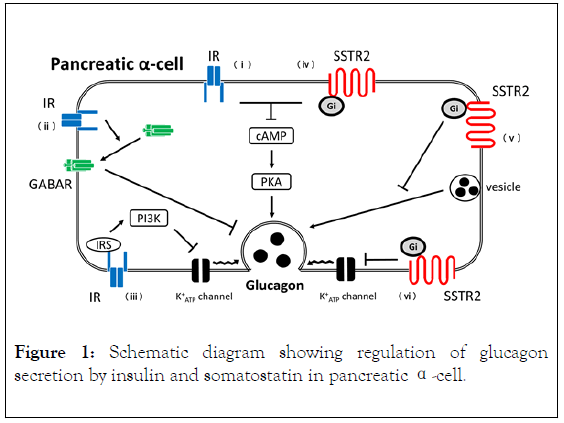
Figure 1. Schematic diagram showing regulation of glucagon secretion by insulin and somatostatin in pancreatic α-cell.
In pancreatic α-cells, insulin suppresses glucagon secretion through IR by (i) Suppressing the cAMP/PKA pathway, (ii) GABAR translocation, and (iii) Suppressing the IRS/PI3KJournal mediated activation of KATP channel. Somatostatin suppresses glucagon secretion through SSTR2-Gi by (iv) Suppressing the cAMP/PKA pathway, (v) Suppressing exocytosis, and (vi) Suppressing the KATP channel. Solid arrow: positive signal, T arrow: negative signal.
Insulin Resistance In Pancreatic A-Cells
In type 2 diabetes, glucagon secretion is enhanced despite hyperglycemia (paradoxical dysregulated glucagon secretion). Recently, insulin resistance in pancreatic α-cells is proposed, which might explain the paradoxical dysregulated glucagon secretion [6]. The pancreatic α-cell-specific IR KO mouse (insulin resistance in pancreatic α-cells model), showed hyperglycemia and enhanced serum glucagon levels under random-fed conditions [20]. Similarly, glucagon secretion is enhanced when IR is knocked down in glucagon secreting α- cell lines [21]. When pancreatic α-cell lines were cultured under high glucose condition, glucagon secretion and glucagon mRNA expression levels are increased [22], indicating high glucose induces glucagon transcription and secretion. These reports suggest that insulin resistance in pancreatic α-cells lead to glucagon dysregulation. In recent years, impaired somatostatin secretion accompanied by decreased surface expression of SSTR2 in pancreatic α-cells in diabetes mellitus have been shown to be involved in increased glucagon secretion [23]. Consequently, in the diabetic state, pancreatic α-cells have resistance to the glucagon-regulating hormones, including not only insulin but also somatostatin. Further studies are needed to be done to elucidate the pathophysiology of diabetes especially in glucagon secretion.
Limitations
Glucagon was discovered in 1923 and has come back into focus in the last decade. Accordingly, the assays used for glucagon measurement has been reviewed. Conventionally, glucagon is measured using radioimmunoassay (RIA) method, which employed antibodies recognizing the glucagon C-terminus. However, RIA method measures glucagon inaccurately because of the presence of glucagon-like peptides (such as glicentin and mini glucagon) which have same amino acid sequences at glucagon C-terminus. Glucagon measurements have become more accurate with a sandwich enzyme-linked immunosorbent assay using antibodies against both C- and N-terminus of glucagon or liquid chromatography/mass spectrometry (LC/MS/MS) [24]. Therefore, previously reported glucagon data might be inaccurate and need to be reevaluated using above mentioned assays.
Conclusion
In diabetes, glucagon maintains the blood glucose level more than insulin (glucagonocentric hypothesis). Glucagon secretion is suppressed by insulin and somatostatin; however, in diabetes, paracrine control is dysregulated, resulting in enhanced glucagon secretion despite hyperglycemia (paradoxical dysregulated glucagon secretion). This can be explained by insulin or somatostatin resistance in pancreatic α-cells. However, former employed glucagon RIA assay is inaccurate, thus glucagon data need to be reevaluated using precise assay.
Acknowledgments
The authors would like to thank members of their laboratory for helpful and constructive advice.
Conflicts of Interest
K.F. received honorarium from Sanofi K.K, Merck Sharpe and Dohme, Taisho Pharmaceutical, Eli Lilly Japan KK, TERUMO Corporation, ARKRAY, AstellasPharma, AstraZeneca, Nippon BoehringerIngelheim, Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical, Novo Nordisk Pharma, Kissei Pharmaceutical, Sumitomo Dainippon Pharma, Kowa Pharmaceutical, Takeda Pharmaceutical, Daiichi Sankyo, Chugai Pharmaceutical, Abbot Japan, Otsuka Pharmaceutical and Kyowa Hakko Kirin. The funders had no role in the design of the study. The authors declare no other conflict of interest.
REFERENCES
- Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol. Endocrinol. 2010; 24: 1605–1614.
- Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011; 60: 391–397.
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J Clin Invest. 2012; 122: 4–12.
- Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013; 369: 362–372.
- Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato K, et al. Possible role of alpha-cell insulin resistance in exaggerated glucagon responses to arginine in type 2 diabetes. Diabetes Care. 2007; 30: 2583–2587.
- Honzawa N, Fujimoto K, Kitamura T. Cell autonomous dysfunction and insulin resistance in pancreatic alpha cells. Int J Mol Sci. 2019; 20: 15.
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. β-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005; 54: 1808–1815.
- Leung YM, Ahmed I, Sheu L, Gao X, Hara M, Tsushima RG, et al. Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 50-triphosphate inhibition. Endocrinol. 2006; 147: 2155–2162.
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006; 3: 47–58.
- Elliott AD, Ustione A, Piston DW. Somatostatin and insulin mediate glucose- inhibited glucagon secretion in the pancreatic alpha- cell by lowering cAMP. Am JPhysiolEndocrinolMetab. 2015; 308: E130–E143.
- Yoshimoto Y, Fukuyama Y, Horio Y, Inanobe A, Gotoh M, Kurachi Y. Somatostatin induces hyperpolarization in pancreatic islet α cells by activating a G protein-gated K+ channel. FEBS Lett. 1999;444: 265–269.
- Gerich JE, Lovinger R, Grodsky GM. Inhibition by somatostatin of glucagon and insulin release from the perfused rat pancreas in response to arginine, isoproterenol and theophylline: evidence for a preferential effect on glucagon secretion. Endocrinol. 1975; 96: 749–754.
- Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009; 58: 403–411.
- Trowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinol, 2000; 141: 111–117.
- DiGruccio MR, Mawla AM, Donaldson CJ, Noguchi GM, Vaughan J, Cowing-Zitron C, et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. MolMetab. 2016; 5: 449e458.
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest. 2013; 123: 1275e1284.
- Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009; 58: 403–411.
- Gromada J, Hoy M, Olsen HL, Gotfredsen CF, Buschard K, Rorsman P, et al. Gi2 proteins couple somatostatin receptors to low-conductance Kþ channels in rat pancreatic a-cells. Pflüg Arch. 2001; 442: 19e26.
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Hih JL, Ford EL, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009; 9: 351–361.
- Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005; 54; 1789–1797.
- Katsura T, Kawamori D, Aida E, Matsuoka TA, Shimomura I. Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS One. 2017; 12: e0176271.
- Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine control of alpha-cell glucagon exocytosis is compromised in human type-2 diabetes. Nat Commun. 2020; 11: 1896.
- Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windelov JA, Plamboeck A, Bojsen-Moller KN, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2017; 57: 1919-1926.
- Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem. 2017; 409: 5911-5918.
Citation: Honzawa N, Fujimoto K (2020) Regulation of Glucagon Secretion and Insulin Resistance in Pancreatic Α-Cells. J Data Mining Genomics Proteomics. 11:224. DOI:10.35248/2153-0602.20.11.224.
Copyright: © 2020 Honzawa N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

