Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2020) Volume 12, Issue 1
Reduce the Risk of Oxidation and Pathogenic Bacteria Activity by Moringa oleifera Different Leaf Extract Grown in Sudan
Rasha Khalid Abbas1,2, Amina AM Al-Mushhin3, Fatma S Elsharbasy4 and Kother Osman Ashiry52Department of Biochemistry, Faculty of Applied and Industrial Science, University of Bahri, Sudan
3Department of Biology, Science and Humanity College in Al –kharj, Prince Sattam bin Abdul-Aziz University Al-Khaarj 11942, Saudi Arabia
4Department of Chemistry, Faculty of Science and Humanity Studies, Prince Sattam Bin Abdul Aziz University Alkarj, Alkharj City 11942, Saudi Arabia
5Department of Chemistry of Natural and Microbial Products, National Research Center, Dokki 12622, Egypt
Received: 26-Oct-2019 Published: 07-Feb-2020, DOI: 10.35248/1948-5948.20.12.427
Abstract
High Performance Liquid Chromatography (HPLC) used in this study to identified Polyphenol constituents of Moringa oleifera leaf extract by different methods (aqueous, ethanol, ethyl acetate and chloroform), it contain gallic acid, Chlorogenic acid, Catechin, Coffeic acid, Rutin, Pyro catechol, Coumaric acid, Vanillin, Ferulic acid1, Naringenin, Propyl Gallate, 4`,7-Dihydroxyisoflavone, and Cinnamic Acid at conc. (µg/15 mg) in all extracts. Ellagic acid gave the highest concentration when extracted by ethyl acetate Caffeine gave the lowest concentration. in all different extract, The effect of moringa (aqueous, ethanol, ethyl acetate and chloroform) leaf extracts against four different pathogenic bacteria Salmonella typhimurium, Pseudomonas aeruginosa, Escherichia coli, and Bacillus cereus, were examined using Mueller Hinton Agar and measuring inhibition zone (diameter mm), were found that, there were a significant different of all moringa leaf extracts against bacteria. The study was conducted to determine the polyphenol constituents of Moringa oleifera aqueous, ethanol, ethyl acetate and chloroform leaf extract. The effect of Moringa oleifera (aqueous, ethanol, ethyl acetate and chloroform) leaf extracts against four different pathogenic bacteria.
Keywords
Moringa oleifera; Polyphenol constituent; Microorganism; Antimicrobial
Introduction
Moringa oleifera species is widely cultivated around the world, in, East, West and Sudan, the origin is India. Moringa belong to family Moringaceae [1]. Flowers, leaves, bark, seeds and roots it used as medicinal purposes and food. Moringa leaves contain important constituent, including carbohydrates, protein, vitamin such as riboflavin, ascorbic acid, thiamine, niacin, mineral such as calcium, phosphorus and iron [2]. Moringa leaves extract reduce the free radicals and the oxidation of blood because it contain polyphenol, the leaves are used in medicinal, against AIDS, fever, respiratory diseases and antimicrobial [3,4]. Moringa contain poly phenol that prevents body against many diseases such as pathogenic bacteria, hypertensive, and cancer. It include carotenoids (including β-carotene or provitamin A) prevent body from free radicals, act as antioxidant are more commonly recognized as phytochemicals [5]. Many studies reported that moringa have chemo preventive properties and has potent cytotoxicity in human cancer cells, the leaves have best used to reduce oxidation, prevent from cancer and degenerative diseases [6]. Moringa leaves extract contain several compounds, including glucopyranoside, and niazimicinreduce reduce the risk of lymphoblastic anemia [7]. Moringa contain flavonoids which are an essential in diet prevent lipid peroxidation that lead to cancer, and thrombosis, flavonoids prevent body from free radical, inflammatory inhibition of oxidative and hydrolytic enzymes (cyclooxygenase, phospholipase A2 and lipoxygenase). In general Phenolic acid classified into hydro benzoic acid contain protocatechuic, gallic, vanillic, syringic acid and p-hydroxybenzoic, the other class is hydroxycinnamic acids contain sinapic, coffeic, coumeric and ferulic acids most of these compound found in moringa [8].
Materials and Methods
Moringa oleifera leaves were purchased in the super market and identified in the Department of Plant Botany, Faculty of Agriculture, Khartoum University, Sudan.
Microorganisms
The bacteria used in this work were isolated from Stak Laboratory (Khartoum), Sudan and identified by conventional biochemical methods [9]. These methods for identification were carried out on all isolates bacteria in the three separate laboratories. The results from the three separate laboratories were from routine clinical microbiology service. According to standard microbiology techniques, these microbes were Pseudomonas aeruginosa, Escherichia coli, Bacillus cereus and Salmonella typhimurium.
Mueller Hinton Agar
Mueller Hinton Agar (MHA) (Becton Dicknson M.D USA), media was prepared according to the manufacturer’s instruction. Sterile Mueller Hinton agar plates were inoculated with the test culture by surface spreading using sterile wire loops and each bacterium evenly spread on the entire surface of the plate to obtain uniformity of the inoculum. Concentrations of 12.5, 25, 50 and 100 mg/ml prepared from the dry leaves powder were used for antibacterial analysis using agar well incorporation methods. Plates of Mueller Hinton agar were prepared and allowed to solidify on Petri dishes. Each plate was then seeded with a test bacterium. Four holes were made in each of the plate with a sterile 2.0 mm diameter cork borers. Each of the four holes was filled with a given concentration of the extract mixed with plane sterile agar. The plates were then incubated at 37°C for 24 hours. The diameters of zones of inhibition were measured using a meter rule and the mean value for each organism was recorded [10].
Preparation of plants extracts
The powdered sample (100 g, of leaf plant) was weighed, and were subjected to different extraction solvents separately extracted with ethyl acetate 80% at 50°C-60°C for 2 h, Chloroform 80% at 50°C-60°C for 2 h, ethanol 98% at 60°C for 2h in a Sox let apparatus. The all solvents extract were evaporated by a Buchi Rotary evaporator under reduced pressure.. The extract was similarly evaporated exhaustively; air dried for about 18 h and the yield was preserved in a covered flask, also the plant was extracted by distilled water over night at room temperature (25-30°C) filtered and dried [11].
Method of analysis by HPLC
In this analysis, High Performance Liquid Chromatography (HPLC) (Shimadzu corporation (Koyoto, Japan) an Agilent 1260 series was used. Temperature 35°C and C18 column (4.6 mm × 250 mm i.d., 5 μm) were used. The mobile phase contained water (A) and 0.02% tri-floro-acetic acid in acetonitrile (B), with a flow rate of 1 ml/ min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5-8 min (40% A); 8-12 min (50% A); 12-14 min (80% A) and 14-16 min (80% A). The wavelength was 280 nm and volume injected 10 μl. Authentic compound were obtained from the Central laboratory of The National Research Centre, Egypt.
Statistical analysis
It was done according to Duncan, Multiple Range Test [12].
Results and Discussion
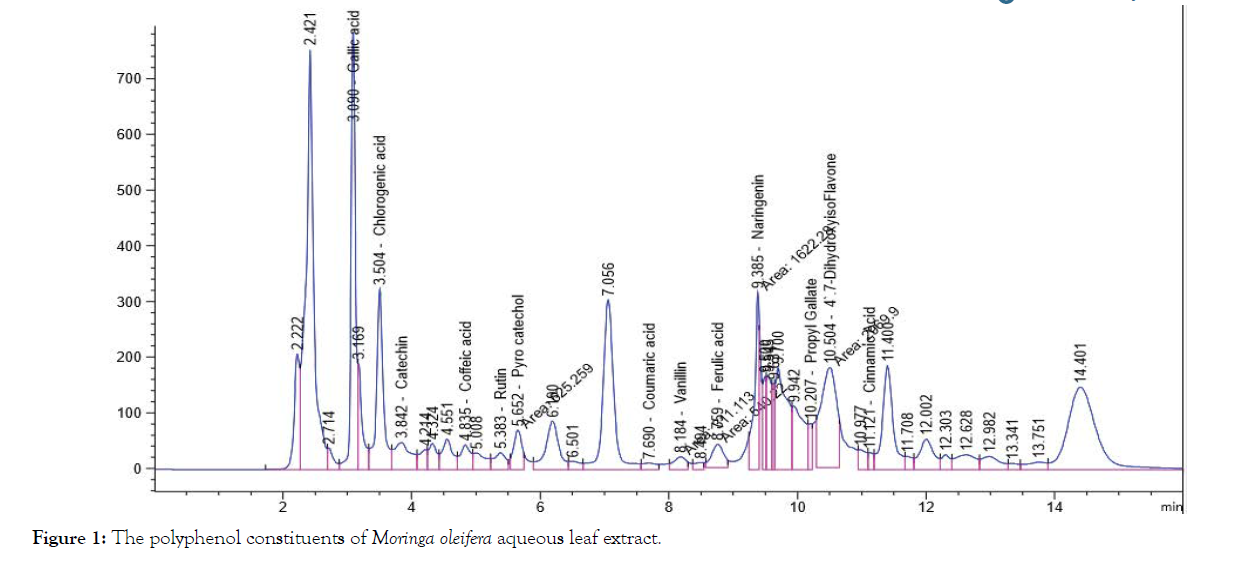
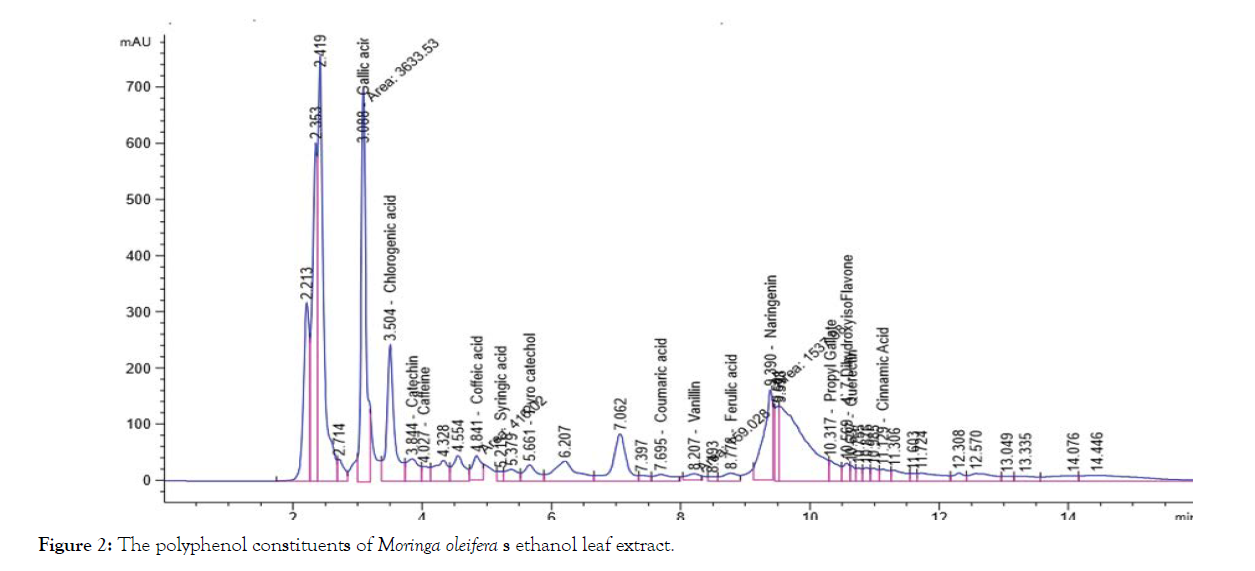
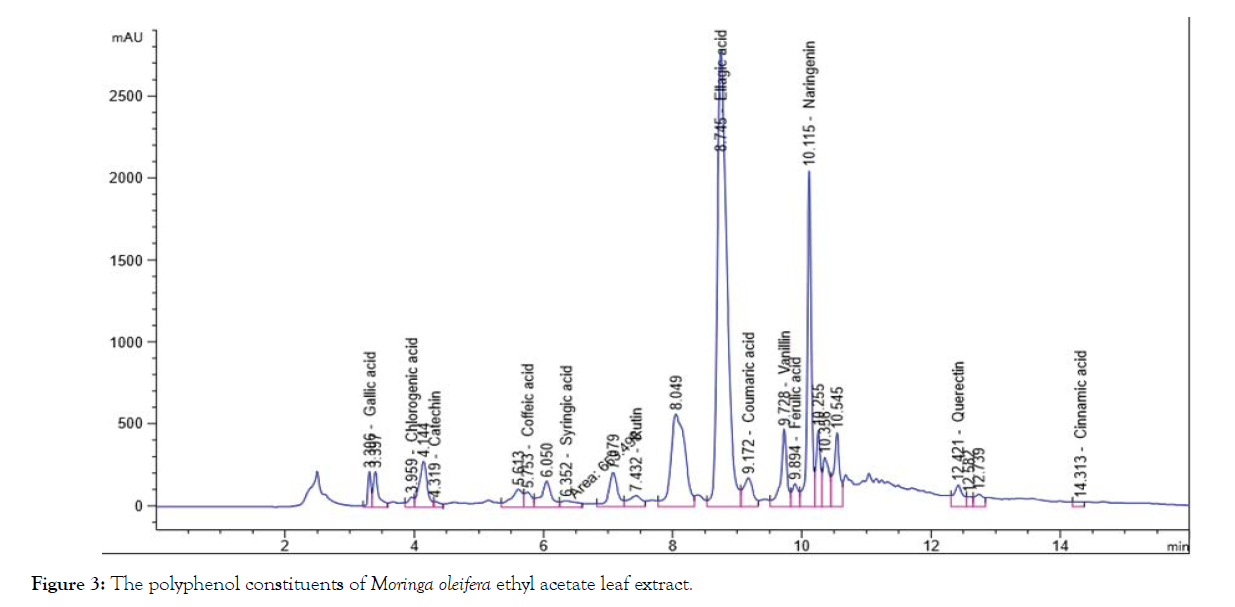
The solvent of the extraction is the main factor in the prognosis of the qualitative and quantitative composition of the isolated phenolic compounds by HPLC, Table 1 and Figures 1-4 shows that the polyphenol constituents of Moringa oleiferan contain gallic acid, chlorogenic acid, catechin, coffeine, coffeic acid, syringic acid, rutin, pyro catechol, ellagic acid, coumaric acid, vanillin, ferulic acid1, naringenin, propyl gallate, 4’,7-Dihydroxyisoflavone, querectin and cinnamic acid in different extract methods (aqueous, ethanol, ethyl acetate and chloroform) at Conc. (μg/15 mg). Ellagic acid gave the highest concentration when extracted by ethyl acetate, Caffeine gave the lowest concentration in all different extracts, these result were confirm with the previous studies [8,13]. Antimicrobial activity of Moringa oleifer leaf extracts by (water, ethanol, ethyl acetate and chloroform) at different concentrations of (12.5, 25, 50 and 100%) against four pathogenic organisms (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium and Bacillus cereus) were examined by using Mueller Hinton Agar and measuring inhibition zone (diameter mm) (Table 1)( Figure 1-4).
Figure 1: The polyphenol constituents of Moringa oleifera aqueous leaf extract.
Figure 2: The polyphenol constituents of Moringa oleifera s ethanol leaf extract.
Figure 3: The polyphenol constituents of Moringa oleifera ethyl acetate leaf extract.
Figure 4: The polyphenol constituents of Moringa oleifera chloroform leaf extract.
| Amount of phenolic compounds of in different method of extract (µg/15mg) | |||||
|---|---|---|---|---|---|
| Phenolic compound | Aqueous Extract µg/ 15 mg | Ethanol Extract µg/15 mg) | Ethyl acetate Conc. (µg/15 mg) | Chloroform Conc. (µg/15 mg) | Mean Phenolic compound |
| Gallic acid | 241.52a | 233.97b | 52.69c | 0.67d | 132.21b |
| Chlorogenic acid | 149.12a | 118.73b | 24.38c | 4.29d | 74.13c |
| Catechin | 144.56a | 86.97b | 37.44c | 6.85d | 68.96c |
| Caffeine | 0.70b | 5.78a | 0.35d | 0.45c | 1.82d |
| Coffeic acid | 16.16b | 13.71c | 24.45a | 4.20d | 14.63d |
| Syringic acid | 0.65d | 3.79c | 23.88a | 5.51b | 8.46d |
| Rutin | 58.36b | 0.33d | 130.00a | 11.45c | 50.04c |
| Pyro catechol | 64.52a | 41.48b | 10.2c | 9.3d | 31.38d |
| Ellagic acid | 0.81c | 0.78d | 1811.52a | 3.57b | 454.17a |
| Coumaric acid | 3.95c | 5.35b | 78.80a | 3.74d | 22.96d |
| Vanillin | 10.92b | 5.58c | 74.96a | 0.50d | 22.99d |
| Ferulic acid | 11.51b | 4.85d | 31.13a | 11.15c | 14.66d |
| Naringenin | 65.87b | 62.45c | 507.55a | 8.81d | 161.17b |
| Propyl Gallate | 8.05b | 8.78a | 7.40c | 6.7d | 7.73d |
| 4`,7-DihydroxyisoFlavone | 74.67a | 5.52b | 0.55d | 0.77c 0 | 20.38d |
| Querectin | 0.91d | 10.55c | 512.45a | 151.37b | 168.82b |
| Cinnamic acid | 1.38d | 1.71c | 2.97b | 531.03a | 134.27b |
| Mean Moringa oleifera leaf extract | 50.22b | 35.90d | 195.92a | 44.73c | |
Note: Values are mean (n = 3). The different letters indicate significant differences between the values (p<0.05).
Table 1: Content of phenolic compounds of Moringa oleifera in different methods of extracts.
Table 2 shows that a significant differences among bacteria when Moringa oleifera aqueous leaf extract were added against microorganism, the highest inhibition zone were detected against Salmonella typhimurium (13 mm) and Escherichia coli (13 mm) while the lowest inhibition zone against Pseudomonas aeruginosa (5 mm) (Table 2).
| Zone of inhibition by microorganism | Concentration of the of Moringa oleifera leaf aqueous extract | Mean microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 10 | 12 | 14 | 16 | 13 |
| Pseudomonas aeruginosa | 0 | 0 | 10 | 10 | 5 |
| Escherichia coli | 10 | 13 | 14 | 15 | 13 |
| Bacillus cereus | 10 | 10 | 14 | 15 | 12.25 |
| Mean Moringa oleifera leaf aqueous extract | 7.5 | 8.75 | 13 | 14 | |
Table 2: Inhibition zone (in mm) for different concentrations of Moringa oleifera leaf aqueous extract.
Table 3 shows a significant differences among bacteria, when moringa ethanol leaf extract were added against microorganism, the highest activity against Pseudomonas aeruginos (13.25 mm) and lowest inhibition detected against Salmonella typhimurium (7.5 mm) (Table 3).
| Zone of inhibition by microorganism | Concentration of the of Moringa oleifera leaf ethanol extract | Mean microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 0 | 10 | 10 | 10 | 7.5 |
| Pseudomonas aeruginosa | 10 | 12 | 15 | 16 | 13.25 |
| Escherichia coli | 10 | 13 | 14 | 15 | 13 |
| Bacillus cereus | 10 | 12 | 14 | 15 | 12.75 |
| Mean Moringa oleifera leaf ethanol extract | 7.5 | 11.75 | 13.25 | 14 | |
Table 3: Inhibition zone (in mm) for different concentrations of Moringa oleifera leaf ethanol extract
Table 4 shows that a significant differences among bacteria when Moringa oleifera ethyl acetate leaf extract were added against microorganism, the highest inhibition zones against Salmonella typhimurium (17 mm) and lowest inhibition detected against Bacillus cereus (13.5 mm) (Table 4).
| Zone of inhibition by microorganism | Concentration of the of Moringa oleifera leaf extract by ethyl acetate | Mean Microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 17 | 17 | 17 | 17 | 17 |
| Pseudomonas aeruginosa | 14 | 15 | 15 | 16 | 15 |
| Escherichia coli | 13 | 13 | 15 | 15 | 14 |
| Bacillus cereus | 13 | 13 | 14 | 14 | 13.5 |
| Mean Moringa oleifera leaf extract by chloroform | 14.25 | 14.5 | 15.25 | 15.5 | |
Table 4: Inhibition zone (in mm) for different concentrations of Moringa oleifera leaf extract by ethyl acetate
Table 5 shows that a significant differences among bacteria when Moringa oleifera chloroform leaf extract were added against microorganism the highest inhibition zones against Salmonella typhimurium (15.25 mm) and lowest inhibition detected against Escherichia coli (13 mm) these results agree with the previous studies showed that the powder from the leaves of Moringa have potential antibacterial activity against the tested gram positive bacteria; Staphylococcus aurous and gram negative bacteria Escherichia coli and Pseudomonas aeruginosa [13-18] (Table 5).
| Zone of inhibition by microorganism | Concentration of the of Moringa oleifera leaf extract by chloroform | Mean Microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 14 | 14 | 16 | 17 | 15.25 |
| Pseudomonas aeruginosa | 13 | 13 | 14 | 16 | 14 |
| Escherichia coli | 12 | 12 | 13 | 15 | 13 |
| Bacillus cereus | 14 | 15 | 15 | 16 | 15 |
| Mean Moringa oleifera leaf extract by chloroform | 13.25 | 13.5 | 14.5 | 16 | |
Table 5: Inhibition zone (in mm) for different concentrations of Moringa oleifera leaf extract by chloroform
Conclusion
As a conclusion, Moringa oleifera contains polyphenol compounds in all extract (aqueous, ethanol, ethyl acetate and chloroform) have antioxidant, anticancer and anti-inflammatory activity, also all extracts of moringa have antibacterial activities.
Acknowledgement
The authors would like to thank the staff of the Laboratories of Chemistry and Microbiology, National Research Centre, Khartoum, Sudan.
REFERENCES
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, Genetic, Ethno pharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int J Mol Sci. 2015;16(6): 12791-835.
- Abbas RK, Elsharbasy FS, Fadlelmula AA. Nutritional values of Moringa oleifera, total protein, amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. J Microb Biochem Technol. 2018;10(2):56-58.
- Madukwe, E.U. Nutrient Composition and Sensory Evaluation of Dry Moringa oleifera Aqueous Extract. Int J Basic App Sci. 2013;13(3):100-102.
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 2009;64(4):303-311.
- Bharali R, Tabassum J, Azad MRH. Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolizing enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2003;4(2):131-139.
- Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, et al. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4(2):164-171.
- Nikkon F, Haque ME, Aragianis K, Mosaddik MA. Isolation of aglycone of deoxy-nia-zimicin from Moringa oleifera and its cytotoxicity. Rev. Latinoamuraunim. 2003;31(1):5-9
- Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, et al. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J Med Food. 2010 Jun;13(3):710-716.
- Cheesebrough M. District laboratory practice in tropical countries Part 2. Cambridge University Press; Cambridge: 2000, pp. 63–70.
- Omenka CA, Osuoha JO. Antimicrobial potency of grapefruit seed extract on five selected pathogens. Nig J Microbiol 2000;14(2):39-40.
- Nobosse P, Fombang EN, Mbofung CMF. The effect of steam blanching and drying method on nutrients, phytochemicals and antioxidant activity of Moringa (Moringa oleifera L.) leaves. J Food Sci Technol. 2017;5(2):53-60.
- https://pdf.usaid.gov/pdf_docs/PNAAR208.pdf
- Leone A, Fiorillo G, Criscuoli F, Ravasenghi S, Santagostini L, et al. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi refugee camps, and Haiti. Int J Mol Sci. 2015;16(8):18923-18937.
- Kiran SIingh, Tafiada GM. Antibacterial activity of Moringa oleifera L leaves extract against some selected. Int J Pharm Pharm Sci. 2013;6(9):52-54.
- Cushine TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrobial Agents. 2005;26(5):343-356.
- Lanciotti R, Patrignani F, Bagnolini F, Guerzoni ME, Gardini F. Evaluation of diacetyl antimicrobial activity against E. coli, L. monocytogenes and S. aureus. Food Microbiol. 2003;20(5):543-575.
- Doughari JH, Pukuma MS, De N. Antibacterial effects of Balanitesaegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. African J Biotechnol. 2007:6(19): 2212-2215.
- Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, et al. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. BioresourTechnol. 2007;98(1):232-236.
Citation: Citation: Abbas RK, Al-Mushhin AAM, Elsharbasy FS, Ashiry KO (2020) Reduce the Risk of Oxidation and Pathogenic Bacteria Activity by Moringa oleifera Different Leaf Extract Grown in Sudan. J Microb Biochem Technol. 12:427. doi: 10.35248/1948-5948.20.12.427
Copyright: Copyright: © 2020 Abbas RK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.