Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 10, Issue 2
Real Structure of Benzene Molecule and the Thickness of Graphene
Ning Wang*, Rui Wang and Dulun WangReceived: 17-Feb-2022, Manuscript No. MCA-22-15657; Editor assigned: 21-Feb-2022, Pre QC No. MCA-22-15657(PQ); Reviewed: 07-Mar-2022, QC No. MCA-22-15657; Revised: 10-Mar-2022, Manuscript No. MCA-22-15657(R); Published: 17-Mar-2022, DOI: 10.35248/2329-6798-22.10.338
Abstract
For more than 100 years, the problem of benzene molecular structure has not been solved. Based on the fact that the essence of chemical bonds is that the nuclei share electrons, this study proposes that each electron shared by the nuclei corresponds to a semi valence bond. Semivalent bonds can be formed between intervening carbon atoms of the benzene ring. A new theory of benzene molecular structure was established. Quantum mechanical calculations quantitatively explain the existence of ground and excited states of benzene, as well as the experimental results of the hydrogenation heat and ultraviolet spectroscopy of benzene. Semivalent bonds are represented by dashed lines, molecular structures and chemical reaction formulas of benzene and its dozens of homologues and derivatives are designed. The method is universal and can also record the chemical reaction process. Under the new theory, benzene stacks may form benzene tubes, and the thickness of the three-layer benzene tubes is calculated to be very close to graphene. Based on other properties of graphene, it is considered more like a three-layer structure.
Keywords
Benzene; Molecular structure; Semivalent bond; Dotted line; Hydrogenation heat; Ultraviolet spectroscopy; Graphene thickness
Introduction
In 1865, F.A.Kekulé proposed that cyclohexatriene is a benzene molecular plane structure, which was questioned because it was inconsistent with the experimental results. Later, some people proposed different structural formulas, but they were all unsuccessful. In particular, the experimental results of the hydrogenation heat and UV spectroscopy of benzene cannot be quantitatively interpreted [1-3]. So much so that it became the mystery of the century [4].
The birth of the semi price bond
Solving the problem of benzene molecular structure should follow the valence bond theory of chemical bonds. According to the valence bond theory at that 1865 time, only integer valence bonds exist. Later, it was believed that the essence of chemical bonds is that the nuclei share electrons [4,5]. Nuclei share a pair of electrons to form a valence bond, also consistent with earlier theories. In this case, Kekule’s cyclohexatriene should be the moyersst perfect. But it does not match the experimental results [6]. It turns out that integer valence bonds don’t work for benzene. In fact, the nature of chemical bonds does not exclude non-integer valence bonds. It is also reasonable if every electron shared by the nucleus corresponds to a semivalence bond. However, it has never been proposed, let alone how to represent such a semivalence bond. We now present it as one of the foundations of a new structural theory.
Ground and excited states of the benzene molecule
According to existing molecular orbital theories [4,7], the 2s and 2p orbitals of each carbon atom in the benzene ring can be hybridized into three sp2 orbitals and a one un hybridized pzorbital. The 12 sp2 orbitals of 6 carbon atoms form 6 σ bonds and connect into a regular hexagonal ring. The other 6 σ bonds are attached to hydrogen atoms outside the ring. The remaining 6 un-hybridized pzorbitals combine linearly into 6 molecular orbitals. Among them, there are 3 bonding orbitals and 3 antibonding orbitals. Their 6 p electrons are all in bonding orbitals, and each orbital can hold 2 electrons with opposite spins. The 3 antibonding orbitals are empty. Molecular structural energy is low and therefore structurally stable [8,9]. The 6 carbon nuclei of benzene ring share 6 p electrons, forming 6 semivalent π bonds. A semivalent π bond can be formed between adjacent p orbitals, and connected into a large bond ring. It should be the ground state of the benzene molecule. When the electron gains enough energy outside the system, one electron in each bonding orbital jumps to the antibonding orbital [7]. The 3 antibonding orbitals are also fully occupied. All electrons become the same spin [7,10]. Active electrons increase the mutual repulsion between adjacent pzorbital electrons. The steric hindrance effect [2,9] causes one of the pzorbitals to be turned to the opposite phase in the plane of the benzene ring. After that, the positive and negative orientation phases each have three spaced carbon atoms pzorbitals, and form a new triangular ring adjacent relationship. In each phase, there are three spaced carbon nuclei sharing three π electrons, forming three semivalent π bonds and their bond rings, and the structure is still stable[2,11]. This should be the excited state of the benzene molecule.
Due to electrons have the high activity in the excited state; they can participate in chemical reactions. The semivalent bonds between interval carbon atoms ensure the carbon tetravalent and covalent bonds saturation [4,11]. In this way, a new theory of the planar structure of the benzene molecule was constructed.
Benzene tube hypothesis
Assuming that in the excited state of benzene, when the flipped ring plane of one molecule overlaps with another molecule, the two benzene rings are parallel and mirror-symmetrical. They correspond to the three pzorbitals of the spaced carbon atoms, butt to form three σ bonds, and connect to form a two-layer benzene tube. The other three spaced pzorbitals of the flipped ring plane are connected to another molecule that is not flipped in the same way, and finally a three-layer benzene tube is formed. The top and bottom of the three-layer benzene tube are exactly the same as the top and bottom of the benzene molecule and it will be as stable as benzene. However, since multiple vertical sigma bonds form a lattice, the physical properties will be like metals. After calculating the thickness of the three-layer benzene tube, it is very close to the thickness of graphene [12]. The physical properties of graphene are also metal-like. Therefore, it is speculated that graphene is more like a three-layer structure.
Materials and Methods
In order to verify the correctness of the new theory of benzene molecular structure, it is necessary to establish the electronic wave function of the benzene ring and its wave equation and solve the equation. Use relevant calculated values to quantitatively explain experimental results [1,9]. In addition, to design a molecular structural formula that conforms to the new theory, the design method must not only have a wide range of adaptability to design any molecule, but also be able to record many details of its chemical reaction process.
Quantum mechanical calculations verify the reliability of the new structural theory
A complex wave equation with multiple electrons, and it is almost impossible to solve it [10,13]. Only a simplified model can be used to approximate the solution.
In the benzene ring, 6 p electrons rotate around the carbon nucleus where they are located, and the movement of each electron has an arc in the same direction along the benzene ring. They will be connected in 6 arcs to form a circle. The interaction of the corresponding 6 carbon nuclei with electrons is regarded as the positive charge of the center. In this way, their overall behavior is similar to an equivalent electron, which moves in a circle around the positive charge along the circum circle of the benzene ring. The ground state and the excited state are the two energy levels of equivalent electrons. Thus, a simplified equivalent electronic model of benzene molecule was established. If the model can be transitioned to the hydrogen-like atom model, the existing method can be used to those calculations [13,14]. However, the kinetic energy of the equivalent electron is unknown. The only data that can be inferred is the work done by the electrons around the circumscribed circle of the benzene ring.

The question is whether it is possible for WH to be seen as kinetic energy EK.
First look, the circumference of the circle is expressed by integral and the force is expressed by the derivative of speed.
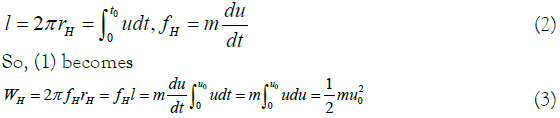
It seems that at the end point of the circle, WH and kinetic energy EK can be equal.

It can also be said that at this point, it is possible to change the equivalent electron model to a hydrogen-like atom model. Similarly, at this point, the kinetic energy EK and the potential energy VH of the electrons outside the hydrogen nucleus are also equal.
In the same way, the kinetic energy EK and the potential energy VH of the electrons outside the hydrogen nucleus are also equal.

In (5), e is the electronic charge, and ε0 is the vacuum permittivity. The force of the equivalent electron should be the same as the general form of the force of the hydrogen atom, and when it is equal to the force of the extra nuclear electron of hydrogen [10,13], it must be:
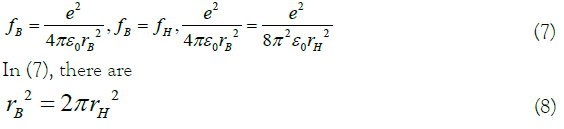
In (8), r only represents feasible in the simplification of the equivalent electron-like hydrogen atom model. However, there is an error between it and the actual situation. Therefore, let g here, as a real coefficient that has nothing to do with r, to represent the error.

This clearly shows that the difference in the expression of the two forces is mainly reflected in the radius r. If the different r at both ends of formula (9) can be regarded as a coordinate transformation, this transformation can make the equivalent electron model transition to the hydrogen-like atom model [13,14].
With reference to (7), (8) and (9), the potential energy of the equivalent electron can also be written more in the general form:
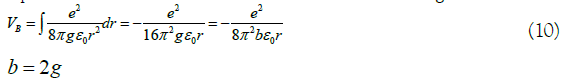
In (10), VB is the equivalent electronic potential energy of the benzene ring. So, as the equivalent electronic coordinate transformation of the benzene ring, refer to (9) and (10), which can be written as a general transformation form [13]:
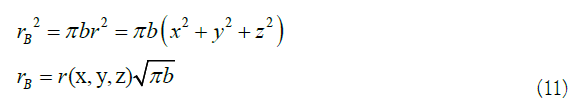
Based on the above-mentioned equivalent electrons, it makes a reciprocating circular motion along the benzene ring, and only at the end of the circle meets the conditions of the above model. According to these conditions, imitating Schrödinger, the equivalent electronic wave function is established [11]. Due to the limitation of (3), the wave function period can only be set to 2nπ. From (4), (10), (11), Planck formula and De Broglie formula 13 and 14, get
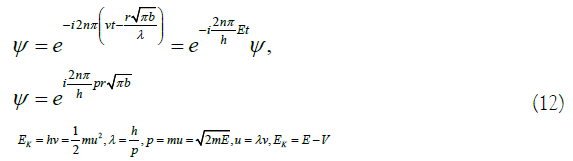
In (12), n is a natural number, λ is the wavelength, ν is the frequency, r is the radius of the benzene ring, h is the Planck constant, p is the electron momentum, m is the static mass of the electron, u is the electron velocity, EK is the kinetic energy, E is the total energy, and V is the potential energy. With reference to (10), (12) and Schrodinger equation [14], the three-dimensional wave equation of the equivalent electron can be obtained:
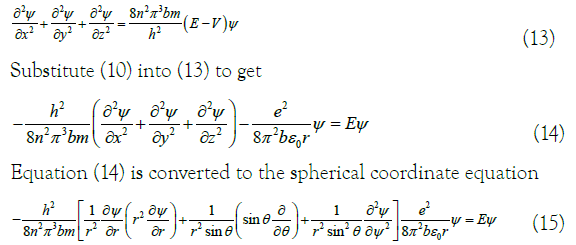
The energy eigenvalues E in equation (15) are solved using the variation method 15, where the Lagrangian undetermined coefficient method is applied [15,16]. The algorithm uses Hamiltonian, which is represented by H, Since H only the r part, of (15) is involved. So, calculate the eigenvalues as follows
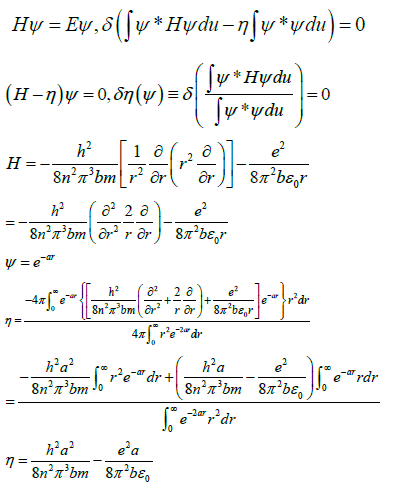
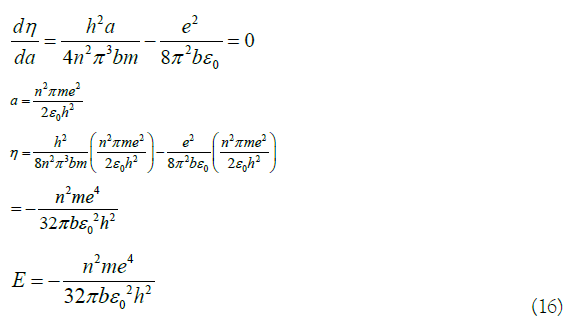
Quantitatively explain the hydrogenation heat of benzene with the quantum mechanics calculation
In (16), E is the energy eigenvalue of the equivalent electron. Turn it into an energy level formula is:
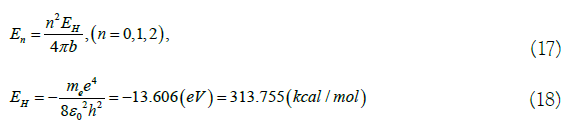
In (17), (18), EH is the lowest energy level of extra nuclear electrons of hydrogen atom [4,14]. M is the static mass of electron, m=9.1095 × 10-31 kg, basic charge e=1.602 × 10-19 C, the vacuum dielectric constant ε0=8.854 × 10-12 F/m, Planck constant h=6.626 × 10-34 J.S. En is the energy level of the equivalent electron. n is the energy level number. When n=2, the energy level is E2, which is the lowest energy level. In the ground state, When n=1, the energy level is E1 and it is in an excited state. Active electrons can participate in chemical reactions. The electrons further increase energy and escape beyond the control of the benzene ring. The benzene ring is destroyed and benzene is hydrogenated to form cyclohexane. If it is also regarded as an extreme state of energy level, it can only be n=0 and energy level E0=0. These energy levels are as follows:
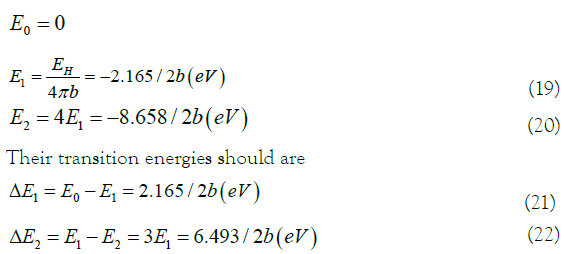
Where ΔE2 is the transition energy of the ground state. ΔE1 is the transition energy of the excited state. The transition energy of the equivalent electron in the excited state was used to quantitative interpretation the experimental results of the hydrogenation heat of benzene [1,2,8]. In (21), the transition energy value ΔE1=2.165/2b (eV) of the excited state is equivalent to the hydrogenation heat released of benzene to cyclohexane. The experimental value of the hydrogenation heat of benzene is 2.160 eV (49.802 kcal/mol). The error rate is only 0.231% (2b=1.00231). It is close enough. The experimental result of the hydrogenation heat of benzene is quantitatively explained here.
If 2b is ignored, the hydrogenation heat of benzene is a constant composed entirely of basic physical constants 14, denoted as BH
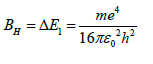
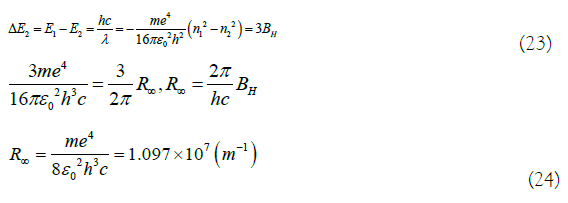
In (23) and (24), c is the speed of light, c=2.998 × 108 m/s. Since the ground state transition energy ΔE2 is related to the ultraviolet light energy absorbed by benzene, the Rydberg constant, R∞ can also be measured by the ultraviolet spectroscopy [14,17].
Quantitative interpretation of the UV spectrum of benzene with quantum mechanics calculation
If the energy level of the equivalent electron is related to the energy level of the molecular orbital, here, through the simplified calculation of the Hückel molecular orbital theory the wave function and energy level of the molecular orbital of benzene are [7,12]:
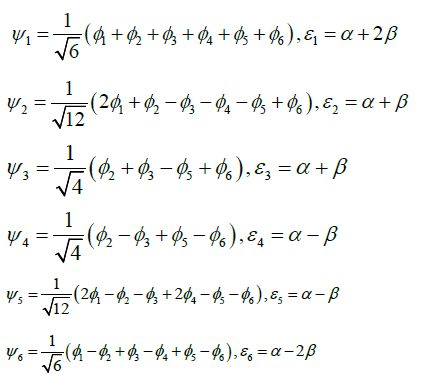
According to the new theory of benzene molecular structure, referring to (19), (20), (25), the corresponding ground state and excited state energy levels are
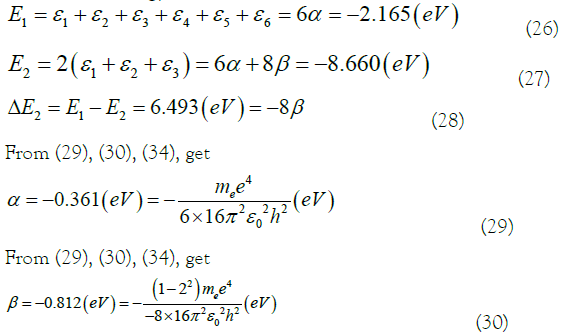
In (29), The Coulomb integral α and the transition integral β in the Hückel molecular orbital theory are constants composed of basic physical constants. The relationship is obtained between α and β
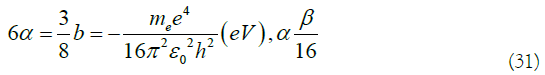
The UV spectrum of benzene shows that it has a strong single peak at 184 nm, a strong dentate peak at 203-212 nm, and a weak dentate peak at 230-270 nm. The infrared spectrum shows that there are skeleton vibration peaks at 6250 nm and 6670 nm, and C-H stretching vibration peaks at 3230 nm and 3300 nm. Their corresponding energy can be calculated using Planck formula [13,17].

In (32), E is the energy, λ is the wavelength of the absorption peak, refer to (18) and (24), c and h and c substitute (32). Calculation results are shown in Table 1.
In the infrared spectra of Table 1,the average value of the skeleton vibration energy representing the fundamental vibration of the benzene ring is E6460=0.192 eV [3,17]. C-H bending vibration can be regarded as the characteristic of expansion and contraction caused by the benzene ring resonance. The average energy is E3265=0.380 eV. The βcorresponds to the energy level difference of the molecular orbital. Combining the combination of these three basic indicators has a clear meaning, which is to simulate and quantitatively explain the results of ultraviolet spectroscopy (Table 2).
The analysis results from Tables 1 and 2 and (24) show that the energy corresponding to the wavelength of the ultraviolet spectrum of benzene is within the range of the ground state transition energy. The combined energy value of three simple indicators shows that the ultraviolet spectrum of benzene is related to whether the benzene molecule absorbs part of the energy of the external system before the ground state transition, and the amount of ultraviolet light energy absorbed during the transition.
It is found that the ratio of energy 5.890 eV corresponding to the median wavelength of 212 nm to the ground state transition energy of 6.493 eV is 0.907. The weird thing is that the ratio of the benzene ring bond length 0.1397 nm to the single bond length 0.154 nm of ordinary chain hydrocarbons is same.
It is found that the ratio of energy 5.890 eV corresponding to the median wavelength of 212 nm to the ground state transition energy of 6.493 eV is 0.907. The weird thing is that the ratio of 0.1397 nm of the benzene ring bond length to 0.154 nm of the ordinary single bond length in chain hydrocarbons is also 0.907.
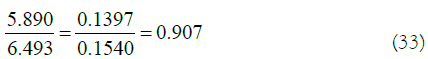
In (33), if 6.493-5.890=0.603 (eV), the difference between these two energies can be regarded as the holding energy of the benzene ring bond length of 0.1397 nm. It also ensures the normality of the planar structure of the benzene molecule. The median wavelength peak at 212 nm may be closely related to the bond length of the benzene ring. So, quantitative relationship of median peak wavelength in UV spectrum of benzene or there may be a quantitative relationship between the bond length of the benzene ring and the average wavelength of the ultraviolet spectrum.
Measuring the bond length of the benzene ring with the median peak wavelength in the ultraviolet spectrum of benzene
The physical concept of the wave function in quantum mechanics can be understood as follows: it must meet the three so-called reasonable conditions of continuous, single value and finite in all variable regions of variable x. Hence, the equation 13,14.
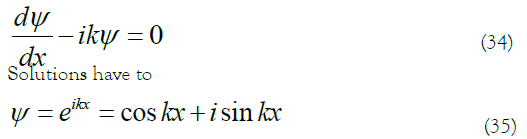
Only when k is not an imaginary number or a complex number can the above three conditions be met. Reference to the equation of momentum in quantum mechanics 13 is
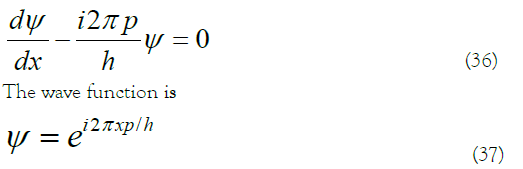
When a free particle moves in a circle along a ring with a radius of r, for the wave function to satisfy the single value condition (like an equivalent electron), the wavelength λ must satisfy 13,
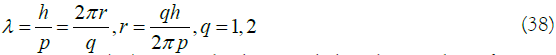
In equation (38), q can also be regarded as the number of energy levels. Substituting equation (38) into equation (37) to obtain the corresponding wave function:

According to the one-dimensional Schrodinger equation 13 should have
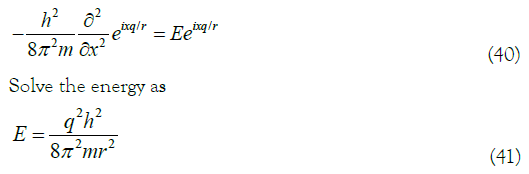
In (41), when this free particle is given two states (such as ground state and excited state of the equivalent electron) and absorbs ultraviolet light energy, the transition energy between the two energy levels (q=1, 2) and the absorption peak wavelength related.
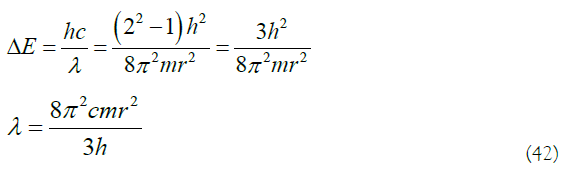
Equations (12), (18), (23), (24), (42), Substituting the constant values of h, m, and c into (42), the relationship between the wavelength of the absorbed light and the radius is obtained:

Substituting the median of the important peak wavelength of the ultraviolet spectrum of benzene at 212 nm, that is, λ=2.12 × 10-7 m, into (43), obtains
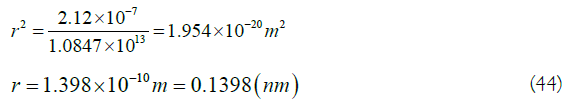
In (44), is the equivalent electron radius calculated using the median wavelength of the ultraviolet spectrum of benzene. It is almost equal to 0.1397 nm of the benzene molecular bond length measured by Raman spectroscopy [2,17]. It shows that the result of two kinds of spectral measurements are consistent.
The semivalent bond between interval carbon atoms of the benzene ring is given by (23) as 0.361 eV, and then the bond length of the semivalent bond is deduced from (44) to be 0.242 nm.
Design and application of new structure of benzene molecule
Design and application of the structural formula of benzene molecule and its homologues and derivatives:
Design of the planar structure of benzene molecule: According to the new structure theory of benzene, the plane structure form of benzene molecule is designed. Semivalent π bonds are represented by dashed lines. The short form of the ground state is a double ring of a regular hexagon. The outer circle is a solid line, and the inner circle is a dashed line. The outer ring of the excited state is the same as the ground state, and the inner ring consists of two dashed equilateral triangles that are inverted to each other. The positive triangle represents the positive phase, and the inverted triangle represents the negative phase (Figure 1) [2,18]. Before the chemical reaction, the benzene in the ground state must absorb energy and be activated to the excited state (Figure 2).
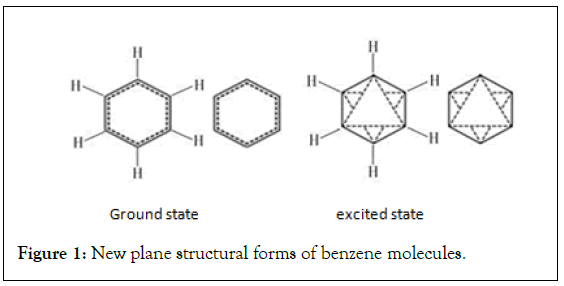
Figure 1: New plane structural forms of benzene molecules.
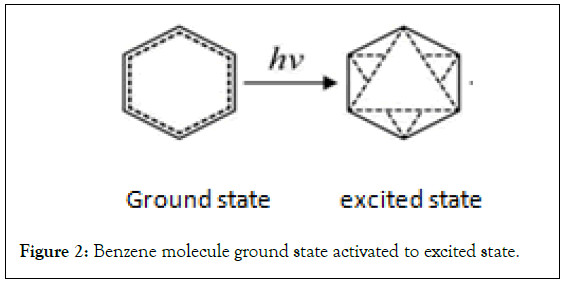
Figure 2: Benzene molecule ground state activated to excited state.
Benzene substitution reaction process: According to the new theory of benzene molecular structure, the half-valent π bonds are formed between interval carbon atoms of the benzene ring. Its crosscovered area of the electron cloud has high electronegativity [2,6]. The positive ions in the reaction solution tend to the dense area of the electron cloud, and form an instantaneous positive ion bridge and interrupt the other two bonds in the triangular bond ring. The electronegativity of the hydrogen connected to the carbon at both ends of the bridge is weakened, and is easy substituted. When the hydrogen at one end is replaced first, and the positive ion of the bridge breaks away immediately. The triangle bond ring is restored and completes the substitution reaction. Because the position at which the substitution reaction occurs is arbitrary on both ends of the bond, there are no isomers in the substitution product (Figure 3) [1,2,4].
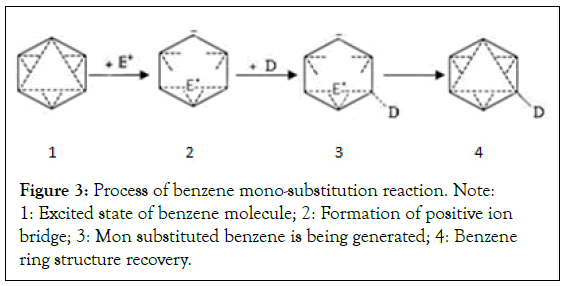
Figure 3: Process of benzene mono-substitution reaction. Note: 1: Excited state of benzene molecule; 2: Formation of positive ion bridge; 3: Mon substituted benzene is being generated; 4: Benzene ring structure recovery.
The multi-substitution reaction is affected by the position of the first substituent. The electron-withdrawing positioning group with high electronegativity determines that the multi-substitution reaction mostly occurs in the Meta position. The electron-repellent positioning group with low electronegativity determines that the multi-substitution reaction mostly occurs in the ortho-para position (Figure 4) [2,10].
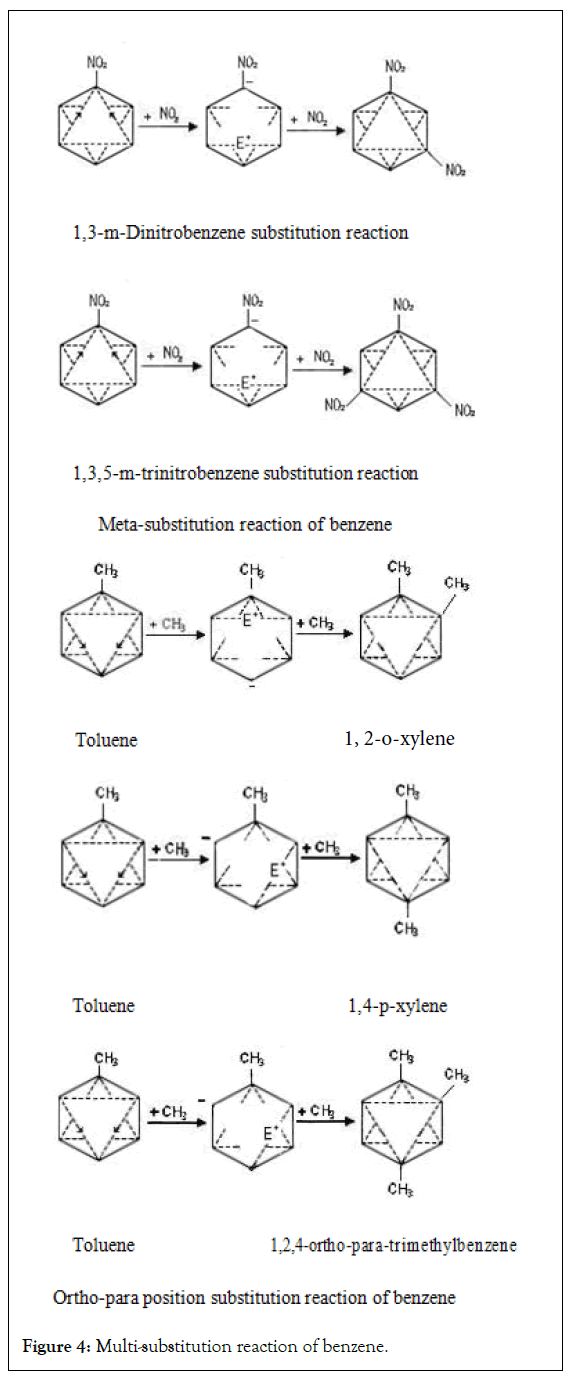
Figure 4: Multi-substitution reaction of benzene.
Benzene addition reaction process: The new structure theory believes that benzene molecules do not contain double bonds and should not undergo addition reactions. But in fact the hydrogenation reaction of benzene is addition reactions [2,6]. The process of benzene to cyclohexane needs to be heated to 300℃ under nickel catalysis. The activation energy [2,4,8] is high and the time is long. In this process, when the absorption energy of benzene exceeds its so-called delocalization energy of 36 kcal/mol, cyclohexatriene with addition reaction conditions is generated, and the addition reaction proceeds immediately to generate cyclohexane. Cyclohexatriene appeared only as a temporary intermediate. This process can be represented by the following reaction formula (Figure 5).
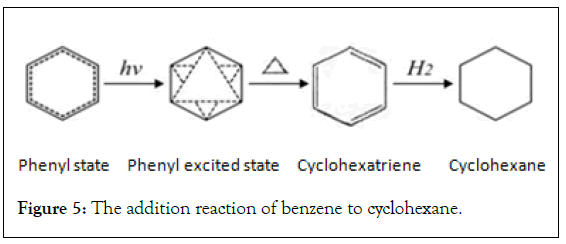
Figure 5: The addition reaction of benzene to cyclohexane.
Structural form design example of polycyclic aromatic hydrocarbons: In Figure 6, the Structural form design example of polycyclic aromatic hydrocarbons is shown [18,19].
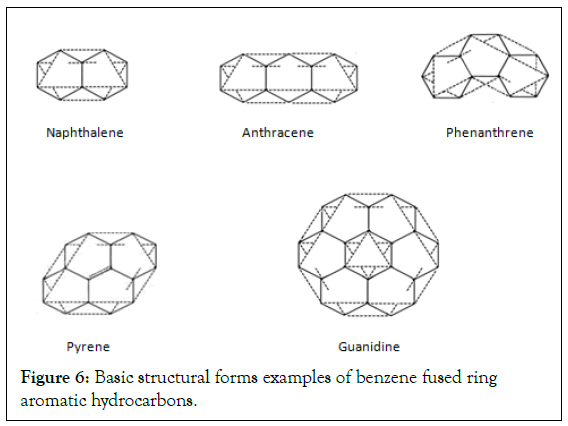
Figure 6: Basic structural forms examples of benzene fused ring aromatic hydrocarbons.
In order to verify the universality of the new structural form design method, the structural forms and chemical reaction formulas of more than 50 various benzene homologs and derivatives were designed [2,19].
Design method of PAHs structural formulas: Although the planar structure of PAH is complex and changeable. The design only needs to be based on the classic solid-line frame of hexagonal fused rings and follow the concepts and laws of valence bond theory (especially carbon four valences). Then it is not difficult to arrange semivalent bonds between the spacer carbon atoms with dashed lines. Because it is also regular. The specific method should be noted as follows:
a) First use equal hexagonal solid lines to splice the basic frame of the molecular.
b) Starting from the outer periphery of the molecule, add dotted lines of the half-valent bond between interval carbon atoms of the benzene ring. Under the condition of guaranteeing the four valence of carbon, try beyond the benzene ring to connect the dashed line into several large polygonal ring until must dashed lines are connected completely.
c)The big dashed polygonal ring is divided into two phases, plus and minus. The dashed circle of the negative phase is interrupted, indicating that the part is covered by the positive phase.
d)In the last remaining central part that can no longer be linked by a dotted line, and the part that still needs to be connected, have to use a solid line to form a double bond to finish.
The design examples in basic structural formulas of PAHs: In Figure 6, The design examples in basic structural formulas of PAHs is shown [2,19].
The design examples of carcinogenic PAH on Pullman’s theory: French chemist Pullman studied 35 kinds of PAH. Summarized the theory of PAH carcinogenic molecular structure. In new structural forms of PAH for 10 carcinogenic effects, all 3 highly carcinogenic PAHs were found to contain rare double bonds (Figure 7).
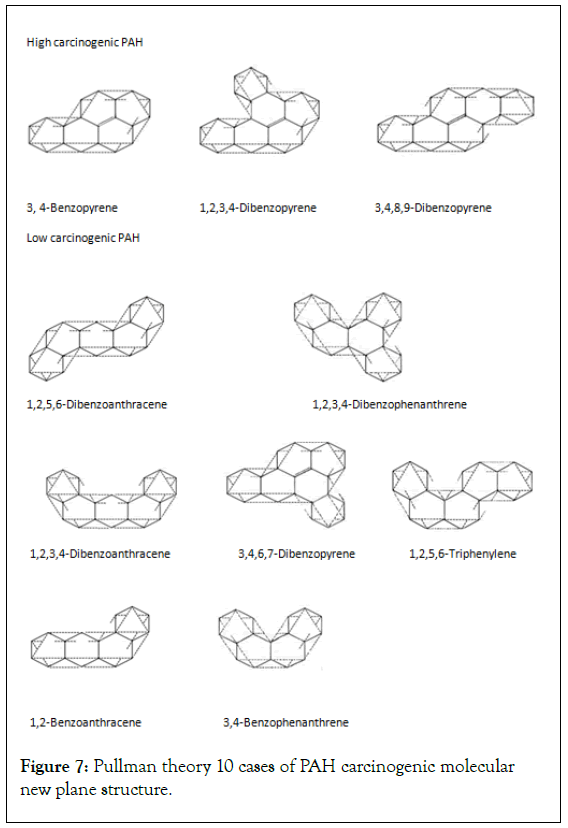
Figure 7: Pullman theory 10 cases of PAH carcinogenic molecular new plane structure.
The variety and complexity of benzene homologues and derivatives test the versatility and practicability of the new design method. However, the design process shows that the structural formula can not only be easily designed, but also can be used to record chemical reaction formulas.
Design examples of Phenyl benzene structural formals and reaction formulas: Phenyl benzene is a special molecular structure composed entirely of a benzene ring. It is not like the fused ring compound of benzene tightly joined together but the structural forms connected to each other through σ bond (Figure 8) [2,18].
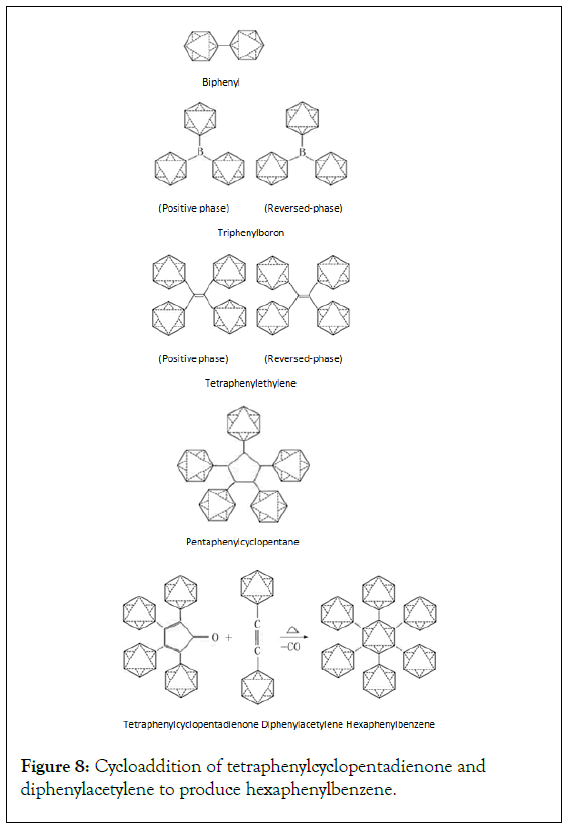
Figure 8: Cycloaddition of tetraphenylcyclopentadienone and diphenylacetylene to produce hexaphenylbenzene.
In Figure 8, Hexaphenylbenzene is a very special phenyl benzene compound. It is difficult to obtain. Only under strict conditions can this reaction be realized, and the desired result can be obtained.
Design examples of structural formals of homologue and derivative of benzene: In Figure 9, Design examples of structural formals of homologue and derivative of benzene is shown [18,19].
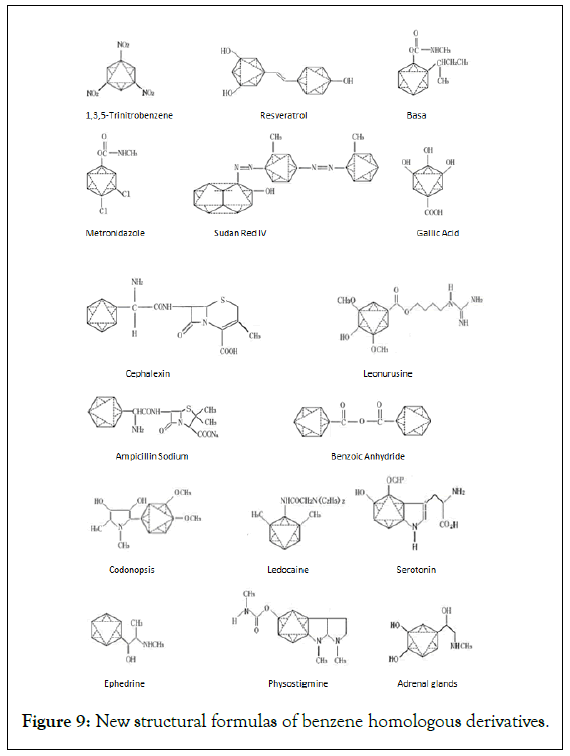
Figure 9: New structural formulas of benzene homologous derivatives.
Design examples of chemical reaction formulas of homolog and derivative of benzene: Although the chemical reaction formulas of homologues and derivatives are also complicated, the structure design is changing based on chemical reaction, and there are regularities, so the design is not difficult (Figure 10) [18,19].
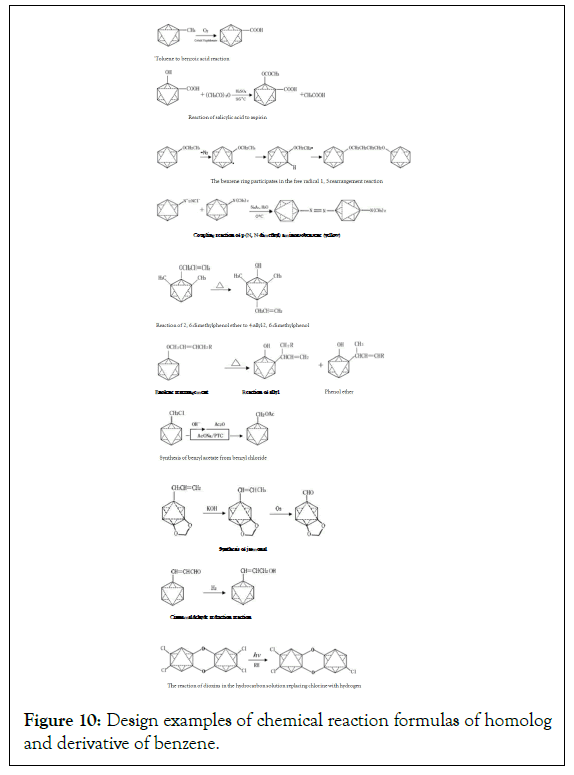
Figure 10: Design examples of chemical reaction formulas of homolog and derivative of benzene.
Three-layer benzene tube and the thickness of grapheme: If benzene can be synthesized into a three-layer benzene tube, its total thickness is easy to calculate [19-21]. The three layers of benzene are connected by the length of 2 σ bonds. The top and bottom pzorbital are in the same direction, only adding one pzorbital to the length [4,7]. That is 0.5 σ bond length. The total thickness should be 2.5 times the σ bond length (Figure 11).
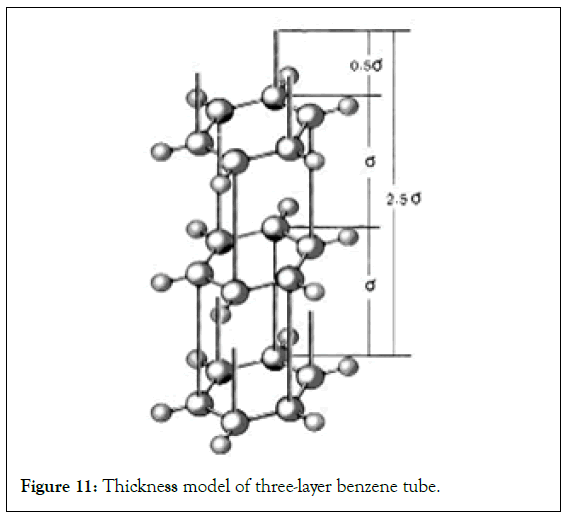
Figure 11: Thickness model of three-layer benzene tube.
What is the energy required to synthesize an n layer benzene tube? Except for the top and bottom half of the benzene ring structure, the remaining n-1 layers are all cyclohexane-type single-ring laminated tubes. 3(n-1) times the energy of C-C bond synthesis must be obtained from outside the system. Based on the general C-C bond energy 2,11 of 3.590 eV (346 kJ/mol), minus the hydrogenation heat released, that is (n-1)BH [2,10]. The total energy required is
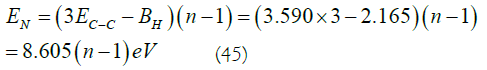
In (45),when n=2, the total energy of 3σ bonds between the two layers is only 8.605 eV, which is much weaker than the total energy of 6 σ bonds in each ring. Therefore, the interlayer structural energy is much smaller than the structural energy of the layer itself.
When calculating the force of the σ bond between 6 carbon atoms towards the center, it is exactly equal to the parallel force of 3 σ bonds. Considering the relationship between the energy and the benzene ring bond length in (33), the interlayer σ bond length should be equal to the bond length of benzene ring, but due to the release of the hydrogenation heat of benzene, the ring will be slightly twisted and tilted, and the vertical projection length of the σ bond may be slightly shortened [2,4,7,22].
So

This is the total thickness of the three-layer benzene tube. And the thickness of graphene is 0.334-0.335 nm, which is very close. If according to the crystal structure constant of graphene 2.46 nm, the calculated σ bond length is 1.420 nm. 2.5 times it is 3.55 nm, which is also very close to the thickness of graphene. This makes people think that many properties of graphene, especially metal-like properties, are not like a one-layer structure, but more likely to be a three-layer structure. In turn, if these three layers of benzene, or if they are replaced by PAHs,such as Guanidine, they may form honeycomb benzene. Since honeycomb benzene is structurally similar to graphene, could they also have the properties of graphene?
Results
Using the equivalent electron-like hydrogen atom simplified model and its wave equation, it is calculated that the ground state energy level is about -8.665 eV, and the excited state energy level is about -2.165 eV. The ground state transition energy is about 6.493 eV. The energy corresponding to the peak wavelength of the ultraviolet spectrum of benzene is between 4.625-6.876 eV, which is within the energy range of the equivalent electronic ground state transition. The excited state transition energy is 2.165 eV, the experimental value of benzene hydrogen heat is 2.160 eV, and the difference is 0.231%. When neglecting this slight difference, the hydrogenation heat of benzene becomes a constant composed of basic physical constants and is proportional to the Rydberg constant. Calculated from the median peak wavelength of the benzene ultraviolet spectrum at 212 nm, the bond length of the benzene ring is 0.1398 nm, which is almost the same as the result of the Raman spectrum. The mirror images of the benzene rings overlap, and multilayer benzene tubes may be synthesized. The calculated energy required is 8.605(n-1) eV. According to the bond length of the benzene ring, the calculated thickness of the three-layer benzene tube is 0.349 nm, or the thickness of the three-layer benzene tube calculated according to the graphene microcrystalline structure constant is 0.355 nm. They are basically close in 0.334-0.335 nm of the thickness of graphene.
Design out plane structural formulas of benzene molecules and its substitution and addition reactions process are 8 examples, and structural formulas of PAH are 15 examples. Designs out structural formulas of phenyl benzene are 6 examples and its 1 chemical reaction formula. Design out structural formulas of homolog and derivative of benzene are 16 examples and third chemical reaction formulas are 10 examples.
Discussion
Based on the nature of chemical bonds, a new concept of covalent bonds is proposed, that is, every electron shared between atomic nuclei corresponds to a semivalent bond. In this way, it breaks the traditional concept of valence bond theory that there are only integer valence bonds, and is more widely applicable to the valence bonds of all molecules, and perfects the valence bond theory. In particular, in the excited state of the benzene molecule, semivalent bonds are formed between interval carbon atoms of the benzene ring, which clarifies the true structure of benzene and completely solves the structural problem of the benzene molecule. It also effectively explains that there are no isomers of benzene mono-substitution, and multi-substitution reactions always occur in the Meta or ortho-para position. The birth of the half-valent bond will have a profound impact on organic chemistry. For example, we found that polycyclic aromatic hydrocarbons are not cyclic molecules joined by cyclohexatrienes, they are each joined differently, and double bonds are rarely present. All of the highly carcinogenic PAHs have double bonds.
Under the new theory, the half-valent bond is represented by a dashed line. The designed plane structure of the benzene molecule is real and novel. The chemical reaction process can also be recorded in detail and become a new symbol of the benzene molecule. The new plane structure formula of the benzene molecule will replace the wrong Kekule structure to use already more than 100 Year, and will discard the useless Robinson structure. Using the new design method, it is easy to design the complex structural formula and chemical reaction formula of benzene and its homologues and derivatives, which has a wide range of applicability and lays a foundation for popularization and application. For more than 100 years, in professional academic exchanges, printing, publishing, database construction, etc., the wrong molecular structure of cyclohexatriene has been used instead of benzene. Now that it has its own structural formula, everything will be changed from scratch. It will be a huge engineering. Some parts require further study or learn from scratch.
The new structural formula, which can be used as the expression of the substitution reaction mechanism through the positive ion bridge effect, can be perfectly explained at the same time, the electrophilic substitution and nucleophile reaction of benzene, and the multi-substitution reaction position mechanism. This will also play a guiding role in the directional synthesis of multiple substitutions of benzene.
Regarding the mechanism of benzene addition reaction, the new theory is that benzene molecules do not have double bonds and cannot undergo addition reactions. Only through high activation energy, benzene turns into a cyclohexatriene intermediate, generates three double bonds, and instantaneous addition reaction occurs to generate monocyclic hydrocarbons or chain hydrocarbons and explain the mechanism of cyclohexatriene’s existence.
In order to avoid the unsolvable problem of the multi-electron Schrodinger equation of benzene, the simplification of the equivalent electron hydrogen-like atom model is used to establish the wave function and wave equation, and solve the equation to obtain the ground state and excited state energy levels and transition energies. In the end, the calculated value of quantum mechanics is highly consistent with the experimental results. It not only verified the reliability of the equivalent electronic model and the new structural theory, but also quantitatively explained the unsolved problems such as the hydrogenation heat of benzene and ultraviolet spectroscopy.
Using the median peak wavelength of the UV spectrum of benzene, the calculated bond length of the benzene ring is almost identical to the measured value of the Raman spectrum, indicating the consistency of the measurement results of the two spectra.
Conclusion
Based on the new structure theory of benzene molecules, the benzene rings are stacked in mirror images, and it is possible to synthesize multilayer benzene tubes. Calculate the thickness of the three-layer benzene tube, which is equivalent to the thickness of graphene. When considering that graphene also exhibits a high degree of chemical stability, good electrical and thermal conductivity, as well as high hardness and toughness, it is much like metal with a complex crystal lattice. Therefore, graphene is more like a three-dimensional structure. It is speculated that there is at least one type of graphene with a three-layer structure. In this three-layer structure, the centerline of the carbon tube formed by a large number of equilateral hexagonal carbon rings may be an undisturbed superconducting channel. It is an ideal superconducting structural material in itself. An in-depth study of the real structure of the benzene molecule may contribute to the study of the graphene structure.
REFERENCES
- Kauffman GB. Essays on the history of organic chemistry (Traynham, James G). J Chem Educ. 1988;65(7):A188.
- Wade LG. Organic Chemistry.2009.
- Chandra S. Molecular spectroscopy. Alpha Science International Ltd. 2009;pp:170-178.
- Lai D, Chen W, Jiang G. Research progress of grapheme-based composite materials. Curr Phys Chem. 2013;3(3):269-282.
- Pauling L. The modern theory of valency. J Chem Soc. 1948; pp:1461-1467.
[Cross Ref] [Google Scholar] [PubMed]
- Szabo A, Ostlund NS. Modern quantum chemistry: Introduction to advanced electronic structure theory. Dover Publications.1996; pp:139-143.
- Bent HA. Molecules and the chemical bond: An introduction to conceptual valence bond theory. Trafford Publishing; 2013.
- Klein DJ, Trinajstic N. Valence-bond theory and chemical structure. J Chem Educ. 1990;67(8):633.
- Sanderson RT. Chemical Bond and Bond Energy (2nd Edition).1976.
- Ira N. Levine. Quantum Chemistry (7th Edition). Pearson, 2013;pp:139-231.
- Putz MV, Cimpoesu F, Ferbinteanu M. Structural Chemistry. 2018.
- Lai D, Chen W, Jiang G. Research progress of graphene-based composite materials. Curr Phys Chem. 2013;3(3):269-282.
- Corry S. Symmetry and quantum mechanics. Chapman and Hall/CRC. 2016.
- Alastair I. M. Rae, Napolitano J. Quantum mechanic. Sixth Edition. CRC Press, 2015;pp:102-119.
- Zeidler E. Nonlinear functional analysis and its application III: Variational methods and optimization. 1985.
- Simon B. Quantum mechanics for hamiltonians defined as quadratic forms. Princeton University Press. 2015.
- Huber KP, Herzberg G. Constants of diatomic molecules. Molecular Spectra Molecular Structure. 1979;8:689.
- Carey FA, Sundberg RJ. Advanced organic chemistry: Structure and mechanisms Part A. 2008.
- Pullman A, Pullman B. Electronic structure and carcinogenic activity of aromatic molecules new developments. Adv Cancer Res. 1955;3: pp:117-169.
[Cross Ref] [Google Scholar] [PubMed]
- Hoard JL, Geller S, Cashin WM. Structures of molecular addition compounds. III. Ammonium–boron trifluoride, H3N–BF3. Acta Crystallogr. 1951;4(5): 396-398.
- Novoselov KS, Geim AK, Morozov SV, Jiang DE, Zhang Y, Dubonos SV, et.al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696): 666-669.
[Cross Ref] [Google Scholar] [PubMed]
- Cao Y, Fatemi V, Fang S, Watanabe K, Taniguchi T, Kaxiras E, et al. Unconventional superconductivity in magic-angle graphene superlattices. Nature. 2018;556(7699):43-50.
[Cross Ref] [Google Scholar] [PubMed]
Citation: Wang D, Wang N, Wang R (2022) Real Structure of Benzene Molecule and the Thickness of Graphene. Mod Chem Appl. 10:338.
Copyright: © 2022 Wang N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


