Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Purification of Syncytia-Producing Peptide as a Potential Therapeutic Agent for Cancer and Viral Infection
Michiko Koga1*, Xiang-Guo Zheng1, Manabu Watanabe1, Sakiko Sato1 and Kazuhiro Tanabe2,32Medical Solution Promotion Department, Medical Solution Segment, LSI Medience Corporation Yokohama, Kanagawa 227-8502, Japan
3Kyushu Pro Search Limited Liability Partnership, Yokohama, Kanagawa 227-8502, Japan
Received: 29-Mar-2021 Published: 20-Apr-2021, DOI: 10.35248/2157-7560.21.s13.002
Abstract
Virus-induced syncytium formation, or cell fusion, has been investigated as a potential therapeutic approach for cancer. HVJ virus infection or transfection of membrane glycoproteins of virions have been known to cause syncytium formation in affected cells. Also, it was known that other enveloped viruses such as Herpes viruses, leukaemia viruses induce cell fusion. However, substances or molecules that directly cause syncytium formation have not been identified to date. Here, we identify a peptide that efficiently induces syncytia and report its structure, as an actionable therapeutic agent for cancer and viral infection. We purified and identified the fusion factor from the exosomes of the cells infected with murine leukaemia virus but not producing viruses by column chromatography, mass spectrometry, and amino acid analyses. We confirmed the peptide purified from the cell culture media and synthesized peptides induce syncytia as well as the Murine Leukaemia Viruses, or the membranes or exosomes of MuLV infected cell lines in RFL cells and several cancer cell lines leading to apoptosis. And this peptide suppresses in vivo growth of cancer cells significantly. Furthermore, we found the synthesized peptide can cause fusion of enveloped virions as well as virus infected cells or cancer cell lines. These results nominate the use of this peptide as a potentially promising therapeutic approach for cancer and viral infection through efficient induction of syncytium formation followed apoptosis.
Keywords
Herpes viruses; Hemagglutinating Virus of Japan (HVJ); Murine leukaemia viruses; Peptide; Apoptosis
Introduction
Cell fusion occurs in wide varieties of essential biological phenomena such as endocytosis, exocytosis, phagocytosis, fertilization and maturation of striated muscles [1]. Furthermore, cell fusion is very useful for making hybrid cells and genetic analyses, and has potential of further applications such as gene transfers [2]. Although cell fusion can cause tumour progression through inducing aneuploidy and genomic instability [1,3-7], implication of the utility of cell fusion for cancer treatment exists [1,8,9]. We previously reported that syncytium formation of RFL cells, which are fibroblasts derived from the lungs of WKA rat embryo, was induced syncytia by infection of Murine Leukaemia Viruses (MuLVs) [10,11]. Cell fusion induced by viruses which has envelopes composed of lipids and proteins, such as paramyxoviruses, orthomyxoviruses [12], herpesvirus [13,14] and retroviruses [15-20], has been widely studied as well. It has been shown that this cell fusion activity was related to the glycoproteins in the envelope. However, the actual substances that induce cell fusion have not been identified [20,21].
Methods
Cell lines
TRFL cell line, an established cell line derived from the embryonal lung of WKA rats, was obtained from R. Takaki, School of Medicine, Kyusyu University. RM4 cells were obtained from a clone of RFL cells infected with Moloney-MuLV which was obtained from N. Ida, Toyo Kogyo Hospital. LLC, CW2, B1203L, and A549 cell lines were obtained from Cell Bank, RIKEN Bio Resource Research Centre. (Tokyo, Japan). RM4 cells induced syncytia with RFL cells, but don’t fuse each other, the RM4 cells resistant against fusion factor of themselves. All cell lines were maintained in RPMI-1640 medium (Wako,189-02025) supplemented with 5% FBS (Biosera, 0158S493) at 37°C in a humidified incubator with 5% CO2.
Treatment with RM4 cell’s conditioned media and the RM4 plasma membrane
RFL cells were induced syncytium by cocultivation with the RM4 cells and cell membranes of RM4 cells and exosomes in the culture medium of RM4 cells. We tried to purify the fusion factor from the exosomes produced by RM4 cells [12].
Purification of a syncytium-inducing peptide and determination of amino acids arrangement from the exosomes produced RM4 cells
Exosomes derived from RM4 cells were collected by filtration of the RM4-conditioned media through Amicon Ultra-15 filters (Merk Millipore LTD.). The collected exosomes were treated with 70% acetone at room temperature and the supernatant which showed the activity of syncytium formation was concentrated by evaporation. The supernatant was applied on QAE Sephadex C-25 anion exchanger and SP Sephadex C-25 cation exchanger (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Then the solubilized fraction which has the activity of syncytium formation was applied on Hitachi LaChrom Elite HPLC system Pump L-2130(Hitachi, Co. Ltd, Tokyo, Japan) using X Bridge C18. 5 μm (Waters, Ireland) and eluted by 10% Acetonitrile. The fraction which has the activity of syncytia formation was further analysed by mass spectrometry (Shimadzu LC-10A). The LC-MS data were acquired on a liquid chromatography system (Agilent HP1200, Agilent Technologies, Palo Alto, CA) equipped with a C18 column (CAPCELL PAK C18 IF, 2 μm, 50 mm × 2.0 mm ID, Shiseido, Tokyo, Japan) coupled with an electrospray ionization quadrupole time-of-flight (Q-TOF) mass spectrometer (Agilent 6520, Agilent Technologies, Palo Alto, CA, USA) 31. Solvent A was 5 mM ammonium acetate in water, and solvent B was acetonitrile. The target peptides were eluted at a flow rate of 0.2 mL/min at 40°C with a following gradient program: 0 to 10 min, 10% to 100% solvent B; and 5 min hold at 100% solvent B. The mass spectrometer was operated in both positive and negative mode with the capillary voltage of 3500 V. The nebulizing gas pressure was 30 psi and the dry gas flow rate was 8 L/min at 350°C.
The injection volume was 10 μL. MS/MS spectra to identify the target peptides which were also obtained with QTOF mass spectrometer. Detected range was set from m/z 70 to 3000. Liquid chromatography (Figure 1a) and mass spectrometry (Figure 1b) of the concentrated supernatant of the solubilized RM4-derived exosomes revealed that certain peptide was analysed by MS/MS. Amino acid analyses were carried out on the Hitachi amino acid analyser L-8900 after hydrolysis of the peptide with 6M HCl at 110℃ for 22 hours in an evacuated sealed tube. The arrangement of the amino acids was determined as follows CH3-Pro-Ile-Val-Ser- Gln-Thr-Thr-Ala-Ile-Ala.
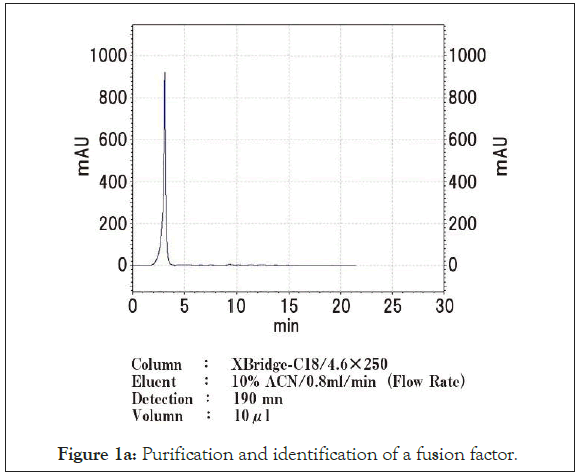
Figure 1a: Purification and identification of a fusion factor.
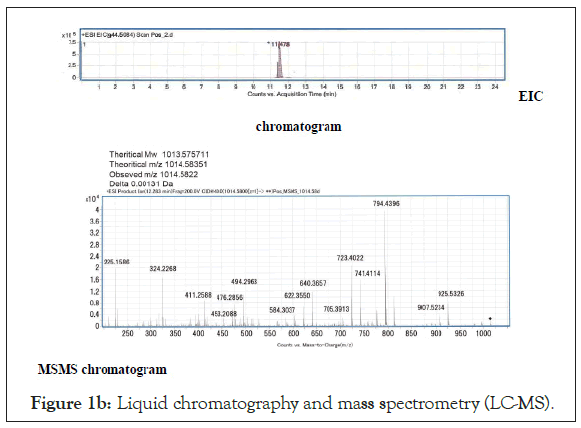
Figure 1b: Liquid chromatography and mass spectrometry (LC-MS).
The synthetic peptide induces syncytia as well as purified peptide from culture media
To validate this purified peptide is responsible for syncytium formation, we synthesized the peptide of the same sequence. The synthetic peptide as well as the plasma membrane or the exosomes derived from RM4 cells induced syncytia formation in RFL cells (Figures 2a and 2b). RM4 cells and murine Lewis Lung Carcinoma (LLC) cells formed syncytia after treatment with this synthesized peptide (Figures 2c-2e). These results were complicated, because RM4 cells did not form syncytia with RM4 cells itself, cell membranes or exosomes derived from RM4cells. The reason why only purified peptide or, synthesized peptide induced syncytia in RM4 cells and several cancer cells is unknown. There may be exist protective mechanism in the membrane to fuse each other or by exosomes of themselves.
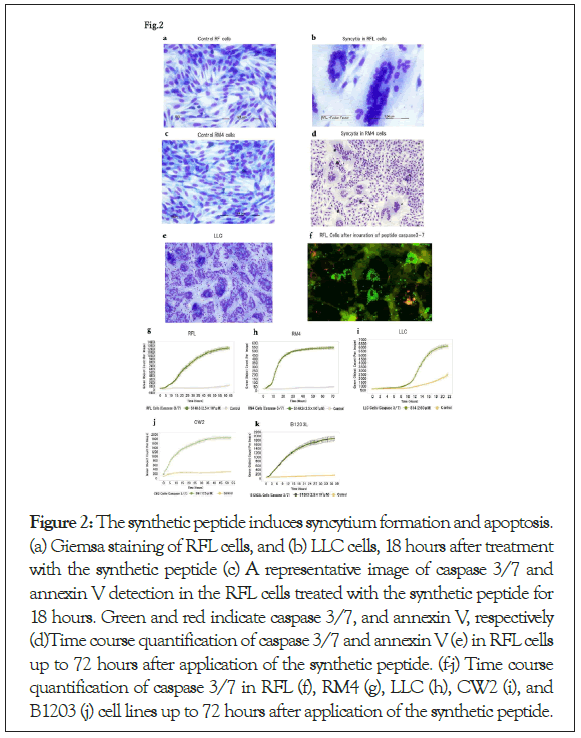
Figure 2: The synthetic peptide induces syncytium formation and apoptosis. (a) Giemsa staining of RFL cells, and (b) LLC cells, 18 hours after treatment with the synthetic peptide (c) A representative image of caspase 3/7 and annexin V detection in the RFL cells treated with the synthetic peptide for 18 hours. Green and red indicate caspase 3/7, and annexin V, respectively (d)Time course quantification of caspase 3/7 and annexin V (e) in RFL cells up to 72 hours after application of the synthetic peptide. (f-j) Time course quantification of caspase 3/7 in RFL (f), RM4 (g), LLC (h), CW2 (i), and B1203 (j) cell lines up to 72 hours after application of the synthetic peptide.
Syncytium formation assay
For the syncytium formation assay, 8 × 104 of RFL or LLC cells per 0.25 ml of the media were plated in each well of 24-well plate (I waki 2820-024). After 30 min, the media was replaced by 50 μL of the media containing the peptide (1 μg/ml). After 16 to 24 hours, the cells were fixed with methanol (Wako, Japan) and Giemsa staining (Muto Chemistry, 15003) was performed according to the manufacturer’s instruction.
The peptide induces apoptosis after syncytium formation
It has been shown that viral-induced syncytium formation lead to apoptosis [22]. Thus we investigated if the treatment with the synthetic peptide lead to apoptosis of the treated cells by measuring caspases [23] and annexin V [24]. Caspase 3/7 and annexin V were increased in RFL cells after treatment with the synthetic peptide (Figure 2f). Induction of apoptosis by the peptides in dose dependent manner were confirmed in RFL cells and RM4 cells (Figures 2g and 2h), as well as several cancer cells lines, such as LLC which is a mouse lung cancer cell, CW2 which is a human colon adenocarcinoma cell line, and B1203L which is a human lung squamous cell carcinoma cell line (Figures 2i and 2j). The sensitivity of apoptosis followed by syncytium formation by the synthetic peptides were higher in cancer cell lines and RM4, which are viral infected cells, as compared with RFL cells, which are non- viral infected, non-cancer cells (Figures 2g-2j), suggesting existence of therapeutic windows of these synthetic peptides against cancer and viral infection.
The peptide induced fusion in viruses as well as cells
HVJ viruses, obtained Ishihara Sangyo Kaisya LTD, and RFL cells were observed with electron microscope (JEM-1400 Plus; JEOL Ltd, Tokyo, Japan) after treatment by synthesized peptides. Figure 3a shows that HVJ viruses fused each other after treatment with the peptide. In Figure 3b shows the syncytia of RFL cells after treatment of the peptide.
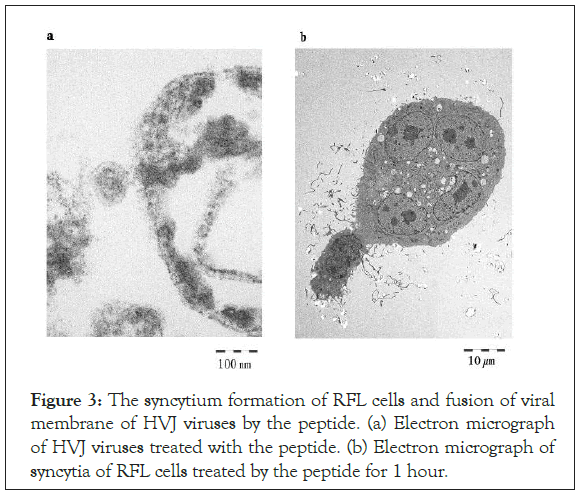
Figure 3: The syncytium formation of RFL cells and fusion of viral membrane of HVJ viruses by the peptide. (a) Electron micrograph of HVJ viruses treated with the peptide. (b) Electron micrograph of syncytia of RFL cells treated by the peptide for 1 hour.
Results
Measurement of the apoptosis by the synthesized peptides
The cells were incubated in IncuCyte® SX1 Live-Cell Analysis System (Essen BioScience, Sartorius Company) at 37°C, 5% CO2. IncuCyte® Caspase-3/7 reagent is DEVD group. When an activated Caspase-3/7 cut the DEVD group reagent, the nuclei of apoptosis cells are put on green fluorescence. Each cell was plated in 96 well plates (Iwaki, 3869-096, Japan) as 5000 cells/well. After 1 hour, the medium of each well were removed and synthesized peptide and 1000 times diluted solution of Casparse-3/7 Green Reagent (SARTORIUS,4440, Unit size:20 ul,5 mM/vial) with RPMI-1640 media (Wako,189-02025) containing 5% fetal bovine serum (Chile Origin, USDA approved) (Biosella, Noailles, France).
The peptide inhibits tumour growth in vivo
To further evaluate in vivo anti-tumour efficacy of the synthetic peptides, we treated nude mice harbouring subcutaneous tumours of B1203L, a human lung cancer cell line. Tumour growth was significantly suppressed in the peptide-treated group compared with the vehicle-treated group (Figures 4a and 4b) without affecting the body weight of the animals (Figure 4c). Histological examination of the tumours presented highly cellular tumours in the vehicle- treated group (Figure 4d) and prominent necrosis in the peptide- treated group (Figure 4e), suggesting in vivo anti-tumour efficacy of this synthetic peptide.
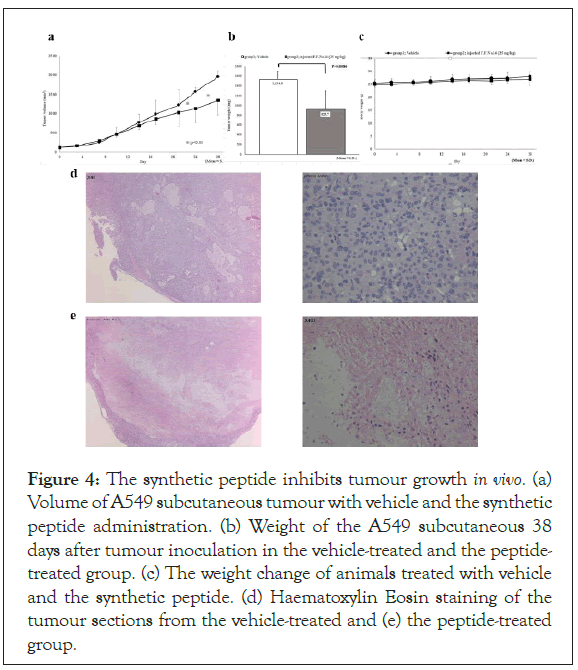
Figure 4: The synthetic peptide inhibits tumour growth in vivo. (a) Volume of A549 subcutaneous tumour with vehicle and the synthetic peptide administration. (b) Weight of the A549 subcutaneous 38 days after tumour inoculation in the vehicle-treated and the peptide- treated group. (c) The weight change of animals treated with vehicle and the synthetic peptide. (d) Haematoxylin Eosin staining of the tumour sections from the vehicle-treated and (e) the peptide-treated group.
Treatment of subcutaneous tumour growth by synthesized peptide
The antitumor experiments in mice were performed by HAMRI Co. LTD (Tsukuba, Japan). The human lung cancer cell line B1203L was provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT/AMED, Japan. Male mice (CAnN.Cg-Foxnlnu/CrlCrlj) were obtained from Japan Charles River Co.LTD. 4 x 106 cells of B1203L cells suspended in 0.1 mL of phosphate buffered saline were injected in the right flank of six nude mice for each of the treatment group and the vehicle group. Either 25 mg/kg of the peptide or the vehicle was administered intravenously from the tail veins every 3 days starting on 14 days after tumour inoculation. The volume of the subcutaneous tumours was measured by vernier callipers.
The animals were euthanized 38 days after tumour implantation and the subcutaneous tumours were excised and weighed. The body weight of all the animals was measured until the end of the experiment. The body weight was not affected by peptide treatment, suggesting that the peptide has not toxicity. The peptide-treated group exhibits necrosis, but vehicle-treated group does not exhibit necrosis, and there are many live cancer cells. The cancer growth in the nude mice was significantly suppressed in the peptide-treated group.
Results and Discussion
In this study we have identified the substance that induce syncytium formation in several cell lines including viral infected cells and cancer cell lines from the exosome in the culture media of MuLV infected cells. Our findings reveal that the short peptide derived from the MuLV-infected cells, which can be artificially synthesized, is an actionable factor that induces syncytium formation and apoptosis in several cell lines with higher sensitivity in cancer and virus-infected cells. In RFL cells MuLV, RM4 cells, cell membranes of RM4 cells, exosomes in culture medium of RM4 cells and purified fusion peptide and synthesized peptide, all can induce syncytia. But in RM4 cells or cancer cells only this peptide can induce syncytia. The reason why RM4 cells and HVJ virions which has fusion factor in the membranes did not fuse each other, and only this peptide can induce syncytia is under investigation.
Conclusion
This Peptide may subversive the harmony of the membranes of RM4 cells and cancer cells. The background mechanisms of this variability in the sensitivity against this peptide among different cell lines are further to be investigated. Nonetheless, this peptide significantly suppressed the tumour growth in the nude mice. Consequently, this peptide could serve as a tool for future therapeutics of neoplastic diseases and viral infections.
Supporting Information
We followed “The ARRIVE Guidelines” for reporting animal data in this manuscript. We had on ethical approval of protocols by ethical committee in Hamuri Co. Tsukuba Research Centre (Tsukuba Japan). We followed SOP of Hamuri Co. Tsukuba Research Centre (Tsukuba Japan).
Acknowledgement
This work was supported by Daiju Ichimaru, e-Evidence Solution Company M3, Inc. and Taku Yoshiya, Peptide Institute, Inc. (Osaka Japan) and Yu Ito, Tokai Electron Microscopy, Inc. (Shizuoka. Japan) and Ryo Tanaka. Hamuri Co. Tsukuba Research Centre (Tsukuba Japan).
Author Contributions
M.K. conceived the study design. M.K., S.T., and M.S. performed experiments, analysed and interpreted data. M.K. wrote the manuscript, and all authors edited and approved the manuscript.
Competing Interests
M.K. is founder, and S.T. and M.W. are employees of Sunkokai Medical Corporation.
REFERENCES
- Platt JL, Zhou X, Lefferts AR, Cascalho M. Cell fusion in the war on cancer: a perspective on the inception of malignancy. Int J Mol Sci. 2016;17(7):1118.
- Goldenberg DM, Rooney RJ, Loo M, Liu D, Chang CH. In-vivo fusion of human cancer and hamster stromal cells permanently transduces and transcribes human DNA. PloS one. 2014;9(9):e107927.
- Dittmar T, Zänker KS. Tissue regeneration in the chronically inflamed tumor environment: Implications for cell fusion driven tumor progression and therapy resistant tumor hybrid cells. Int J Mol Sci. 2015;16(12):30362-30381.
- Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66(16):8274-8279.
- Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, et al. Fusion between intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71(4):1497-1505.
- Gao P, Zheng J. Oncogenic virus-mediated cell fusion: New insights into initiation and progression of oncogenic viruses-related cancers. Cancer Lett. 2011;303(1):1-8.
- Berndt B, Zanker KS, Dittmar T. Cell fusion is a potent inducer of aneuploidy and drug resistance in tumor cell/normal cell hybrids. Crit Rev Oncog. 2013;18(1-2).
- Harris H, Miller OJ, Klein G, Worst P, Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969;223(5204):363-368.
- Lin EH, Salon C, Brambilla E, Lavillette D, Szecsi J, Cosset FL, et al. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: A potential therapy agent for lung cancer. Cancer Gene Ther. 2010;17(4):256-265.
- Koga M. Multinucleated giant cell formation in cultured cells by murine leukemia virus infection. Jpn J Cancer Res. 1973;64(3):321-322.
- Koga M. Titration of murine leukemia viruses with rat cell line RFL. J Virol. 1977;23(2):436-438.
- Zheng XG, Watanabe M, Koga S, Koga M. Purification and structural analysis of a fusion factor derived from a cell line infected with murine leukemia viruses.2019.
- Gething MJ, Doms RW, York D, White J. Studies on the mechanism of membrane fusion: Site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986;102(1):11-23.
- Bayliss GJ, Wolf H. An Epstein--Barr virus early protein induces cell fusion. Proc Natl Acad Sci. 1981;78(11):7162-7165.
- Pertel PE. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J Virol. 2002;76(9):4390-4400.
- Rand KH, Long C. Syncytial assay for the putative human C-type virus, RD-114, utilizing human cells transformed by Rous sarcoma virus. Nat New Biol. 1972;240(101):187-190.
- Roy NH, Chan J, Lambelé M, Thali M. Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion. J Virol. 2013;87(13):7516-7525.
- Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci. 1983;80(23):7337-7341.
- Ogura H. XC cell fusion by murine leukemia viruses: Fusion from without. Med Microbiol Immunol. 1976;162(3):175-181.
- Johnson GS, Friedman RM, Pastan I. Analysis of the fusion of XC cells induced by homogenates of murine leukemia virus-infected cells and by purified murine leukemia virus. J Virol. 1971;7(6):753-758.
- Zhou X, Platt JL. Molecular and cellular mechanisms of mammalian cell fusion. Cell Fusion in Health and Disease. 2011:33-64.
- Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19(10):514-522.
- Nardacci R, Perfettini JL, Grieco L, Thieffry D, Kroemer G, Piacentini M. Syncytial apoptosis signaling network induced by the HIV-1 envelope glycoprotein complex: An overview. Cell Death & Disease. 2015;6(8):e1846
- Yuan J, Najafov A, Py BF. Roles of caspases in necrotic cell death. Cell. 2016;167(7):1693-1704.
- Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016;2016(11):087288.
Citation: Koga M, Zheng XG, Watanabe M, Sato S, Tanabe K (2021) Purification of Syncytia-Producing Peptide as a Potential Therapeutic Agent for Cancer and Viral Infection. J Vaccines Vaccin. S13:002.
Copyright: © 2021 Koga M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

