Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
PU and Cpsf Blend UF Membranes. II. Pore Statistics, MWCO and Application Studies
Duraikkannu Shanthana Lakshmi1*, Latha C. S2 and Mohan D32Department of Chemistry, QuaidE Millath Government College for Women, Tamil Nadu, India
3Department of Science and Humanities, Rajalakshmi Engineering College, Tamil Nadu, India
Received: 30-Apr-2020, Manuscript No. JMST-24-4056; Editor assigned: 05-May-2020, Pre QC No. JMST-24-4056 (PQ); Reviewed: 19-May-2020, QC No. JMST-24-4056; Revised: 15-Jul-2024, Manuscript No. JMST-24-4056 (R); Published: 12-Aug-2024, DOI: 10.35248/2155-9589.24.14.394
Abstract
The performance of membranes for a specific application can be determined with the help of structural properties such as Molecular Weight Cutoff (MWCO), morphology and pore statistics. Heavy metal ions from aqueous streams can be separated with the help of ultrafiltration membranes. MWCOs and pore statistics of Polyurethane (PU) and Carboxylated Polysulfone (Cpsf) blend ultrafiltration in the presence and absence of the various composition of the additive poly (ethylene glycol) 600 were studied with the help of dextran of different molecular weights ranging from 19 kDa to 150 kDa. The derived pore size, porosity and number of pores have a remarkable relationship with the MWCO, morphology and the flux performance of the membranes. Certain toxic divalent heavy metal ions such as copper, cadmium, nickel and zinc were subjected to rejection by the blend membranes by complexing them a polymeric ligand, Poly (Ethyleneimine) (PEI). The effect of the polymer blend compositions and additive concentrations on the rejection and permeate flux of metal ions are discussed.
Keywords
Ultrafiltration; Blend membrane; MWCO; Pore statistics; Toxic metal ion separation
Abbreviations
PU: Polyurethane; Cpsf: Carboxylated Polysulfone; PEG: Polyethylene Glycol; MWCO: Molecular Weight Cutoff; UF: Ultrafiltration; MF: Microfiltration
Introduction
Pore statistics, Molecular Weight Cutoff (MWCO) and morphology are the structural properties of membranes that are essential for the application of the membrane process with specific permeate qualities. Several ultrafiltration, microfiltration and reverse osmosis membrane systems using different polymeric membranes were evaluated for their pore statistical studies, MWCO, morphology and formation mechanism. The average pore size, MWCO and morphological studies have been carried out for polyacrylonitrile/polyurethane blend membranes and applied for water purification. Thus it has been considered as a key parameter of membranes, which is useful for variety of pharmaceutical, food and biotechnological applications [1].
In the present investigation, the solute retention method is used for determining the pore statistics because of its simplicity and its advantage in determining MWCOs. Further, the filtration characteristics of dextran with different molecular weights can also be used to correlate MWCO and pore statistics.
Materials and Methods
Dextran with molecular weight 19 kDa, 42 kDa, 77 kDa and 150 kDa were procured from Sigma-Aldrich company (U.S.A) and stored at a suitable temperature before use. Sulfuric acid and phenol were procured from SRL Chemicals Ltd. and used as received for the analysis of dextran.
Poly (ethyleneimine) (Mw=600000-1000000) 50% aqueous solutions was procured from Fluka chemicals, AR grade (steinheim) and used as a 1 weight% aqueous solutions for the metal complexation studies.
Copper (II) sulfate (AR), nickel (II) sulfate (AR) and zinc (II) sulfate (AR), were procured from Merck Ltd. and used as such for the preparation of aqueous metal ion solutions. Cadmium (II) chloride (AR) was procured from Qualigens fine chemicals Ltd., India and used as such.
Deionized and distilled water were employed for the preparation of dextran, metal ion solution and 1 weight% PEI aqueous solution [2].
Characterization
Conditions and specifications of materials under investigation: PU/Cpsf blend composition of 80%/20% and 75%/25% with 0, 2.5, 5 and 7.5 weight% PEG 600 concentrations only were taken for investigation because Cpsf composition less than 20% was found to yield negligible pure water flux and more than 25% was not compatible with PU. Thickness of membranes was maintained at 0.20 mm ± 0.02 mm.
Ion Exchange Capacity (IEC): The dried sample of membrane is immersed in saturated sodium chloride solution for a day to liberate the H+ ions. The mixture was then titrated with 0.1 M Sodium Hydroxide (NaOH) solution to determine the H+ content and the IEC was calculated as follows
IEC=(NaOH consumed × molarity of NaOH)/weight of dried membrane (meq/g)
Metal ion rejection: Rejection and flux experiments were carried out at room temperature, using an ultrafiltration kit of 450 ml capacity and holdup volume of 10 ml, supplied by Amicon model 8400, Millipore Ltd., Bangalore, India. The effective membrane area was found to be 38.5 cm2, the applied pressure was 345 kPa and the agitation was kept uniform [3].
Aqueous solution of Cu2+, Ni2+, Cd2+ and Zn2+ were prepared at an approximate concentration of 1000 ppm in 1 weight% solution of PEI in deionized water. The pH of these aqueous solutions was adjusted to 6 ± 0.25 by adding small amount of either 0.1 M HCl or 0.1 M NaOH. Solutions containing PEI and individual metal ions were thoroughly mixed and left standing for 5 days to complete binding.
During ultrafiltration, for each run, the first few ml of permeate were discarded. For the pre-setting of all the membranes and to maintain the constant flux, each metal ion-PEI chelate solutes were run in the ultrafiltration kit at 345 kPa (with compressed air). The permeate flux and percentage rejection were determined by analyzing concentrations of the feed and permeate [4].
The concentrations of each metal ion in permeate and feed was measured using an atomic absorption spectrophotometer. The pH of the feed and permeate solutions were measured with an Elico pH meter. In the absence of metal ions, the concentration of PEI was also confirmed by UV-visible spectrophotometer (Hitachi model U-2000) at λmax=269 nm.
Results and Discussion
During dextran rejection studies for the determination of pore statistics and MWCOs, uniform agitation in the feed side was performed or else concentration polarization and cake formation would take place on the membrane surface which would affect flux and ultimately affect the partition coefficient and aggregate size of pores.
Due to larger pore size it is impossible for ionic level rejection of metal ions. Hence to enhance the size and consequently the rejection of metal ions, a water soluble chelating polymer poly ethyleneimine (PEI) was used for the complexation of metal ions such as Cu2+, Ni2+, Zn2+ and Cd2+ and were subjected individually from aqueous streams by the PU/Cpsf blend membranes [5].
Blend membranes of 80%/20% and 75%/25% composition in the absence and in the presence of various concentrations of additive, PEG 600 from 0 to 7.5 wt% were subjected to the rejection of metal chelates and the results are given in figures I and II. All the experiments were conducted in triplicate in order to get reproducibility with negligible small deviation (Figures 1 and 2).
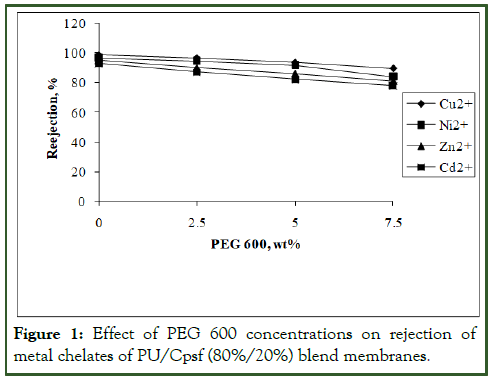
Figure 1: Effect of PEG 600 concentrations on rejection of metal chelates of PU/Cpsf (80%/20%) blend membranes.
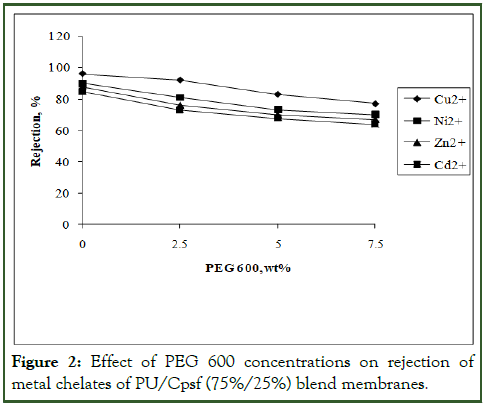
Figure 2: Effect of PEG 600 concentrations on rejection of metal chelates of PU/Cpsf (75%/25%) blend membranes.
Pore statistics
Effect of polymer blend composition: In the blend system when the composition of Cpsf is increased from 20% to 25% changes were found in pore statistics. Thus when Cpsf composition in the blend system was increased from 20 to 25% the pore radius of the blend membranes also increased from 32.68 Å to 61.86 Å as shown in table. Similarly, the percentage porosity had also been increased. However, the number of pores per unit area decreased from 2.941 × 10-10 to 1.648 × 10-10 with an increase of Cpsf from 20% to 25% as shown in table. Thenumber of pores is less at 75%/25% composition and in the absence of additive. The increase in pore radius, porosity and decrease in the number of pores per unit area due to addition of Cpsf in the system may be due to the phase separation between the polymeric components also formation of inhomogeneity leading to cavities in the sub layer of membranes. The same trend was observed in flux values of our previous studies (Table 1) [6].
| Blend composition | PEG 600, Wt% | Pore radius, R (Å) | Porosity, Ɛ (%) | No. of pores/cm2, n (Х 10-10) | MWCO, (kDa) | |
|---|---|---|---|---|---|---|
| PU | Cpsf | |||||
| 80 | 20 | 0 | 32.68 | 0.0112 | 2.941 | 42 |
| 75 | 25 | 0 | 61.86 | 0.0184 | 1.648 | 42 |
| 80 | 20 | 2.5 | 47.14 | 0.019 | 1.728 | 42 |
| 75 | 25 | 2.5 | 66.1 | 0.0206 | 1.187 | 77 |
| 80 | 20 | 5 | 49.4 | 0.021 | 1.632 | 42 |
| 75 | 25 | 5 | 69.34 | 0.0241 | 1.056 | >77 |
| 80 | 20 | 7.5 | 50.12 | 0.0383 | 1.56 | >42 |
| 75 | 25 | 7.5 | 73.02 | 0.0434 | 0.943 | >77 |
Table 1: Pore statistics and molecular weight cutoff of PU/Cpsf blend membranes.
Effect of additive concentration: Pore statistics of blend membranes has been changed by the addition of PEG 600. Hence the increase of Cpsf from 20% to 25% at 2.5 wt% PEG 600 concentration increases the porosity and the pore radius whereas number of pores per unit area decreases. This is due to the formation of large size pores in smaller number due to the inhomogeneity between Cpsf and PU in the presence of PEG 600. The same trend is obtained for 5 wt% and 7.5 wt% and similar performance were observed for flux measurements too [7].
Molecular weight cutoff
MWCO of a membrane is a parameter to specify the rejection behavior of membrane and determined by using an inert solute of stable molecules having various range of molecular weights. In general, the MWCO of a membrane is determined by the identification of an inert solute, which has the lowest molecular weight and has a solute rejection of 80%-100% in steady state UF experiments. Thus dextrans of different molecular weights such as 19 kDa, 42 kDa, 77 kDa and 150 kDa were chosen to calculate MWCO of all PU/Cpsf membranes.
Effect of polymer blend composition: Membrane of 80%/20% with 0 wt% additive PU/Cpsf composition showed highest separation for 42 kDa and the rejection was very low for 19 kDa dextran i.e., less than 80%. Hence MWCO of 80%/20% blend membrane was considered as 42 kDa. In a similar manner membrane with 75%/25% PU/Cpsf composition in the absence of additive exhibited a higher percentage separation of 92% for 42 kDa dextran but resulted low percentage separation for dextran of 19 kDa.
Effect of additive concentration: The MWCO values had a change in magnitude when additive was added into the casting solution of PU/Cpsf blend membranes. However when the additive concentration is increased from 0 to 7.5 wt% at 2.5% increment there was no appreciable change in MWCO value, since the percentage separation of 42 kDa itself fell in the least needed 80% separation to fix the MWCO. For 75%/25% PU/ Cpsf membranes, when the additive was increased to 2.5 wt% the MWCO also showed an increase in value of 77 kDa. On further increase in additive showed no appreciable change in MWCO. This phenomenon concludes that upon increase of additive increases the pore size. This increase in MWCO upon increase of additive may indicate an increase in pore size which may be due to the initiation of surface tension gradient at the gelation medium for the convective mass transfer phenomena of non-solvent to solvent or vice versa. This convective flow is influenced by the higher density of the nascent membrane, leading to the formation of macro voids and lowering the MWCO, similar results have also been observed for PU membranes by other researchers [8].
Ion Exchange Capacity (IEC)
IEC for all membranes was calculated by simple titration method.
Effect of polymer blend composition: IEC for 80/20 PU/Cpsf blend membrane was found to be 1.84 meqg-1 and for 75/25 PU/Cpsf blend membrane is 2.59 meqg-1 at 0% additive. This increase may be due to higher concentration of Cpsf in blend which may lead to liberate more number of H+ ions.
Effect of additive concentration: On increasing PEG 600 concentration to 2.5 wt% the IEC of 80/20 PU/Cpsf is slightly increased to 2.3 meqg-1. Similarly for 2.5 increment of PEG 600 the IEC value of 75/25 PU/Cpsf was found to be increased to 3.2 meqg-1. This increase in IEC with increase in PEG 600 concentration may be due to the formation of macro voids due to leaching out of PEG 600 from the membrane during gellation which becomes the domain of H+ ions. A similar trend was observed for other membranes too. It has been concluded that as more pores are opened the number of H+ ions release also increased.
Metal ion rejection studies
All the metal salts were prepared at the concentration of 1000 ppm and were complexed with a water soluble chelating polymer PEI as the ionic level separation through UF process is not possible due to larger pore size of membranes which are not suitable to reject ions and hence the salts were complexed with PEI and subsequently rejected individually from aqueous streams by the PU/Cpsf blend UF membranes.
PU/Cpsf blend UF membranes of 80%/20% and 75%/25% composition in the absence and in the presence of various concentrations of additive PEG 600 from 0 to 7.5 wt% were subjected to the rejection of metal chelates and the results were given in Figures 1 and 2. Metal ion rejection studies and permeates flux measurements were repeated twice for the sake of reproducibility. The results are reproducible with negligible small deviations [9].
Role of polymer blend composition: The rejection of metal ions were carried out with PU/Cpsf membranes in absence of additive after complexation of metal ions with the polymeric water soluble ligand PEI and the results of rejection studies are given in Figures 1 and 2.
At 20% Cpsf composition in the blend Cu2+ exhibited 99% rejection while Ni2+, Zn2+ and Cd2+ exhibited 97%, 95% and 93% respectively. On further increase to 25% Cpsf showed a decrease in rejection for all metal ions. The rejection for Cu2+, Ni2+, Zn2+ and Cd2+ are 96%, 90%, 88% and 85%. This lower rejection efficiency of 75%/25% PU/Cpsf blend membranes compared to 80%/20% blend membranes attributed to higher amount of Cpsf in the blend resulting in relatively high negatively charged membranes which would less effectively reject divalent cations. This phenomenon is referred as the Donnan effect and has been confirmed with PU/Cpsf blend membranes. The results also have good correlation with the pore statistics [10].
From this experiment, it is obvious that in all the membranes Cu2+ exhibited higher rejection than Ni2+ which in turn was higher than Zn2+. Cd2+ exhibited the lowest rejection and the size of the ion and its complex suggest the reason for the observation.
Role of additive concentration: Metal ion rejections by PU/ Cpsf membranes of various additive concentrations are shown in Figures 1 and 2.
For 80%/20% PU/Cpsf blend membranes when the additive was increased from 2.5 wt% to 7.5 wt% the rejection of Cu2+ decreased linearly from 97% to 90%. The decrease in rejection upon the increase of PEG 600 concentration in the casting solution or blend membranes may be due to the fact that the higher amount of non-solvent additive leads to formation of bigger pores during gellation because of thermo dynamical instability and to lower the free energy of the system.
In the same manner when Cpsf concentration was increased from 20% to 25% in the blend at 2.5 wt% additive concentration the rejection has decreased from 97% to 92% for copper. The same trend is observed for all the additive concentrations and may be due to the fact that the Cpsf matrix has a larger segmental gap due to its swelling behaviour and that the solvent, non-solvent replacement during gellation takes place rapidly leading to the formation of macro pores. The decrease in rejection in the presence of additive may be attributed to the rapid leaching out of pore former creating larger pores [11].
The above studies showed that the binding capacity of copper with PEI is stronger than that of other metals in the order Cu2+>Ni2+>Zn2+>Cd2+. The complexing capacity depends on the number of functional groups in the macromolecular complex and the atomic weight of the metal. Similar observation have been reported for poly (methacrylate) system. In all the cases, the metal ion complexed with PEI exhibited better rejections as compared to pure metal ion solutions as feed, due to complex formation with PEI based on Jahn-Teller distortion effect.
Metal ion permeate flux studies
The permeate flux of metal ion is essential for predicting the economics of the membrane processes and to specify the product rate. During metal rejection studies, the permeate flux studies were also been carried out simultaneously for 80%/20% and 75%/25%. The permeate flux values of PU/Cpsf membranes in the absence and in the presence of additive are shown in Figures 3 and 4.
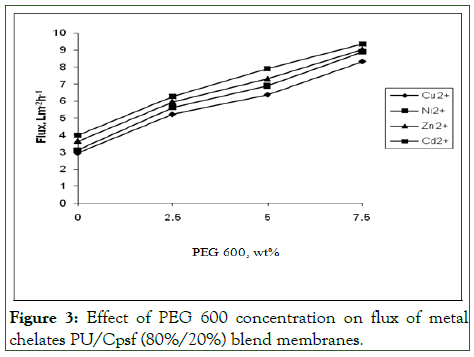
Figure 3: Effect of PEG 600 concentration on flux of metal chelates PU/Cpsf (80%/20%) blend membranes.
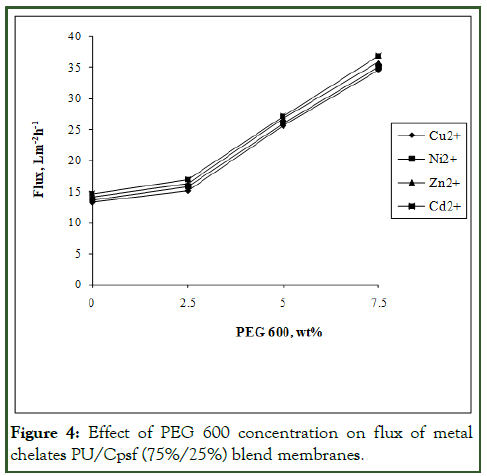
Figure 4: Effect of PEG 600 concentration on flux of metal chelates PU/Cpsf (75%/25%) blend membranes.
Role of polymer blend composition: The 80%/20% PU/Cpsf blend membrane in the absence of additive showed lower flux of 2.94 Lm-2h-1 for copper ions where the values were higher for Ni2+ (3.12), Zn2+ (3.64) and Cd2+. The flux value was highest for Cd2+ with a value of 3.99 Lm-2h-1 as depicted in Figure 3.
When Cpsf content was increased to 25 wt%, the flux value of Cu2+ also increased to 13.24 Lm-2h-1 as shown in Figure 4. All other metal ions also exhibited similar trend for the system. The increase in flux upon increase of Cpsf composition may be due to the formation of higher hydrophilicity incorporated by carboxylated polysulfone. However blend membranes yielded a highest permeate flux value for Cd2+ than for the rest of the metal ions. This trend corresponds to the decreasing metal ion size and chelating behavior with the polymeric ligand, PEI.
Role of additive concentration: As shown in the Figure 3, the additive played a major role in enhancing the permeate flux values of PU/Cpsf membranes. Thus for 80%/20% PU/Cpsf at 2.5 wt% additive copper yielded a flux of 5.21 Lm-2h-1 which is much higher than for the membrane of same composition without additive (2.94 Lm-2h-1). Further, the flux increased to 6.36 Lm-2h-1 and 8.31 Lm-2h-1 respectively when the additive concentration was increased to 5 wt% and 7.5 wt% and similar trend observed for other metal ions also.
In the same manner, for the blend membrane 75%/25% PU/ Cpsf, at 2.5 wt% additive concentrations, the flux value was 15.12 Lm-2h-1 and at 7.5 wt%, the value was 34.65 Lm-2h-1. This increase in flux due to increase in additive is because of the pore former which got leached out during gellation, creating pores. The order of flux for the metal chelate is Cd2+>Zn2+>Ni2+>Cu2+ which was primarily due to the larger metal chelate size for Cu and lowest size for Cd [12].
Similarly, for a given additive concentration of 2.5 wt%, when the Cpsf composition in the blend was increased from 20% to 25%, the flux also increased from 5.21 Lm-2h-1 to 15.12 Lm-2h-1 for the copper ion.
Similar observations were also seen for other additive concentrations. The other metal ions such as Ni, Zn and Cd showed a similar trend with flux values of 5.63 Lm-2h-1, 5.96 Lm-2h-1 and 6.26 Lm-2h-1 and of 15.86 Lm-2h-1, 16.27 and 16.93 Lm-2h-1respectively at 20% and 25% Cpsf content in the blend. Thus, the increase in flux with increasing Cpsf composition in blend favors phase separation, thereby facilitating generation of macro voids.
Conclusion
Ultrafiltration blend membranes based on polyurethane and carboxylated polysulfone in the presence and absence of additive polyethylene glycol 600 were prepared. The 80%/20% and 75%/25% PU/Cpsf blend membranes were found to be suitable composition and the maximum compatible concentration of additive was found to be 7.5 wt%. The molecular weight cutoff and pore statistical studies of blend membranes were calculated with the help of dextran of different molecular weights ranging from 19 kDa to 150 kDa, depending on the composition of polymers and concentration of the additive, PEG 600. Also the blend membranes were subjected to the rejection of toxic heavy metal ions such as Cu2+, Ni2+, Zn2+ and Cd2+. Toxic heavy metal ions were separated by complexing them with poly ethyeleneimine. The permeate flux studies have also been carried out. The polymercomposition and additive concentration were found to possess considerable impact on the rejection and permeate flux of metal ions.
Acknowledgement
The authors gratefully acknowledge Prof. Michel Guiver, national research council of canada and chemplast (I) Ltd., India, for the generous gift samples of Cpsf and PU. The first author (C.S.L) thanks the Council of Scientific and Industrial Research (CSIR), government of India, New Delhi, for the award of extended senior research fellowship.
References
- Agarwal GP, Cooney CL. Study of protein transmission through ultrafiltration membranes. J Membr Sci. 1993;85(2):111-128.
- McCoy BJ. Membrane sieving of a continuous polydisperse mixture through distributed pores. Separat Sci Technol. 1995;30(4):487-507.
- Bhattacharjee S, Kim AS, Elimelech M. Concentration polarization of interacting solute particles in cross-flow membrane filtration. J Colloid Interface Sci. 1999;212(1):81-99.
[Crossref] [Google Scholar] [PubMed]
- Broens L, Altena FW, Smolders CA, Koenhen DM. Asymmetric membrane structures as a result of phase separation phenomena. Desalination. 1980;32:33-45.
- Brousse CL, Chapurlat R, Quentin JP. New membranes for reverse osmosis I. Characteristics of the base polymer: Sulphonated polysulphones. Desalination. 1976;18(2):137-153.
- Dizman C, Tasdelen MA, Yagci Y. Recent advances in the preparation of functionalized polysulfones. Polym Int. 2013;62(7):991-1007.
- Chen MH, Chiao TC, Tseng TW. Preparation of sulfonated polysulfone/polysulfone and aminated polysulfone/polysulfone blend membranes. J Appl Polym Sci. 1996;61(7):1205-1209.
- Han MJ, Bhattacharyya D. Characterization of reverse osmosis cellulose acetate membranes by gas adsorption method: Effect of casting variables and chlorine damage. J Membr Sci. 1991;62(3):325-346.
- Hernandez AC, Calvo JI, Pradanos P, Tejerina F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J Membr Sci. 1996;112(1):1-2.
- Hong S, Faibish RS, Elimelech M. Kinetics of permeate flux decline in crossflow membrane filtration of colloidal suspensions. J Colloid Interface Sci. 1997;196(2):267-277.
[Crossref] [Google Scholar] [PubMed]
- Juang RS, Chen MN. Measurement of binding constants of poly (ethylenimine) with metal ions and metal chelates in aqueous media by ultrafiltration. Ind Eng Chem Res. 1996;35(6):1935-1943.
- Latha CS, Shanthanalakshmi D, Mohan D, Balu K, Kumarasamy MD. Polyurethane and carboxylated polysulfone blend ultrafiltration membranes. I. Preparation and characterization. J Appl Polym Sci. 2005;97(3):1307-1315.
Citation: Lakshmi DS, Latha CS, Mohan D (2024) PU and Cpsf Blend UF Membranes. II. Pore Statistics, MWCO and Application Studies. J Membr Sci Technol. 14:394.
Copyright: © 2024 Lakshmi DS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.